The Role of p53 Protein in the Realization of the Exogenous Heat Shock Protein 70 Anti-Apoptotic Effect during Axotomy
Abstract
1. Introduction
2. Methods
2.1. Expression and Purification of Recombinant Hsp70
2.2. Fluorescent Labeling of Recombinant Hsp70
2.3. Isolation of Crayfish MRN
2.4. Assessing the Effect of Exogenous Hsp70 on Necrosis and Apoptosis
2.5. Application of p53 Activators
2.6. Immunohistochemical Analysis of Crayfish MRN
2.7. Bilaterally Axotomized Ganglia of Crayfish Ventral Nerve Cord
2.8. Immunoblotting
2.9. Statistical Analysis
3. Results
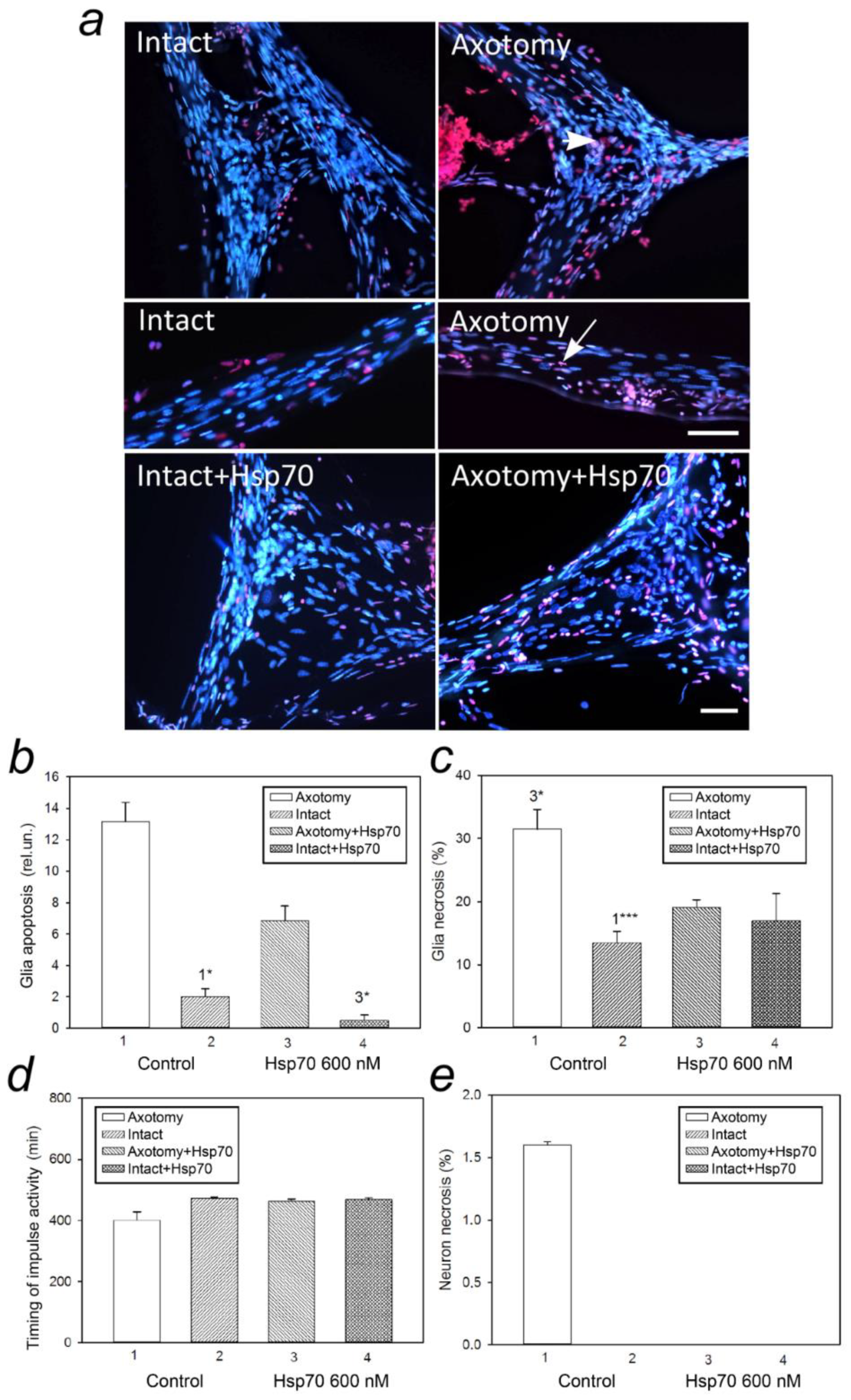
3.1. eHsp70 Reduces Apoptosis and Necrosis of Glial Cells after Axotomy
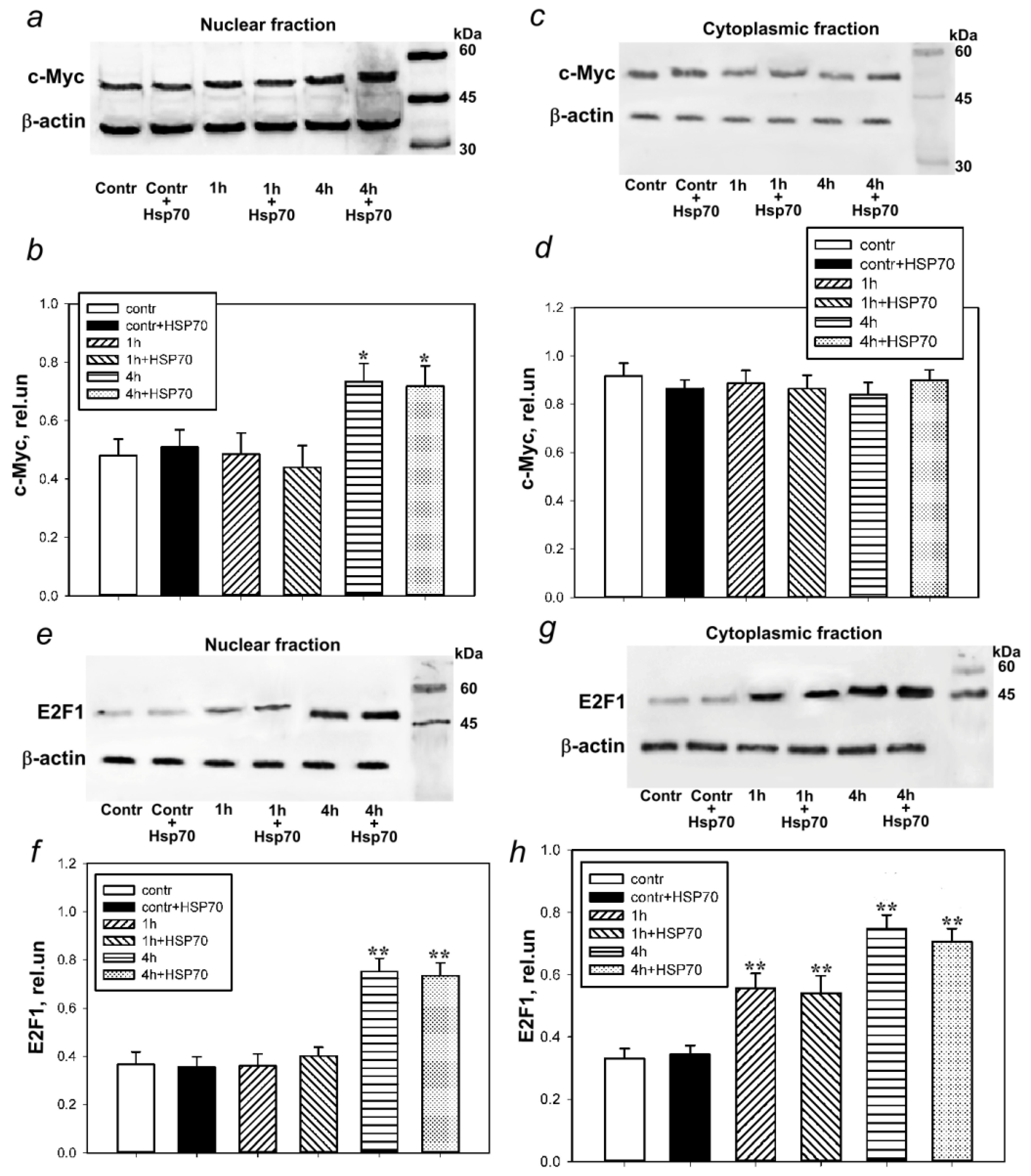
3.2. Hsp70 Downregulates p53 Expression in Bilaterally Axotomized Crayfish Ventral Nerve Cord Ganglia without Affecting c-Myc and E2F1 Expression
3.3. eHsp70 Downregulates p53 Expression in the Mechanoreceptor Neuron of the Crayfish Stretch Receptor
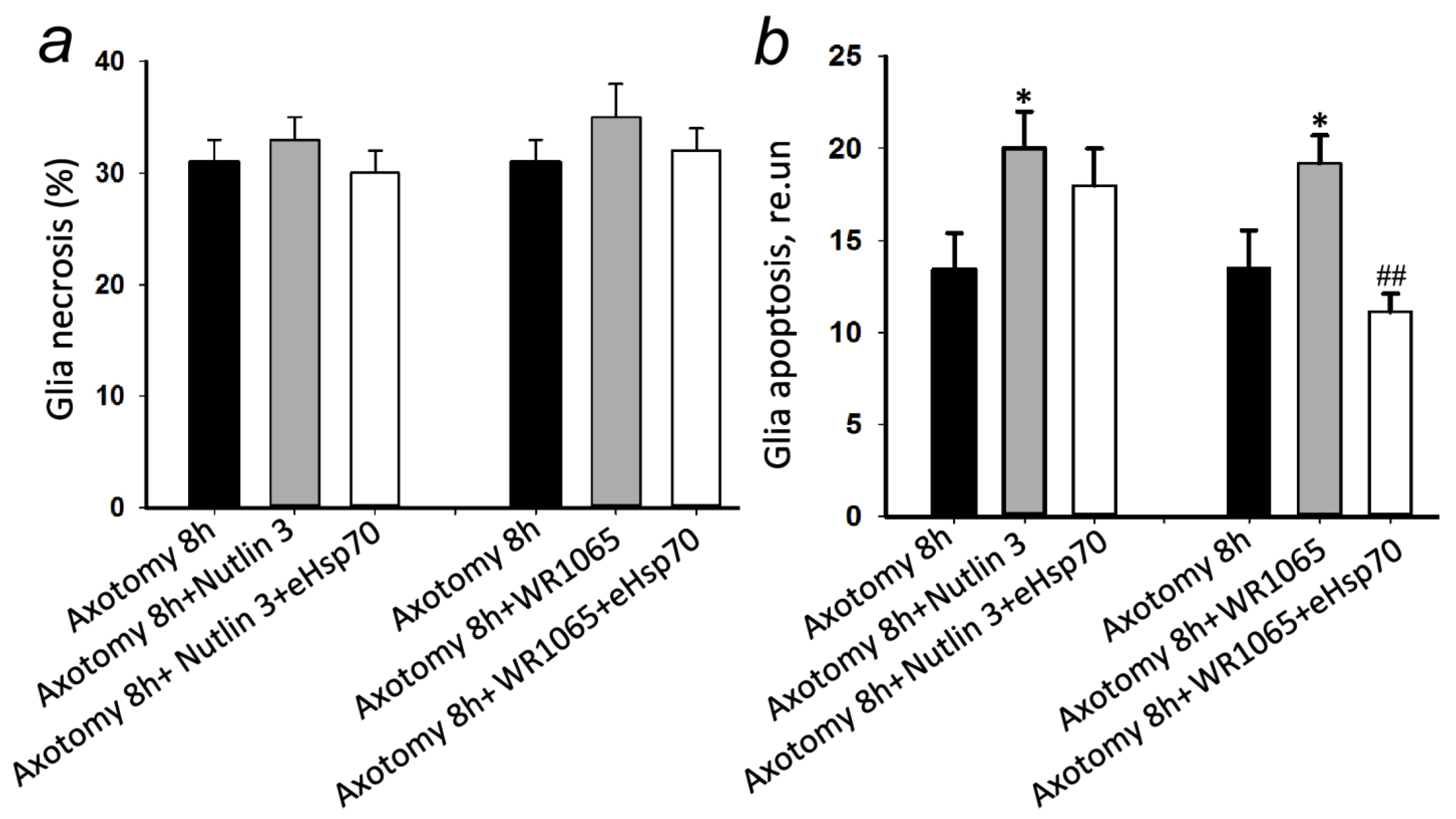
3.4. eHsp70 Reduces Glial Apoptosis Caused by the Synergistic Effect of Axotomy and p53 Activator WR1065
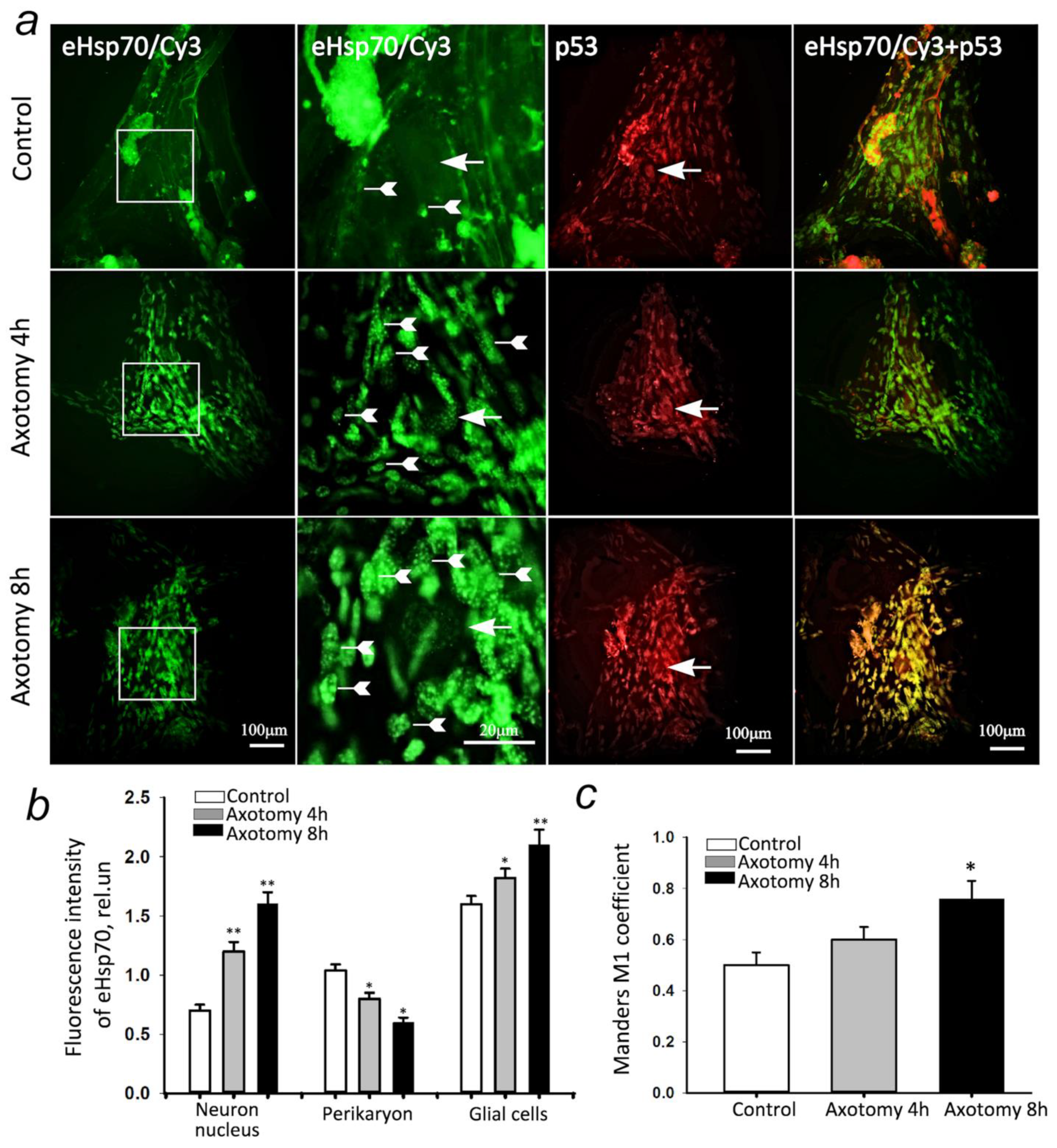
3.5. eHsp70 Accumulates in the Neurons and Glial Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berkner, J.; Mannix, R.; Qiu, J. Clinical traumatic brain injury in the preclinical setting. Methods Mol. Biol. 2016, 1462, 11–28. [Google Scholar] [CrossRef]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 317–318. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Pan, Y.; Chen, L.; Zhang, Z.; Lu, L. Peripheral nerve injuries treatment: A systematic review. Cell Biochem. Biophys. 2014, 68, 449–454. [Google Scholar] [CrossRef]
- Casas, C.; Isus, L.; Herrando-Grabulosa, M.; Mancuso, F.M.; Borrás, E.; Sabidó, E.; Forés, J.; Aloy, P. Network-based proteomic approaches reveal the neurodegenerative, neuroprotective and pain-related mechanisms involved after retrograde axonal damage. Sci. Rep. 2015, 18, 9185. [Google Scholar] [CrossRef]
- Demyanenko, S.; Dzreyan, V.; Uzdensky, A. Axotomy-induced changes of the protein profile in the crayfish ventral cord ganglia. J. Mol. Neurosci. 2019, 68, 667–678. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Rodkin, S.; Khaitin, A.; Pitinova, M.; Dzreyan, V.; Guzenko, V.; Rudkovskii, M.; Sharifulina, S.; Uzdensky, A. The Localization of p53 in the Crayfish Mechanoreceptor Neurons and Its Role in Axotomy-Induced Death of Satellite Glial Cells Remote from the Axon Transection Site. J. Mol. Neurosci. 2020, 70, 532–541. [Google Scholar] [CrossRef]
- Wadhwa, R.; Yaguchi, T.; Hasan, M.K.; Mitsui, Y.; Reddel, R.R.; Kaul, S.C. Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein. Exp. Cell Res. 2002, 274, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bonner, H.P.; Concannon, C.G.; Bonner, C.; Woods, I.; Ward, M.W.; Prehn, J.H. Differential expression patterns of Puma and Hsp70 following proteasomal stress in the hippocampus are key determinants of neuronal vulnerability. J. Neurochem. 2010, 114, 606–616. [Google Scholar] [CrossRef]
- Amadio, M.; Scapagnini, G.; Laforenza, U.; Intrieri, M.; Romeo, L.; Govoni, S.; Pascale, A. Post-transcriptional regulation of HSP70 expression following oxidative stress in SH-SY5Y cells: The potential involvement of the RNA-binding protein HuR. Curr. Pharm. Des. 2008, 14, 2651–2658. [Google Scholar] [CrossRef]
- Smedowski, A.; Liu, X.; Podracka, L.; Akhtar, S.; Trzeciecka, A.; Pietrucha-Dutczak, M.; Lewin-Kowalik, J.; Urtti, A.; Ruponen, M.; Kaarniranta, K.; et al. Increased intraocular pressure alters the cellular distribution of HuR protein in retinal ganglion cells—A possible sign of endogenous neuroprotection failure. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A. Heat shock proteins and neuroprotection. Adv. Exp. Med. Biol. 2002, 513, 281–299. [Google Scholar] [CrossRef]
- Mohammadi, F.; Nezafat, N.; Negahdaripour, M.; Dabbagh, F.; Haghghi, A.B.; Kianpour, S.; Banihashemi, M.; Ghasemi, Y. Neuroprotective Effects of Heat Shock Protein70. CNS Neurol. Disord. Drug Targets 2018, 17, 736–742. [Google Scholar] [CrossRef]
- Houenou, L.J.; Li, L.; Lei, M.; Kent, C.R.; Tytell, M. Exogenous heat shock cognate protein Hsc 70 prevents axotomy-induced death of spinal sensory neurons. Cell Stress Chaperones 1996, 1, 161–166. [Google Scholar] [CrossRef]
- Zatsepina, O.G.; Evgen’ev, M.B.; Garbuz, D.G. Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells 2021, 10, 1638. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Saidi, L.-J.; Wahlster, L. Molecular chaperones and protein folding as therapeutic targets in Parkinson’s disease and other synucleinopathies. Acta Neuropathol. Commun. 2013, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, J.L.; Houenou, L.J.; Tytell, M. Administration of Hsp70 in vivo inhibits motor and sensory neuron degeneration. Cell Stress Chaperones 2004, 9, 88–98. [Google Scholar] [CrossRef]
- Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS ONE 2013, 8, e62687. [Google Scholar] [CrossRef]
- Gurskiy, Y.G.; Garbuz, D.G.; Soshnikova, N.V.; Krasnov, A.N.; Deikin, A.; Lazarev, V.F.; Sverchinskyi, D.; Margulis, B.A.; Zatsepina, O.G.; Karpov, V.L.; et al. The development of modified human Hsp70 (HSPA1A) and its production in the milk of transgenic mice. Cell Stress Chaperones 2016, 21, 1055–1064. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Gehrmann, M.; Brunet, M.; Multhoff, G.; Garrido, C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukoc. Biol. 2007, 81, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Garbuz, D.G.; Zatsepina, O.G.; Evgen’ev, M.B. The Major Human Stress Protein Hsp70 as a Factor of Protein Homeostasis and a Cytokine-Like Regulator. Mol. Biol. 2019, 53, 200–217. [Google Scholar] [CrossRef]
- Demyanenko, S.; Nikul, V.; Rodkin, S.; Davletshin, A.; Evgen’ev, M.B.; Garbuz, D.G. Exogenous recombinant Hsp70 mediates neuroprotection after photothrombotic stroke. Cell Stress Chaperones 2021, 26, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Evgen’ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Snoeckx, L.H.; Cornelussen, R.N.; Van Nieuwenhoven, F.A.; Reneman, R.S.; Van Der Vusse, G.J. Heat shock proteins and cardiovascular pathophysiology. Physiol. Rev. 2001, 81, 1461–1497. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, G.M.; Uzdensky, A.B. Ultrastructure of neuroglial contacts in crayfish stretch receptor. Cell Tissue Res. 2009, 337, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Rudkovskii, M.V.; Fedorenko, A.G.; Khaitin, A.M.; Pitinova, M.A.; Uzdensky, A.B. The effect of axotomy on firing and ultrastructure of the crayfish mechanoreceptor neurons and satellite glial cells. Mol. Cell Neurosci. 2020, 107, 103534. [Google Scholar] [CrossRef] [PubMed]
- Sheller, R.A.; Smyers, M.E.; Grossfeld, R.M.; Ballinger, M.L.; Bittner, G.D. Heat-shock proteins in axoplasm: High constitutive levels and transfer of inducible isoforms from glia. J. Comp. Neurol. 1998, 396, 1–11. [Google Scholar] [CrossRef]
- Fang, D.A.; Wang, Q.; He, L.; Wang, J.; Wang, Y. Characterization of heat shock protein 70 in the red claw crayfish (Cherax quadricarinatus): Evidence for its role in regulating spermatogenesis. Gene 2012, 492, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lee, J.S.; Ko, Y.G.; Kim, J.I.; Seo, J.S. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J. Biol. Chem. 2000, 275, 25665–25671. [Google Scholar] [CrossRef]
- Saleh, A.; Srinivasula, S.M.; Balkir, L.; Robbins, P.D.; Alnemri, E.S. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2000, 2, 476–483. [Google Scholar] [CrossRef]
- Omori, H.; Otsu, M.; Nogami, H.; Shibata, M. Heat shock response enhanced by cell culture treatment in mouse embryonic stem cell-derived proliferating neural stem cells. PLoS ONE 2021, 16, e0249954. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Meriin, A.B.; Sherman, M.Y.; Morimoto, R.I.; Massie, B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell Biol. 2000, 20, 7146–7159. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, L.; Gurbuxani, S.; Susin, S.A.; Maisse, C.; Daugas, E.; Zamzami, N.; Mak, T.; Jäättelä, M.; Penninger, J.M.; Garrido, C.; et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 2001, 3, 839–843. [Google Scholar] [CrossRef]
- Russell, J.L.; Powers, J.T.; Rounbehler, R.J.; Rogers, P.M.; Conti, C.J.; Johnson, D.G. ARF differentially modulates apoptosis induced by E2F1 and Myc. Mol. Cell Biol. 2002, 22, 1360–1368. [Google Scholar] [CrossRef]
- Hong, S.; Pusapati, R.V.; Powers, J.T.; Johnson, D.G. Oncogenes and the DNA damage response: Myc and E2F1 engage the ATM signaling pathway to activate p53 and induce apoptosis. Cell Cycle 2006, 5, 801–803. [Google Scholar] [CrossRef][Green Version]
- Ma, L.; Yu, H.J.; Gan, S.W.; Gong, R.; Mou, K.J.; Xue, J.; Sun, S.Q. p53-Mediated oligodendrocyte apoptosis initiates demyelination after compressed spinal cord injury by enhancing ER-mitochondria interaction and E2F1 expression. Neurosci. Lett. 2017, 644, 55–61. [Google Scholar] [CrossRef]
- Garimella, R.; Liu, X.; Qiao, W.; Liang, X.; Zuiderweg, E.R.; Riley, M.I.; Van Doren, S.R. Hsc70 contacts helix III of the J domain from polyomavirus T antigens: Addressing a dilemma in the chaperone hypothesis of how they release E2F from pRb. Biochemistry 2006, 45, 6917–6929. [Google Scholar] [CrossRef]
- Wang, D.B.; Kinoshita, C.; Kinoshita, Y.; Morrison, R.S. p53 and mitochondrial function in neurons. J. Biochim. Biophys. Acta 2014, 1842, 1186–1197. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Moll, U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta 2009, 1787, 414–420. [Google Scholar] [CrossRef]
- Dzreyan, V.; Rodkin, S.; Nikul, V.; Pitinova, M.; Uzdensky, A. The Expression of E2F1, p53, and Caspase 3 in the Rat Dorsal Root Ganglia After Sciatic Nerve Transection. J. Mol. Neurosci. 2021, 71, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.Y.; Adler, V.; Buschmann, T.; Yin, Z.; Wu, X.; Jones, S.N.; Ronai, Z. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 1998, 12, 2658–2663. [Google Scholar] [CrossRef]
- Arya, A.K.; El-Fert, A.; Devling, T.; Eccles, R.M.; Aslam, M.A.; Rubbi, C.P.; Vlatković, N.; Fenwick, J.; Lloyd, B.H.; Sibson, D.R.; et al. Nutlin-3, the small-molecule inhibitor of MDM2, promotes senescence and radiosensitises laryngeal carcinoma cells harbouring wild-type p53. Br. J. Cancer 2010, 103, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Haaland, I.; Opsahl, J.A.; Berven, F.S.; Reikvam, H.; Fredly, H.K.; Haugse, R.; Thiede, B.; McCormack, E.; Lain, S.; Bruserud, O.; et al. Molecular mechanisms of nutlin-3 involve acetylation of p53, histones and heat shock proteins in acute myeloid leukemia. Mol. Cancer 2014, 13, 116. [Google Scholar] [CrossRef]
- Pluquet, O.; North, S.; Bhoumik, A.; Dimas, K.; Ronai, Z.; Hainaut, P. The cytoprotective aminothiol WR1065 activates p53 through a non-genotoxic signaling pathway involving c-Jun N-terminal kinase. J. Biol. Chem. 2003, 278, 11879–11887. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, E.H.; Poot, A.A.; Feijen, J.; Vermes, I. Hsp70- and p53-reponses after heat treatment and/or X-irradiation mediate the susceptibility of hematopoietic cells to undergo apoptosis. Int. J. Radiat. Biol. 2008, 84, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Requena, J.M.; Martin, J.; Alonso, C.; López, M.C. Cytoplasmic-nuclear translocation of the Hsp70 protein during environmental stress in Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 1993, 196, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Willsie, J.K.; Clegg, J.S. Small heat shock protein p26 associates with nuclear lamins and HSP70 in nuclei and nuclear matrix fractions from stressed cells. J. Cell. Biochem. 2002, 84, 601–614. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demyanenko, S.V.; Pitinova, M.A.; Dzreyan, V.A.; Kalyuzhnaya, Y.N.; Eid, M.A.; Abramov, A.Y.; Evgen’ev, M.B.; Garbuz, D.G. The Role of p53 Protein in the Realization of the Exogenous Heat Shock Protein 70 Anti-Apoptotic Effect during Axotomy. Cells 2022, 11, 93. https://doi.org/10.3390/cells11010093
Demyanenko SV, Pitinova MA, Dzreyan VA, Kalyuzhnaya YN, Eid MA, Abramov AY, Evgen’ev MB, Garbuz DG. The Role of p53 Protein in the Realization of the Exogenous Heat Shock Protein 70 Anti-Apoptotic Effect during Axotomy. Cells. 2022; 11(1):93. https://doi.org/10.3390/cells11010093
Chicago/Turabian StyleDemyanenko, Svetlana V., Maria A. Pitinova, Valentina A. Dzreyan, Yuliya N. Kalyuzhnaya, Moez A. Eid, Andrey Y. Abramov, Michael B. Evgen’ev, and David G. Garbuz. 2022. "The Role of p53 Protein in the Realization of the Exogenous Heat Shock Protein 70 Anti-Apoptotic Effect during Axotomy" Cells 11, no. 1: 93. https://doi.org/10.3390/cells11010093
APA StyleDemyanenko, S. V., Pitinova, M. A., Dzreyan, V. A., Kalyuzhnaya, Y. N., Eid, M. A., Abramov, A. Y., Evgen’ev, M. B., & Garbuz, D. G. (2022). The Role of p53 Protein in the Realization of the Exogenous Heat Shock Protein 70 Anti-Apoptotic Effect during Axotomy. Cells, 11(1), 93. https://doi.org/10.3390/cells11010093







