Elastic Laminar Reorganization Occurs with Outward Diameter Expansion during Collateral Artery Growth and Requires Lysyl Oxidase for Stabilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models and Tissue Harvest
2.2. Human Arterial Tissue
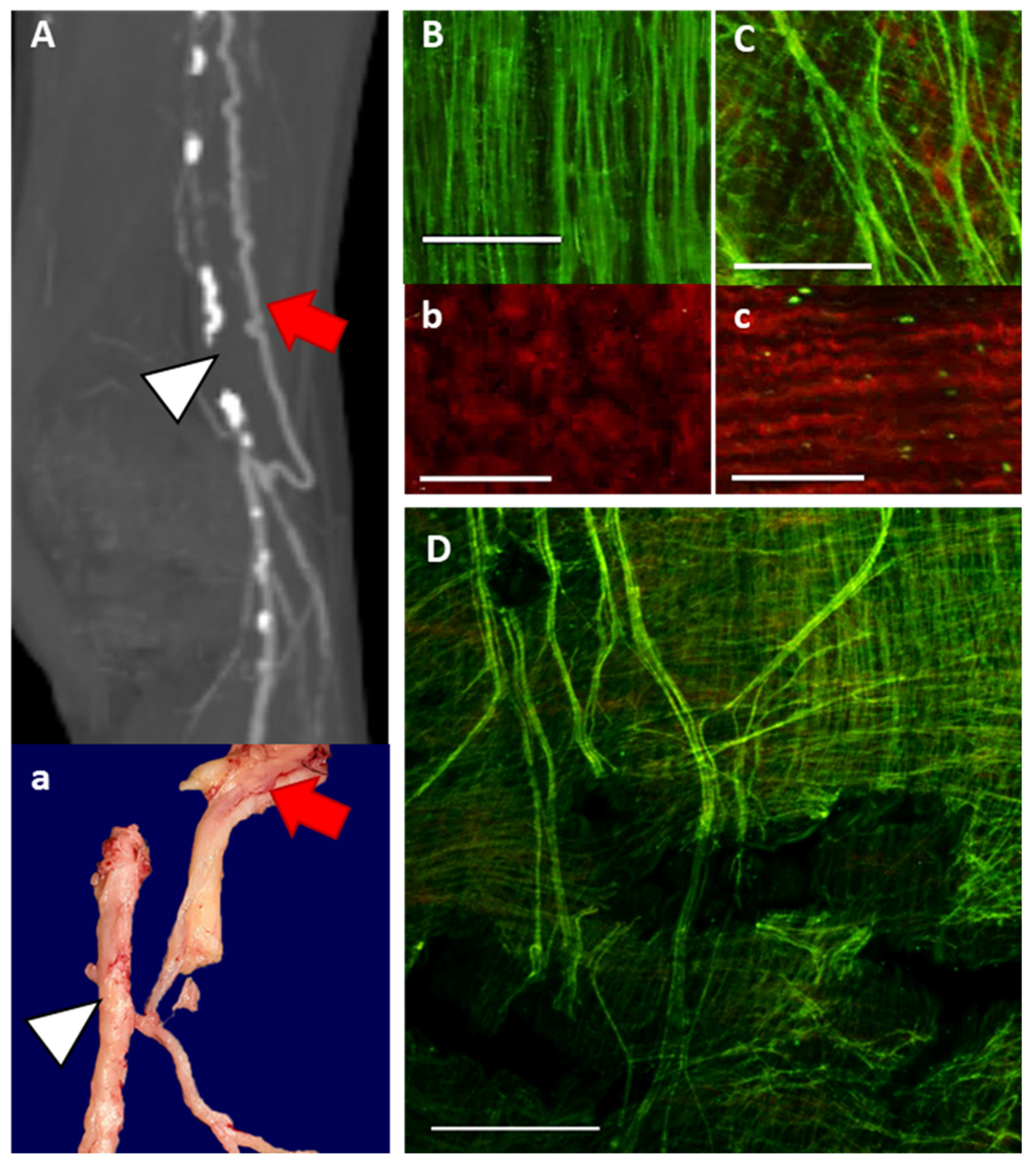
2.3. Multiphoton Microscopy
2.4. Isolation of Desmosine from Blood and Tissue
2.5. Morphometric Analysis of Internal Elastic Lamina Fenestrae and Adventitial Collagens
2.6. Microfil Casting of Arterial Networks
2.7. mRNA Harvest and qPCR
2.8. Statistical Analysis
3. Results
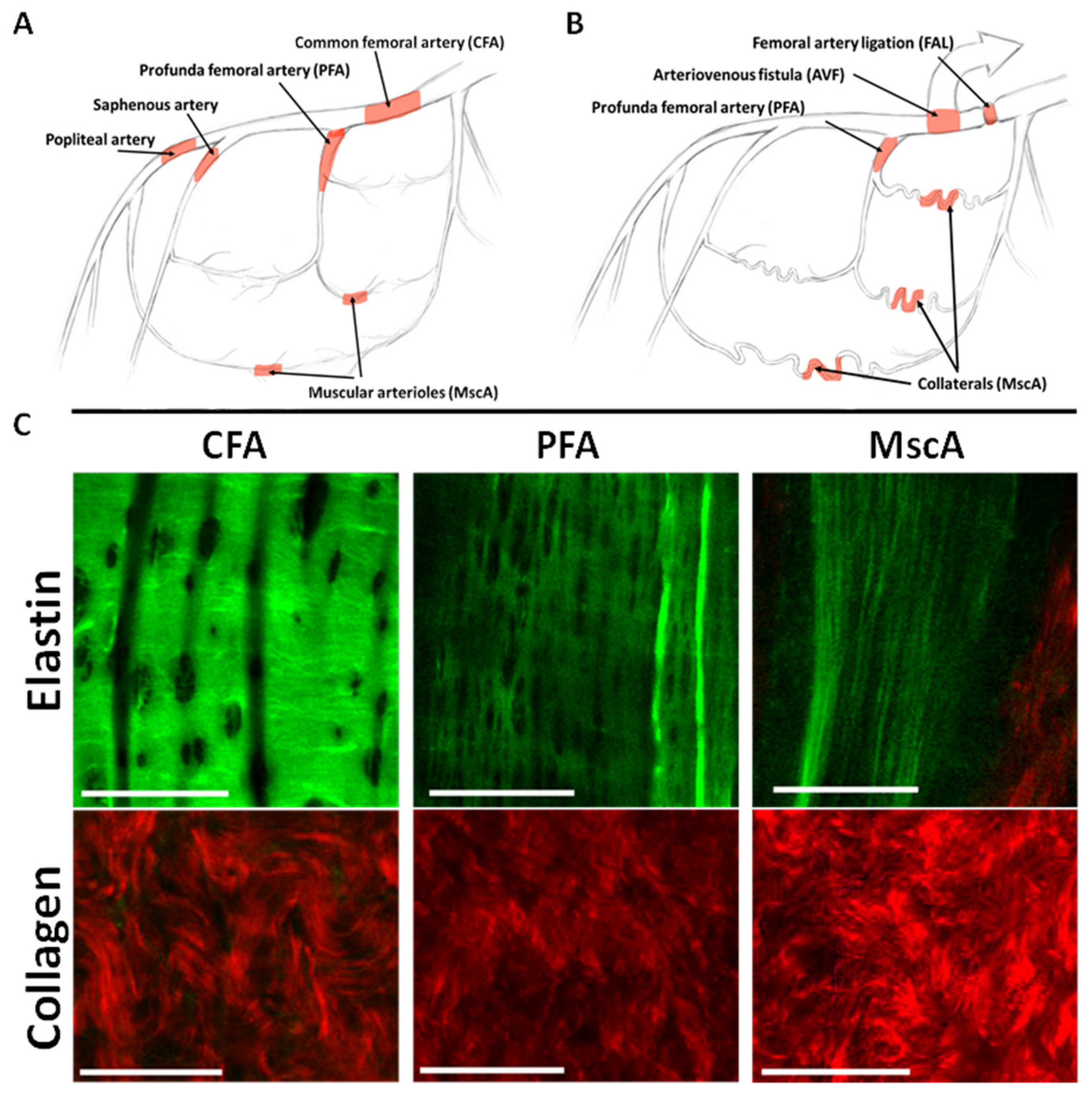
3.1. Baseline IEL and Collagen Morphology and Composition in Lower Limb Arterial Tree
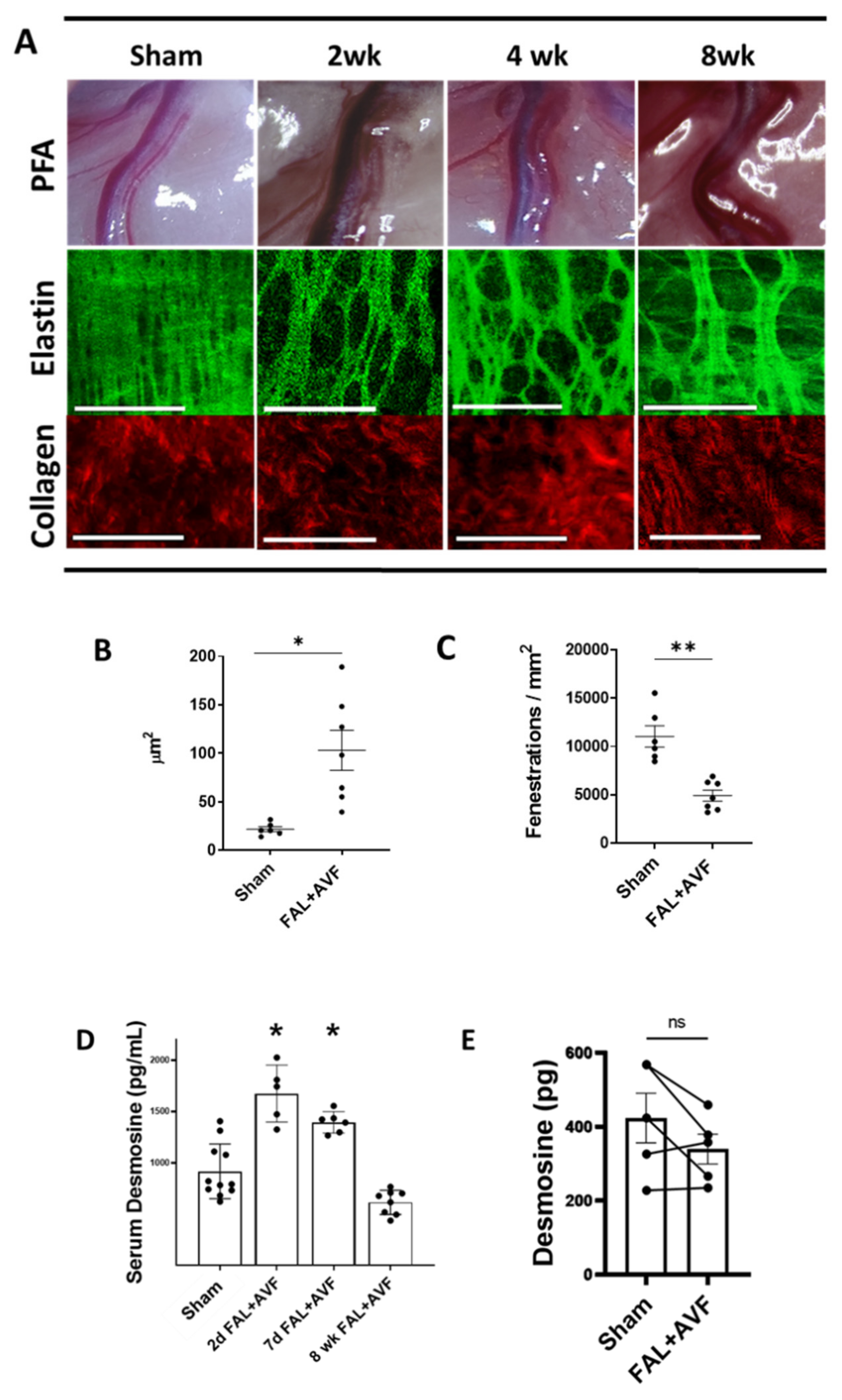
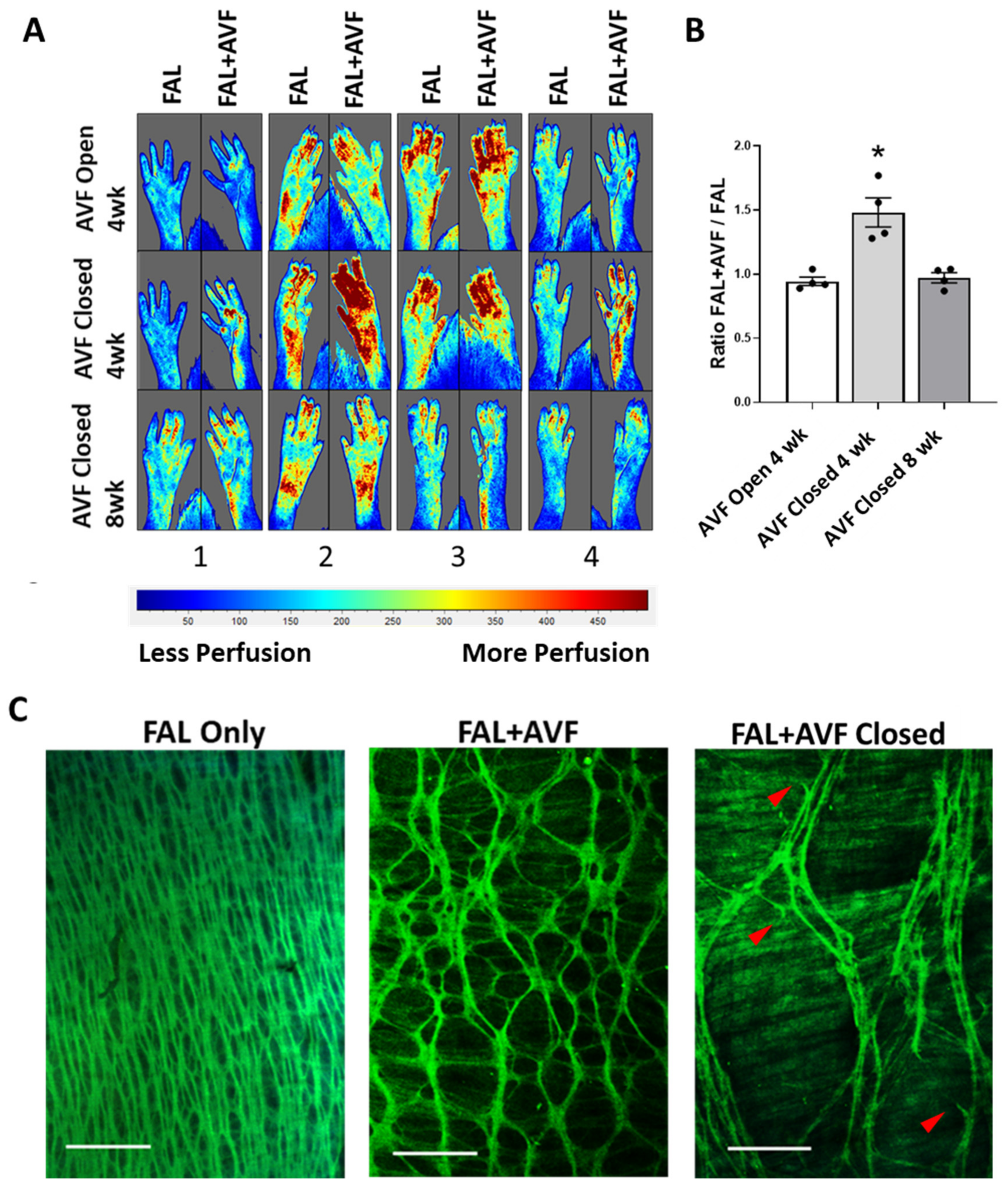
3.2. Structural Remodeling of IEL during Arteriogenesis
3.3. Collagen Strain Increases as Function of Fold-Increase over Normal Diameter in Remodeled Inter-Arteriolar Collaterals
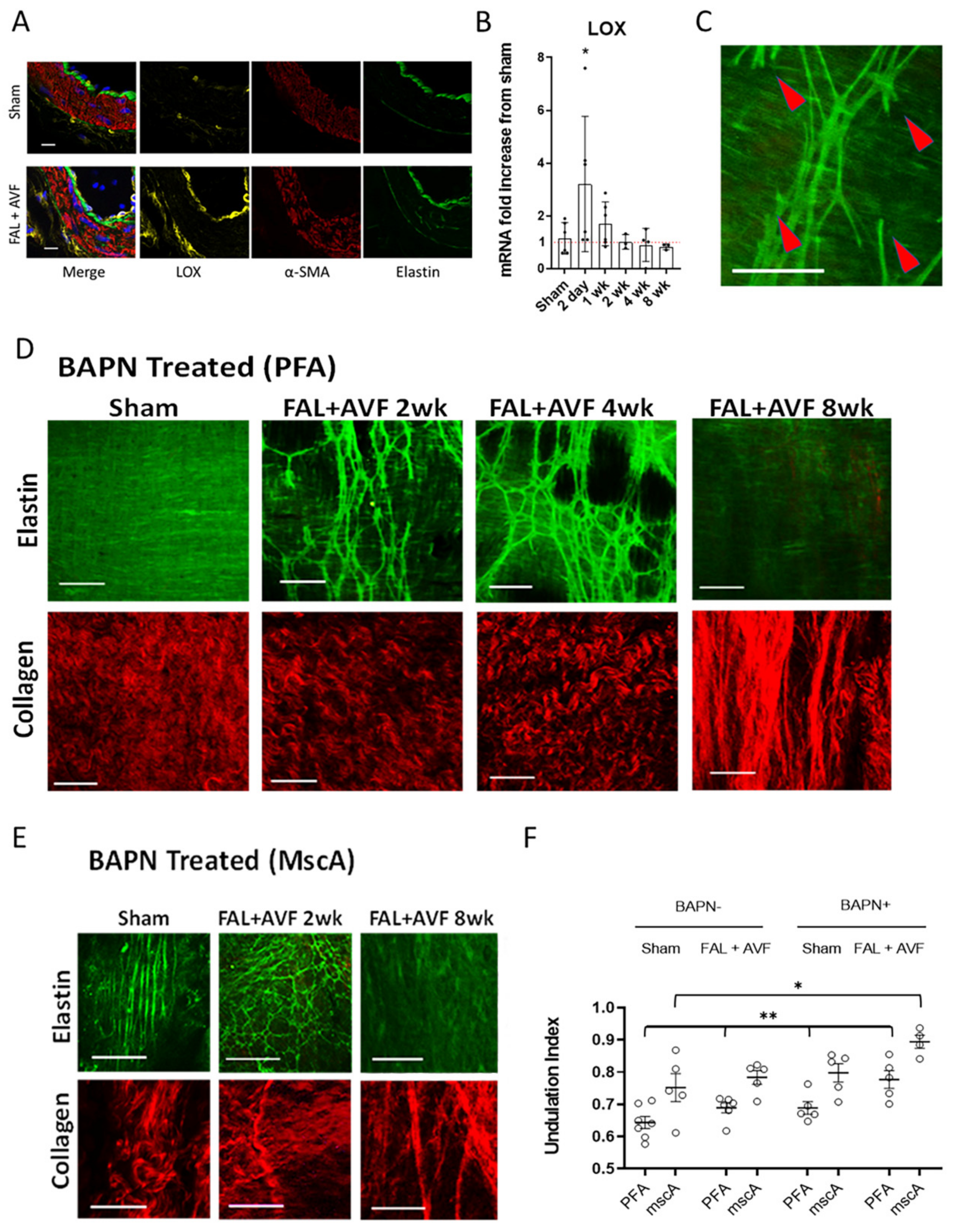
3.4. LOX Is Required for Elastic Tissue Stabilization during Outward Remodeling
3.5. ECM Characteristics of Human Collateral Arteries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoop, W.; Schaper, W.; Schaper, J. Collateral Circulation: Heart, Brain, Kidneys, Limbs; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Qiaozhen, L.; Liu, Q.; Hu, T.; Huang, X.; Zhang, H.; Tian, X.; Yan, Y.; Wang, L.; Huang, Y.; Miquerol, L.; et al. Genetic lineage tracing discloses arteriogenesis as the main mechanism for collateral growth in the mouse heart. Cardiovasc. Res. 2016, 109, 419–430. [Google Scholar] [CrossRef]
- Berry, C.; Balachandran, K.P.; L’Allier, P.L.; Lespérance, J.; Bonan, R.; Oldroyd, K.G. Importance of collateral circulation in coronary heart disease. Eur. Heart J. 2007, 28, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Ito, W.D.; Arras, M.; Scholz, D.; Winkler, B.; Htun, P.; Schaper, W. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am. J. Physiol. 1997, 273, H1255–H1265. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Ziegelhoeffer, T.; Helisch, A.; Wagner, S.; Friedrich, C.; Podzuweit, T.; Schaper, W. Contribution of Arteriogenesis and Angiogenesis to Postocclusive Hindlimb Perfusion in Mice. J. Mol. Cell. Cardiol. 2002, 34, 775–787. [Google Scholar] [CrossRef]
- Buschmannn, I.; Heil, M.; Jost, M.; Schaper, W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation 2003, 10, 371–379. [Google Scholar] [CrossRef]
- Heil, M.; Schaper, W. Influence of Mechanical, Cellular, and Molecular Factors on Collateral Artery Growth (Arteriogenesis). Circ. Res. 2004, 95, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Schaper, W. Collateral circulation: Past and present. Basic Res. Cardiol. 2009, 104, 5–21. [Google Scholar] [CrossRef]
- Dodd, T.; Jadhav, R.; Wiggins, L.; Stewart, J.; Smith, E.; Russell, J.C.; Rocic, P. MMPs 2 and 9 are essential for coronary collateral growth and are prominently regulated by p38 MAPK. J. Mol. Cell. Cardiol. 2011, 51, 1015–1025. [Google Scholar] [CrossRef]
- Cai, W.-J.; Vosschulte, R.; Afsah-Hedjri, A.; Koltai, S.; Kocsis, E.; Scholz, D.; Kostin, S.; Schaper, W.; Schaper, J. Altered Balance Between Extracellular Proteolysis and Antiproteolysis is Associated with Adaptive Coronary Arteriogenesis. J. Mol. Cell. Cardiol. 2000, 32, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Schaper, W. Insights into pathways of arteriogenesis. Curr. Pharm. Biotechnol. 2007, 8, 35–42. [Google Scholar] [CrossRef]
- López-Guimet, J.; Andilla, J.; Loza-Alvarez, P.; Egea, G. High-Resolution Morphological Approach to Analyse Elastic Laminae Injuries of the Ascending Aorta in a Murine Model of Marfan Syndrome. Sci. Rep. 2017, 7, 1505. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.K.; Murthy, S.; Phan, S.; Xu, C.; Buchanan, J.; Spilker, R.; Dalman, R.L.; Zarins, C.K.; Denk, W.; Taylor, C.A. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biol. 2008, 27, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.A.; Green, B.R.; Eskay, M.A.; Kotlarczyk, M.; Hill, M.; Robertson, A.; Watkins, S.; Vorp, D.; Gleason, T.G. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 2014, 147, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Boulesteix, T.; Pena, A.-M.; Pagès, N.; Godeau, G.; Sauviat, M.-P.; Beaurepaire, E.; Schanne-Klein, M.-C. Micrometer scaleEx Vivo multiphoton imaging of unstained arterial wall structure. Cytom. Part A 2006, 69A, 20–26. [Google Scholar] [CrossRef]
- McEnaney, R.M.; McCreary, D.; Tzeng, E. A modified rat model of hindlimb ischemia for augmentation and functional measurement of arteriogenesis. J. Biol. Methods 2018, 5, e89. [Google Scholar] [CrossRef]
- Eitenmüller, I.; Volger, O.; Kluge, A.; Troidl, K.; Barancik, M.; Cai, W.-J.; Heil, M.; Pipp, F.; Fischer, S.; Horrevoets, A.J.G.; et al. The Range of Adaptation by Collateral Vessels After Femoral Artery Occlusion. Circ. Res. 2006, 99, 656–662. [Google Scholar] [CrossRef]
- Tinker, D.; Rucker, R.B. Role of selected nutrients in synthesis, accumulation, and chemical modification of connective tissue proteins. Physiol. Rev. 1985, 65, 607–657. [Google Scholar] [CrossRef]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; Van De Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2011, 11, 461–473. [Google Scholar] [CrossRef]
- Longland, C.J. The collateral circulation of the limb; Arris and Gale lecture delivered at the Royal College of Surgeons of England on 4th February, 1953. Ann. R. Coll. Surg. Engl. 1953, 13, 161–176. [Google Scholar]
- Arribas, S.M.; Briones, A.M.; Bellingham, C.; Gonzalez-Garcia, M.C.; Salaices, M.; Liu, K.; Wang, Y.; Hinek, A. Heightened aberrant deposition of hard-wearing elastin in conduit arteries of prehypertensive SHR is associated with increased stiffness and inward remodeling. Am. J. Physiol. Circ. Physiol. 2008, 295, H2299–H2307. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; González, J.M.; Somoza, B.; Giraldo, J.; Daly, C.J.; Vila, E.; González, M.C.; McGrath, J.; Arribas, S.M. Role of Elastin in Spontaneously Hypertensive Rat Small Mesenteric Artery Remodelling. J. Physiol. 2003, 552, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Schaper, W.; Scholz, D. Factors Regulating Arteriogenesis. Arter. Thromb. Vasc. Biol. 2003, 23, 1143–1151. [Google Scholar] [CrossRef]
- Zeinali-Davarani, S.; Wang, Y.; Chow, M.-J.; Turcotte, R.; Zhang, Y. Contribution of Collagen Fiber Undulation to Regional Biomechanical Properties Along Porcine Thoracic Aorta. J. Biomech. Eng. 2015, 137, 0510011–05100110. [Google Scholar] [CrossRef]
- Broisat, A.; Toczek, J.; Mesnier, N.; Tracqui, P.; Ghezzi, C.; Ohayon, J.; Riou, L.M. Assessing low levels of mechanical stress in aortic atherosclerotic lesions from apolipoprotein E-/- mice--brief report. Arter. Thromb Vasc. Biol. 2011, 31, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H.; Glagov, S. Structural Basis for the Static Mechanical Properties of the Aortic Media. Circ. Res. 1964, 14, 400–413. [Google Scholar] [CrossRef]
- Roach, M.R. The Structure and Elastic Properties of Arterial Junctions. Connect. Tissue Res. 1986, 15, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Krasny, W.; Morin, C.; Magoariec, H.; Avril, S. A comprehensive study of layer-specific morphological changes in the microstructure of carotid arteries under uniaxial load. Acta Biomater. 2017, 57, 342–351. [Google Scholar] [CrossRef]
- Chow, M.-J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen. Biophys. J. 2014, 106, 2684–2692. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Vascular Extracellular Matrix and Arterial Mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef]
- Shadwick, R.E. Mechanical design in arteries. J. Exp. Biol. 1999, 202, 3305–3313. [Google Scholar] [CrossRef]
- Roach, M.R.; Burton, A.C. The reason for the shape of the distensibility curves of arteries. Can. J. Biochem. Physiol. 1957, 35, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Mecham, R.P. New insights into elastic fiber assembly. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 229–240. [Google Scholar] [CrossRef]
- Kozel, B.A.; Mecham, R.P. Elastic fiber ultrastructure and assembly. Matrix Biol. 2019, 84, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Cleary, E.; Cliff, W. The substructure of elastin. Exp. Mol. Pathol. 1978, 28, 227–246. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef]
- Ooshima, A.; Fuller, G.C.; Cardinale, G.J.; Spector, S.; Udenfriend, S. Increased Collagen Synthesis in Blood Vessels of Hypertensive Rats and Its Reversal by Antihypertensive Agents. Proc. Natl. Acad. Sci. USA 1974, 71, 3019–3023. [Google Scholar] [CrossRef]
- Nissen, R.; Cardinale, G.J.; Udenfriend, S. Increased turnover of arterial collagen in hypertensive rats. Proc. Natl. Acad. Sci. USA 1978, 75, 451–453. [Google Scholar] [CrossRef]
- Roach, M.R. The Pattern of Elastin in the Aorta and Large Arteries of Mammals. Novartis Found. Symp. 1983, 100, 37–55. [Google Scholar] [CrossRef]
- Sandow, S.L.; Gzik, D.J.; Lee, R.M.K.W. Arterial internal elastic lamina holes: Relationship to function? J. Anat. 2009, 214, 258–266. [Google Scholar] [CrossRef]
- Kirby, B.S.; Bruhl, A.; Sullivan, M.N.; Francis, M.; DiNenno, F.A.; Earley, S. Robust Internal Elastic Lamina Fenestration in Skeletal Muscle Arteries. PLoS ONE 2013, 8, e54849. [Google Scholar] [CrossRef]
- Van Baardwijk, C.; Roach, M.R. Medial elastin in the thoracic and abdominal aorta of sheep and lambs. Can. J. Physiol. Pharmacol. 1983, 61, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.C.; Langille, B.L. Developmental remodeling of the internal elastic lamina of rabbit arteries: Effect of blood flow. Circ. Res. 1996, 78, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Zhuang, Y.-J.; Singh, T.M.; Kawamura, K.; Murakami, M.; Zarins, C.K.; Glagov, S. Adaptive Remodeling of Internal Elastic Lamina and Endothelial Lining During Flow-Induced Arterial Enlargement. Arter. Thromb. Vasc. Biol. 1999, 19, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Z.S.; Gotlieb, A.I.; Langille, B.L. Wall Tissue Remodeling Regulates Longitudinal Tension in Arteries. Circ. Res. 2002, 90, 918–925. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chen, C.-C.; Hsu, L.-A.; Chang, G.-J.; Ko, Y.-H.; Chen, C.-F.; Chen, M.-Y.; Yang, S.-H.; Pang, J.-H.S. Degradation of the Internal Elastic Laminae in Vein Grafts of Rats with Aortocaval Fistulae: Potential Impact on Graft Vasculopathy. Am. J. Pathol. 2009, 174, 1837–1846. [Google Scholar] [CrossRef][Green Version]
- Ritz-Timme, S.; Laumeier, I.; Collins, M. Aspartic acid racemization: Evidence for marked longevity of elastin in human skin. Br. J. Dermatol. 2003, 149, 951–959. [Google Scholar] [CrossRef]
- Morris, S.M.; Thomas, K.M.; Rich, C.B.; Stone, P.J. Degradation and repair of elastic fibers in rat lung interstitial fibroblast cultures. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1998, 250, 397–407. [Google Scholar] [CrossRef]
- Stone, P.J.; Morris, S.M.; Martin, B.M.; McMahon, M.P.; Faris, B.; Franzblau, C. Repair of protease-damaged elastin in neonatal rat aortic smooth muscle cell cultures. J. Clin. Investig. 1988, 82, 1644–1654. [Google Scholar] [CrossRef]
- Alves, C.; Araújo, A.D.; Oliveira, C.L.N.; Imsirovic, J.; Bartolák-Suki, E.; Andrade, J.S.; Suki, B. Homeostatic maintenance via degradation and repair of elastic fibers under tension. Sci. Rep. 2016, 6, 27474. [Google Scholar] [CrossRef]
- Lavin, B.; Lacerda, S.; Andia, M.E.; Lorrio, S.; Bakewell, R.; Smith, A.; Rashid, I.; Botnar, R.; Phinikaridou, A. Tropoelastin: An in vivo imaging marker of dysfunctional matrix turnover during abdominal aortic dilation. Cardiovasc. Res. 2019, 116, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Krettek, A.; Sukhova, G.K.; Libby, P. Elastogenesis in human arterial disease: A role for macrophages in disordered elastin synthesis. Arter. Thromb Vasc. Biol. 2003, 23, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, X.; Piao, L.; Inoue, A.; Xu, W.; Yu, C.; Nakamura, K.; Hu, L.; Sasaki, T.; Wu, H.; et al. Cathepsin S Deficiency Mitigated Chronic Stress–Related Neointimal Hyperplasia in Mice. J. Am. Heart Assoc. 2019, 8, e011994. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cheng, X.W.; Hu, L.; Takeshita, K.; Hu, C.; Du, Q.; Li, X.; Zhu, E.; Huang, Z.; Yisireyili, M.; et al. Cathepsin S Activity Controls Injury-Related Vascular Repair in Mice via the TLR2-Mediated p38MAPK and PI3K−Akt/p-HDAC6 Signaling Pathway. Arter. Thromb. Vasc. Biol. 2016, 36, 1549–1557. [Google Scholar] [CrossRef]
- Cai, W.-J.; Koltai, S.; Kocsis, E.; Scholz, D.; Kostin, S.; Luo, X.; Schaper, W.; Schaper, J. Remodeling of the adventitia during coronary arteriogenesis. Am. J. Physiol. Circ. Physiol. 2003, 284, H31–H40. [Google Scholar] [CrossRef][Green Version]
- Meisner, J.; Annex, B.H.; Price, R.J. Despite normal arteriogenic and angiogenic responses, hind limb perfusion recovery and necrotic and fibroadipose tissue clearance are impaired in matrix metalloproteinase 9-deficient mice. J. Vasc. Surg. 2015, 61, 1583–1594.e10. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.C.; Kumar, S.; Cassatt, S.; Parker, J.L. Temporal expression of extracellular matrix metalloproteinases and tissue plasminogen activator in the development of collateral vessels in the canine model of coronary occlusion. Can. J. Physiol. Pharmacol. 1996, 74, 983–995. [Google Scholar] [CrossRef]
- McCreary, D.D.; Skirtich, N.O.; Andraska, E.A.; Tzeng, E.; McEnaney, R.M. Survey of the extracellular matrix architecture across the rat arterial tree. JVS Vasc. Sci. 2021. [Google Scholar] [CrossRef]
- Stone, P.J.; Morris, S.M.; Thomas, K.M.; Schuhwerk, K.; Mitchelson, A. Repair of Elastase-digested Elastic Fibers in Acellular Matrices by Replating with Neonatal Rat-lung Lipid Interstitial Fibroblasts or Other Elastogenic Cell Types. Am. J. Respir. Cell Mol. Biol. 1997, 17, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Protack, C.D.; Foster, T.R.; Hashimoto, T.; Yamamoto, K.; Lee, M.Y.; Kraehling, J.R.; Bai, H.; Hualong, B.; Isaji, T.; Santana, J.; et al. Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency. Sci. Rep. 2017, 7, 15386. [Google Scholar] [CrossRef]
- Kulkarni, R.; Andraska, E.; McEnaney, R. Structural Remodeling of the Extracellular Matrix in Arteriogenesis: A Review. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McEnaney, R.M.; McCreary, D.D.; Skirtich, N.O.; Andraska, E.A.; Sachdev, U.; Tzeng, E. Elastic Laminar Reorganization Occurs with Outward Diameter Expansion during Collateral Artery Growth and Requires Lysyl Oxidase for Stabilization. Cells 2022, 11, 7. https://doi.org/10.3390/cells11010007
McEnaney RM, McCreary DD, Skirtich NO, Andraska EA, Sachdev U, Tzeng E. Elastic Laminar Reorganization Occurs with Outward Diameter Expansion during Collateral Artery Growth and Requires Lysyl Oxidase for Stabilization. Cells. 2022; 11(1):7. https://doi.org/10.3390/cells11010007
Chicago/Turabian StyleMcEnaney, Ryan M., Dylan D. McCreary, Nolan O. Skirtich, Elizabeth A. Andraska, Ulka Sachdev, and Edith Tzeng. 2022. "Elastic Laminar Reorganization Occurs with Outward Diameter Expansion during Collateral Artery Growth and Requires Lysyl Oxidase for Stabilization" Cells 11, no. 1: 7. https://doi.org/10.3390/cells11010007
APA StyleMcEnaney, R. M., McCreary, D. D., Skirtich, N. O., Andraska, E. A., Sachdev, U., & Tzeng, E. (2022). Elastic Laminar Reorganization Occurs with Outward Diameter Expansion during Collateral Artery Growth and Requires Lysyl Oxidase for Stabilization. Cells, 11(1), 7. https://doi.org/10.3390/cells11010007





