The Effects of a Preconception Lifestyle Intervention on Childhood Cardiometabolic Health—Follow-Up of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Lifestyle Intervention
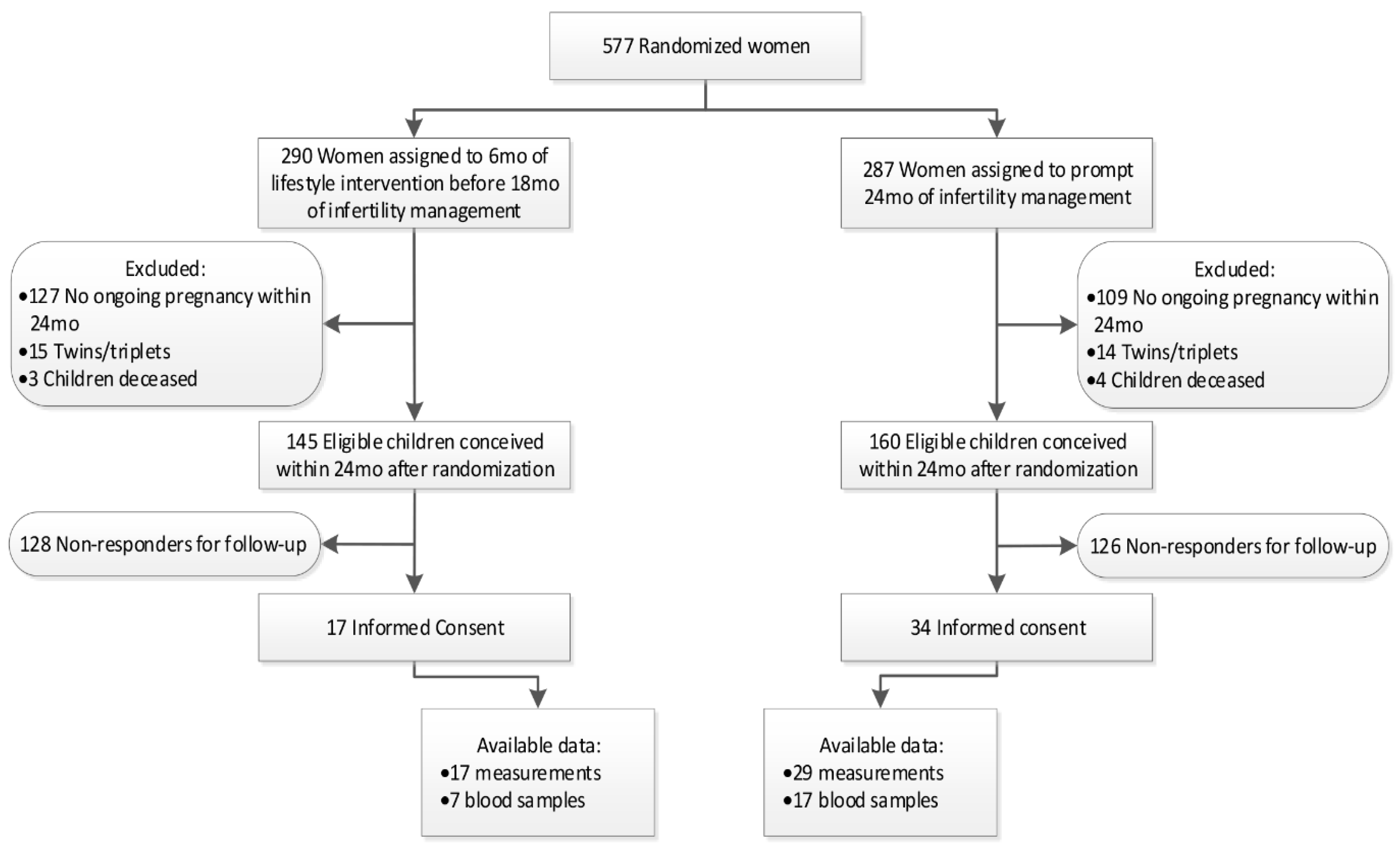
2.2. Eligibility for Follow-Up
2.3. Follow-Up Assessment
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Bentham, J.; Di Cesare, M.; Bilano, V.; Bixby, H.; Zhou, B.; Stevens, G.A.; Riley, L.M.; Taddei, C.; Hajifathalian, K.; Lu, Y.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 1289 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Ayer, J.; Charakida, M.; Deanfield, J.E.; Celermajer, D.S. Childhood obesity and cardiovascular risk. Eur. Heart J. 2015, 36, 1371–1376. [Google Scholar] [CrossRef]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef]
- Luke, B.; Brown, M.B.; Stern, J.E.; Missmer, S.A.; Fujimoto, V.Y.; Leach, R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum. Reprod. 2011, 26, 245–252. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef]
- Reynolds, R.; Allan, K.; Raja, E.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Bhattacharya, S.; Norman, J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1,323,275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- World Health Organization. Ending Childhood Obesity Report of the Commission ON; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- Heslehurst, N.; Rankin, J.; Wilkinson, J.R.; Summerbell, C.D. A nationally representative study of maternal obesity in England, UK: Trends in incidence and demographic inequalities in 619,323 births. Int. J. Obes. 2010, 34, 420–428. [Google Scholar] [CrossRef]
- Hanson, M.; Barker, M.; Dodd, J.M.; Adelaide, N.; Australia, S.; Kumanyika, S.; Norris, S.; Steegers, E.; Stephenson, J. Interventions to prevent maternal obesity prior to conception, during pregnancy, and postpartum. Lancet Diabetes Endocrinol. 2017, 5, 65–67. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Mitchell, M.D. Developmental origins of health and disease: Reducing the burden of chronic disease in the next generation. Genome Med. 2010, 2, 14. [Google Scholar] [CrossRef]
- Zambrano, E.; Martínez-Samayoa, P.M.; Rodríguez-González, G.L.; Nathanielsz, P.W. RAPID REPORT: Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J. Physiol. 2010, 588, 1791–1799. [Google Scholar] [CrossRef]
- Vega, C.; Reyes-Castro, L.A.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int. J. Obes. 2015, 39, 712–719. [Google Scholar] [CrossRef]
- Patel, N.; Godfrey, K.; Pasupathy, D.; Levin, J.; Flynn, A.C.; Hayes, L.; Briley, A.L.; Bell, R.; Lawlor, D.A.; Oteng-Ntim, E.; et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int. J. Obes. 2017, 41, 1018–1026. [Google Scholar] [CrossRef]
- Vesco, K.K.; Leo, M.C.; Karanja, N.; Gillman, M.W.; McEvoy, C.T.; King, J.C.; Eckhardt, C.L.; Smith, K.S.; Perrin, N.; Stevens, V.J. One-year postpartum outcomes following a weight management intervention in pregnant women with obesity. Obesity 2016, 24, 2042–2049. [Google Scholar] [CrossRef]
- Dalrymple, K.V.; Martyni-Orenowicz, J.; Flynn, A.C.; Poston, L.; O’Keeffe, M. Can antenatal diet and lifestyle interventions influence childhood obesity? A systematic review. Matern. Child Nutr. 2018, 14, e12628. [Google Scholar] [CrossRef]
- Mutsaerts, M.A.Q.; van Oers, A.M.; Groen, H.; Burggraaff, J.M.; Kuchenbecker, W.K.H.; Perquin, D.A.M.; Koks, C.A.M.; van Golde, R.; Kaaijk, E.M.; Schierbeek, J.M.; et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N. Engl. J. Med. 2016, 374, 1942–1953. [Google Scholar] [CrossRef]
- Van Elten, T.M.; Karsten, M.D.A.; Geelen, A.; Gemke, R.J.B.J.; Groen, H.; Hoek, A.; Van Poppel, M.N.M.; Roseboom, T.J. Preconception lifestyle intervention reduces long term energy intake in women with obesity and infertility: A randomised controlled trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 3. [Google Scholar] [CrossRef]
- Van Dammen, L.; Wekker, V.; van Oers, A.M.; Mutsaerts, M.A.Q.; Painter, R.C.; Zwinderman, A.H.; Groen, H.; van de Beek, C.; Muller Kobold, A.C.; Kuchenbecker, W.K.H.; et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: A randomized controlled trial. PLoS ONE 2018, 13, e0190662. [Google Scholar] [CrossRef]
- Wekker, V.; Huvinen, E.; Van Dammen, L.; Rono, K.; Painter, R.C.; Zwinderman, A.H.; Van De Beek, C.; Sarkola, T.; Mol, B.W.J.; Groen, H.; et al. Long-term effects of a preconception lifestyle intervention on cardiometabolic health of overweight and obese women. Eur. J. Public Health 2019, 29, 308–314. [Google Scholar] [CrossRef]
- Van De Beek, C.; Hoek, A.; Painter, R.C.; Gemke, R.J.B.J.; Van Poppel, M.N.M.; Geelen, A.; Groen, H.; Willem Mol, B.; Roseboom, T.J. Women, their Offspring and iMproving lifestyle for Better cardiovascular health of both (WOMB project): A protocol of the follow-up of a multicentre randomised controlled trial. BMJ Open 2018, 8, e016579. [Google Scholar] [CrossRef]
- Hoftiezer, L.; Hof, M.; Dijs-Elsinga, J.; Hogeveen, M.; Hukkelhoven, C.W.; Van Lingen, R.A. From population reference to national standard: New and improved birthweight charts. Am. J. Obstet. Gynecol. 2019, 220, 383.e1–383.e17. [Google Scholar] [CrossRef]
- Cole, T.J. The LMS method for constructing normalized growth standards. Eur. J. Clin. Nutr. 1990, 44, 45–60. [Google Scholar] [PubMed]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- De Beer, M.; Timmers, T.; Weijs, P.J.; Gemke, R.J. Validation of total body water analysis by bioelectrical impedance analysis with deuterium dilution in (pre) school children. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2011, 6, e223–e226. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, D.; Pan, Y.; Ding, W.; Wei, Q.; Li, H.; Gao, P.; Mi, J. Validation of Omron HBP-1300 professional blood pressure monitor based on auscultation in children and adults. BMC Cardiovasc. Disord. 2016, 16, 9. [Google Scholar] [CrossRef]
- The National High Blood Pressure Education Program Coordinating Committee Member Organizations. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents; NIH publications: Bethesda, MA, USA, 2005. [Google Scholar]
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.C.; Boileau, P.; Le Bouc, Y.; Deal, C.L.; Lillycrop, K.; et al. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 2011, 32, 159–224. [Google Scholar] [CrossRef] [PubMed]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J. Clin. Investig. 2013, 123, 5052–5060. [Google Scholar] [CrossRef]
- Kleijkers, S.H.M.; Van Montfoort, A.P.A.; Smits, L.J.M.; Viechtbauer, W.; Roseboom, T.J.; Nelissen, E.C.M.; Coonen, E.; Derhaag, J.G.; Bastings, L.; Schreurs, I.E.L.; et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum. Reprod. 2014, 29, 661–669. [Google Scholar] [CrossRef]
- Magnus, M.C.; Wilcox, A.J.; Fadum, E.A.; Gjessing, H.K.; Opdahl, S.; Juliusson, P.B.; Romundstad, L.B.; Håberg, S.E. Growth in children conceived by ART. Hum. Reprod. 2021, 36, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Alcaraz, B.; Serafini, A.; Sepulveda-Martínez, A.; Casals, G.; Rodríguez-López, M.; Garcia-Otero, L.; Cruz-Lemini, M.; Bijnens, B.; Sitges, M.; Balasch, J.; et al. Postnatal persistence of fetal cardiovascular remodelling associated with assisted reproductive technologies: A cohort study. BJOG An Int. J. Obstet. Gynaecol. 2019, 126, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Kabani, C.; Vigé, A.; Gross, M.-S.; Boileau, C.; Rabes, J.-P.; Fruchart-Najib, J.; Jais, J.-P.; Junien, C. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am. J. Physiol. Metab. 2007, 292, E1095–E1100. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C. Effects of a Maternal Pre-pregnancy Dietary and Pharmacological Intervention on Maternal Fecundity and Offspring Health in Sprague-Dawley Rats. Available online: http://hdl.handle.net/11023/956 (accessed on 18 October 2021).
- Xu, H.; Fu, Q.; Zhou, Y.; Xue, C.; Olson, P.; Lynch, E.C.; Zhang, K.K.; Wu, C.; Murano, P.; Zhang, L.; et al. A long-term maternal diet intervention is necessary to avoid the obesogenic effect of maternal high-fat diet in the offspring. J. Nutr. Biochem. 2018, 62, 210–220. [Google Scholar] [CrossRef]
- Mustila, T.; Raitanen, J.; Keskinen, P.; Saari, A.; Luoto, R. Lifestyle counselling targeting infant’s mother during the child’s first year and offspring weight development until 4 years of age: A follow-up study of a cluster RCT. BMJ Open 2012, 2, e000624. [Google Scholar] [CrossRef]
- Tanvig, M.; Vinter, C.A.; Jørgensen, J.S.; Wehberg, S.; Ovesen, P.G.; Lamont, R.F.; Beck-Nielsen, H.; Christesen, H.T.; Jensen, D.M. Anthropometrics and Body Composition by Dual Energy X-Ray in Children of Obese Women: A Follow-Up of a Randomized Controlled Trial (the Lifestyle in Pregnancy and Offspring [LiPO] Study). PLoS ONE 2014, 9, e89590. [Google Scholar] [CrossRef][Green Version]
- Rauh, K.; Günther, J.; Kunath, J.; Stecher, L.; Hauner, H. Lifestyle intervention to prevent excessive maternal weight gain: Mother and infant follow-up at 12 months postpartum. BMC Pregnancy Childbirth 2015, 15, 265. [Google Scholar] [CrossRef]
- Horan, M.K.; Donnelly, J.M.; McGowan, C.A.; Gibney, E.R.; McAuliffe, F.M. The association between maternal nutrition and lifestyle during pregnancy and 2-year-old offspring adiposity: Analysis from the ROLO study. J. Public Health 2016, 24, 427–436. [Google Scholar] [CrossRef]
- Kolu, P.; Raitanen, J.; Puhkala, J.; Tuominen, P.; Husu, P.; Luoto, R. Effectiveness and Cost-Effectiveness of a Cluster-Randomized Prenatal Lifestyle Counseling Trial: A Seven-Year Follow-Up. PLoS ONE 2016, 11, e0167759. [Google Scholar] [CrossRef]
- Ronnberg, A.-K.; Hanson, U.; Nilsson, K. Effects of an antenatal lifestyle intervention on offspring obesity—A 5-year follow-up of a randomized controlled trial. Acta Obstet. Gynecol. Scand. 2017, 96, 1093–1099. [Google Scholar] [CrossRef]
- Dalrymple, K.V.; Tydeman, F.A.S.; Taylor, P.D.; Flynn, A.C.; O’Keeffe, M.; Briley, A.L.; Santosh, P.; Hayes, L.; Robson, S.C.; Nelson, S.M.; et al. Adiposity and cardiovascular outcomes in three-year-old children of participants in UPBEAT, an RCT of a complex intervention in pregnant women with obesity. Pediatr. Obes. 2021, 16, e12725. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines Committee to Reexamine IOM Pregnancy Weight Guidelines Food and Nutrition Board and Board on Children, Youth, and Families; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Hochner, H.; Friedlander, Y.; Calderon-Margalit, R.; Meiner, V.; Sagy, Y.; Avgil-Tsadok, M.; Burger, A.; Savitsky, B.; Siscovick, D.S.; Manor, O. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: The jerusalem perinatal family follow-up study. Circulation 2012, 125, 1381–1389. [Google Scholar] [CrossRef]
- Smith, J.; Cianflone, K.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Lescelleur, O.; Biertho, L.; Simard, S.; Kral, J.G.; et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4275–4283. [Google Scholar] [CrossRef]
- Barisione, M.; Carlini, F.; Gradaschi, R.; Camerini, G.; Adami, G.F. Body weight at developmental age in siblings born to mothers before and after surgically induced weight loss. Surg. Obes. Relat. Dis. 2012, 8, 387–391. [Google Scholar] [CrossRef]
- Gaillard, R.; Welten, M.; Oddy, W.H.; Beilin, L.J.; Mori, T.A.; Jaddoe, V.W.V.; Huang, R.C. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: A prospective cohort study. BJOG An Int. J. Obstet. Gynaecol. 2016, 123, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Tanvig, M.; Vinter, C.A.; Jørgensen, J.S.; Wehberg, S.; Ovesen, P.G.; Beck-Nielsen, H.; Christesen, H.T.; Jensen, D.M. Effects of lifestyle intervention in pregnancy and anthropometrics at birth on offspring metabolic profile at 2.8 years: Results from the lifestyle in pregnancy and offspring (LiPO) study. J. Clin. Endocrinol. Metab. 2015, 100, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araújo-Soares, V.; Sniehotta, F.F. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef]
- Menezes, M.M.; Lopes, C.T.; de Souza Nogueira, L. Impact of educational interventions in reducing diabetic complications: A systematic review. Rev. Bras. Enferm. 2016, 69, 726–737. [Google Scholar]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef]
- Furness, P.J.; McSeveny, K.; Arden, M.A.; Garland, C.; Dearden, A.M.; Soltani, H. Maternal obesity support services: A qualitative study of the perspectives of women and midwives. BMC Pregnancy Childbirth 2011, 11, 69. [Google Scholar] [CrossRef]
- Canoy, D.; Buchan, I. Challenges in Obesity Epidemiology. Obes. Rev. 2007, 8 (Suppl. 1), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Barker, M. Why is changing health-related behaviour so difficult? Public Health 2016, 136, 109–116. [Google Scholar] [CrossRef] [PubMed]
| n | Intervention | n | Control | n | Non-Participants | p-Value # | |
|---|---|---|---|---|---|---|---|
| Maternal baseline characteristics: | |||||||
| Age, years—mean (SD) | 17 | 29.9 (3.4) | 29 | 29.3 (4.1) | 259 | 29.1 (4.3) | 0.51 |
| Caucasian—no (%) | 17 | 16 (94.1) | 29 | 28 (96.6) | 259 | 227 (87.6) | 0.13 |
| Education—no (%) | 17 | - | 29 | - | 246 | - | 0.36 |
| Primary school | - | 0 (0.0) | - | 0 (0.0) | - | 10 (4.1) | - |
| Secondary education | - | 2 (11.8) | - | 8 (27.6) | - | 59 (24.0) | - |
| Intermediate vocation education | - | 11 (64.7) | - | 15 (51.7) | - | 115 (46.7) | - |
| Higher education | - | 2 (13.3) | - | 6 (20.7) | - | 62 (25.2) | - |
| Smoker—no. (%) | 17 | 3 (17.6) | 29 | 5 (17.2) | 255 | 56 (22.0) | 0.56 |
| BMI (kg/m2)—mean (SD) | 17 | 36.0 (2.7) | 29 | 35.6 (3.0) | 259 | 35.9 (3.5) | 0.80 |
| Pregnancy related characteristics: | |||||||
| Maternal age at time of pregnancy (years)—mean (SD) | 17 | 30.5 (3.4) | 29 | 29.8 (4.3) | 254 | 29.8 (4.4) | 0.69 |
| Nulliparous—no. (%) | 17 | 13 (76.5) | 29 | 21 (72.4) | 258 | 207 (80.2) | 0.43 |
| Delta baseline BMI and periconceptional BMI—mean (SD) | 15 | −0.7 (2.8) | 24 | −0.9 (1.5) | 103 | −1.0 (2.7) | 0.24 |
| Gestational weight gain (kg)—mean (SD) | 10 | 11.8 (6.1) | 18 | 11.3 (5.8) | 195 | 9.9 (6.3) | 0.64 |
| Gestational diabetes—no. (%) | 17 | 4 (23.5) | 29 | 7 (24.1) | 252 | 44 (17.5) | 0.31 |
| Gestational age at birth (weeks)—mean (SD) | 17 | 39.0 (1.7) | 29 | 39.2 (1.7) | 254 | 39.0 (2.1) | 0.79 |
| Birth weight (grams)—mean (SD) | 17 | 3234 (497) * | 29 | 3652 (454) * | 253 | 3391 (585) | 0.25 |
| Conception mode—no (%) | 17 | - | 29 | - | 255 | - | 0.78 |
| Natural | - | 10 (58.8) | - | 8 (27.6) | - | 97 (38.0) | - |
| Ovulation Induction | - | 5 (29.4) | - | 11 (37.9) | - | 78 (30.6) | - |
| IUI | - | 2 (11.8) | - | 5 (17.2) | - | 37 (14.5) | - |
| IVF/ICSI/CRYO | - | 0 (0.0) | - | 5 (17.2) | - | 43 (16.9) | - |
| Breastfeeding +—no (%) | 17 | 4 (23.5) | 29 | 9 (31.0) | 259 | 70 (27.0) | 0.86 |
| Anthropometry | |||||
|---|---|---|---|---|---|
| n | Intervention | n | Control | 95% CI | |
| BMI (Z-score)— mean (SD) | 16 | 0.69 (1.17) | 28 | 0.62 (1.04) | −0.62–0.76 |
| Waist circumference (cm)— mean (SD) | 17 | 53.4 (4.3) | 29 | 53.4 (5.3) | −3.04–3.10 |
| Hip circumference (cm)— mean (SD) | 17 | 58.3 (4.4) | 29 | 58.4 (6.9) | −3.90–3.66 |
| Body-fat (%)— mean (SD) | 16 | 20.7 (7.8) | 26 | 21.2 (9.4) | −6.16–5.16 |
| Cardiovascular | |||||
| n | Intervention | n | Control | 95% CI | |
| SBP (Z-score)— mean (SD) | 16 | 0.46 (0.65) | 27 | 0.54 (0.57) | −0.46–0.30 |
| DBP (Z-score)— mean (SD) | 16 | 0.91 (0.66) | 27 | 0.96 (0.57) | −0.44–0.33 |
| PWV (m/sec)— mean (SD) | 12 | 4.51 (0.83) | 22 | 4.50 (1.14) | −0.75–0.77 |
| Metabolic | |||||
| n | Intervention | n | Control | 95% CI | |
| Triglycerides (mmol/L)— mean (SD) | 7 | 0.71 (0.63) | 17 | 0.53 (0.17) | −0.39–0.76 |
| Total cholesterol (mmol/L)— mean (SD) | 7 | 4.26 (0.79) | 17 | 4.07 (0.54) | −0.39–0.77 |
| LDL cholesterol (mmol/L)— mean (SD) | 7 | 2.46 (0.65) | 17 | 2.36 (0.40) | −0.35–0.54 |
| HDL cholesterol (mmol/L)— mean (SD) | 7 | 1.48 (0.20) | 17 | 1.48 (0.26) | −0.22–0.24 |
| Insulin (µIU/mL)— mean (SD) | 7 | 5.52 (3.12) | 12 | 4.21 (2.87) | −1.66–4.29 |
| Glucose (mmol/L)— mean (SD) | 7 | 4.70 (0.33) | 17 | 4.47 (0.42) | −0.13–0.60 |
| HOMA-IR— mean (SD) | 7 | 1.19 (0.75) | 12 | 0.87 (0.64) | −0.37–1.00 |
| Study Identifier | Animal (A) or Human (H) | Intervention | Results |
|---|---|---|---|
| Gallou-Kabani et al., (2007) [36] | A | Dietary at time of conception/pregnancy and lacation | Female, not male offspring, had a higher proportion that remained lean on postnatal high fat diet and improved glycemic indices and lipids. |
| Zambrano et al., (2010) [14] | A | Dietary (30 days prior pregancy) | (Partial) normalization of fat mass, triglycerides, leptin and insulin |
| Dennison et al., (2013) [37] | A | Dietary (low fat high fiber) and/or sitagliptin (8 weeks prior pregnancy) | No changes in offspring body weight. Diet had no significant effects on energy intake, leptin, fasting glucose, however some microbiome change were seen. Sitagliptin alone had largest reduction in glucemic control. |
| Vega et al., (2015) [15] | A | Exercise (30 days prior pregnancy) | Reduced leptin, triglycerides, glucose |
| Xu et al., (2018) [38] | A | Dietary (up to 9 weeks prior preganncy) | Longer maternal diet intervention showed normalization of offspring’s glucose and lipid metabolism |
| Mustilla et al., (2012) [39] | H | Lifestyle intervention on diet and physical activity durng pregnancy | At 24–48 months, the offpsring in the intervention group had slower gains in BMI z score. Over the 0–48 months there was no differences in BMI z score gain between groups. (Follow-up rate, 72%) |
| Tanvig et al., (2014) [40] | H | Diet, exercise and coaching during pregnancy (RCT) | At 2.8 years follow-up, there were no differences between groups in BMI z-scores, nor in skinfold, anthropometrics, total fat mass, lean mass or fat percentage. (Follow-up rate 29%) |
| Rauh et al., (2015) [41] | H | Lifestyle intervention including dietary and physical acivity counseling twice during pregnancy | At 10–12 months after birth, there were no significant differences in offspring’s weight. (Follow-up rate, 85%) |
| Horan et al., (2016) [42] | H | Dietary intervention during pregnancy in women with previous LGA infant | No effects on offspring at 6 months, at 2 years improvement of anthropometrics indices with heatlhier dietary intake during pregancy. (Follow-up rate, 35%) |
| Kolu et al., (2016) [43] | H | Lifestyle intervention of diet and physical activity during 5 antenatel visits during pregnancy | No differences in child’s BMI up to 7 years. Children of mothers who adhered to all lifestyle aims had signifcantly lower BMIs. (Follow-up rate, 43%) |
| Vesco et al., (2016) [17] | H | Weekly weight management intervention focused on diet and exercise during pregnancy | At 1 year of age there was significant reduction in weight-for-age z scores in children in the intervention group, but no differences in weight-for-height z score between groups. |
| Ronnberg et al., (2017) [44] | H | Lifestyle intervention on diet and physical activity during pregnancy, focus on healthy gestational weight gain | Follow-up of children’s BMI until 5 years of age showed no differences between groups in child’s BMI z score. (Follow-up rate, 80%) |
| Dalrymple et al., (2021) [45] | H | Diet and physical activity intervention of 8 weeks during pregnancy (RCT) | 6 months lower skinfold measures in interventions, at 3 year follow-up no significant differences in BMI or skinfold between groups. Significan lower pulse rate in offspring of intervention (Follow-up rate, 33%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintjens, S.; van Poppel, M.N.M.; Groen, H.; Hoek, A.; Mol, B.W.; Painter, R.C.; Gemke, R.J.B.J.; Roseboom, T.J. The Effects of a Preconception Lifestyle Intervention on Childhood Cardiometabolic Health—Follow-Up of a Randomized Controlled Trial. Cells 2022, 11, 41. https://doi.org/10.3390/cells11010041
Mintjens S, van Poppel MNM, Groen H, Hoek A, Mol BW, Painter RC, Gemke RJBJ, Roseboom TJ. The Effects of a Preconception Lifestyle Intervention on Childhood Cardiometabolic Health—Follow-Up of a Randomized Controlled Trial. Cells. 2022; 11(1):41. https://doi.org/10.3390/cells11010041
Chicago/Turabian StyleMintjens, Stijn, Mireille N. M. van Poppel, Henk Groen, Annemieke Hoek, Ben Willem Mol, Rebecca C. Painter, Reinoud J. B. J. Gemke, and Tessa J. Roseboom. 2022. "The Effects of a Preconception Lifestyle Intervention on Childhood Cardiometabolic Health—Follow-Up of a Randomized Controlled Trial" Cells 11, no. 1: 41. https://doi.org/10.3390/cells11010041
APA StyleMintjens, S., van Poppel, M. N. M., Groen, H., Hoek, A., Mol, B. W., Painter, R. C., Gemke, R. J. B. J., & Roseboom, T. J. (2022). The Effects of a Preconception Lifestyle Intervention on Childhood Cardiometabolic Health—Follow-Up of a Randomized Controlled Trial. Cells, 11(1), 41. https://doi.org/10.3390/cells11010041







