Extracellular Vesicles Derived from Bone Marrow in an Early Stage of Ionizing Radiation Damage Are Able to Induce Bystander Responses in the Bone Marrow
Abstract
:1. Introduction
2. Materials and Methods
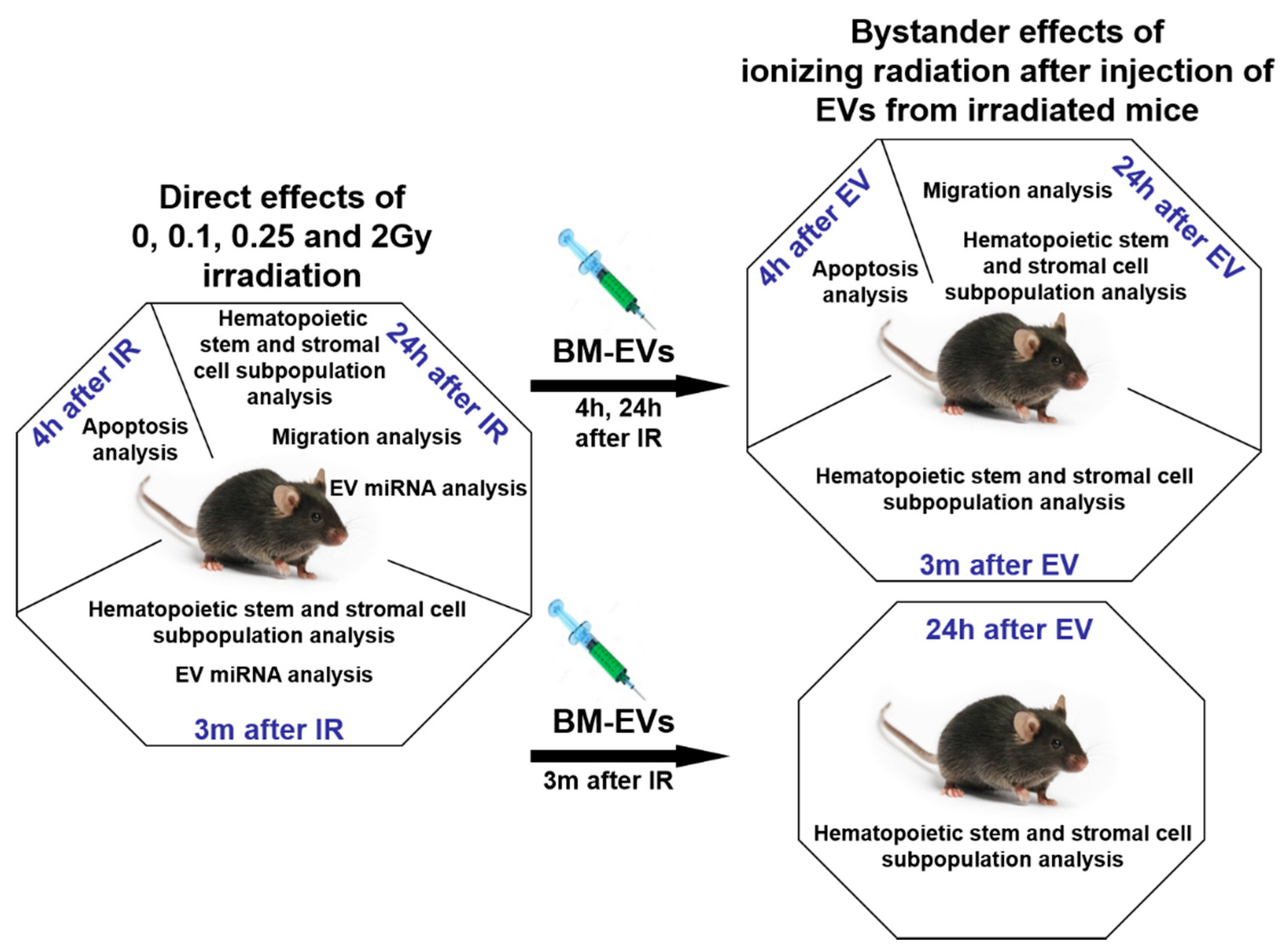
2.1. Murine Model and Irradiation Protocol
2.2. Isolation of Mouse Bone Marrow Cells and Splenocytes
2.3. Isolation, Validation and In Vivo Transfer of EVs
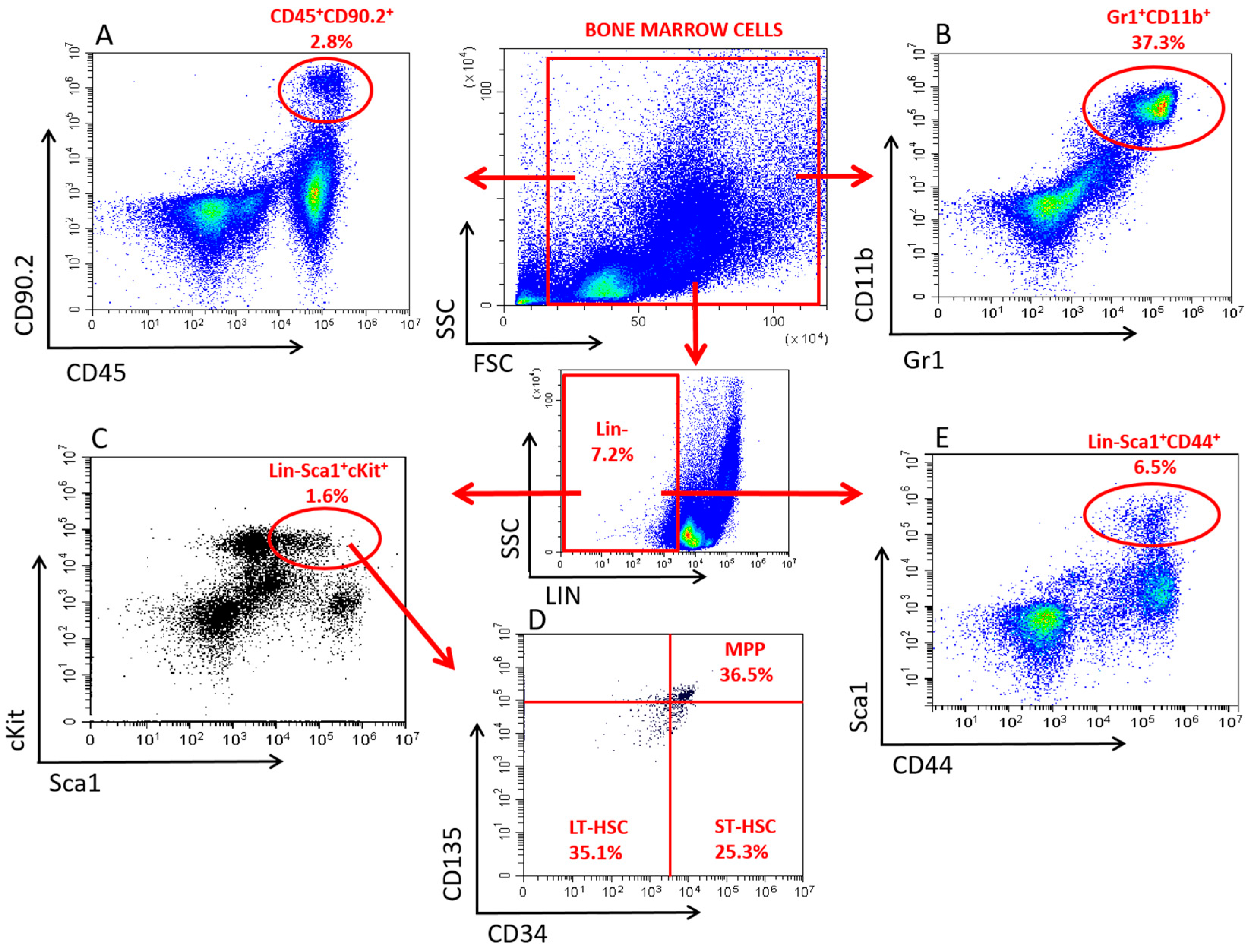
2.4. Immune Phenotypical Analysis of BMCs and Splenocytes
2.5. Analysis of Apoptosis
2.6. Analysis of miRNA Expression from BM-Derived EVs
2.7. Statistical Analysis
3. Results
3.1. Validation of Bone Marrow-Derived EVs
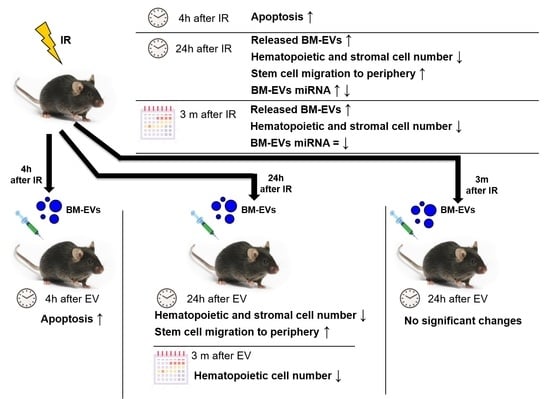
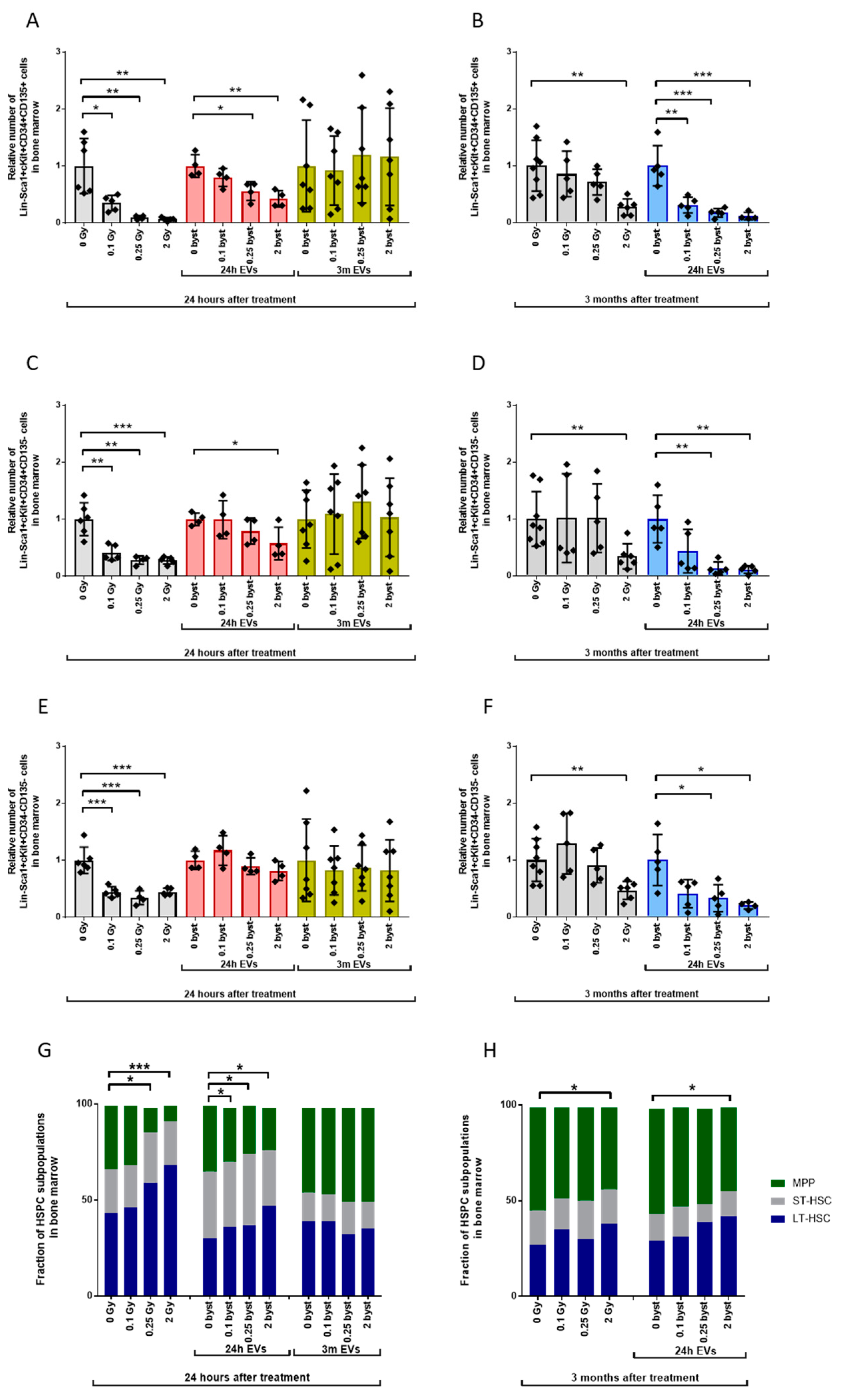
3.2. Radiation-Induced Bystander Effects Are Elicited by BM-Derived EVs Isolated from Mice 24 Hours but Not 3 Months after Irradiation
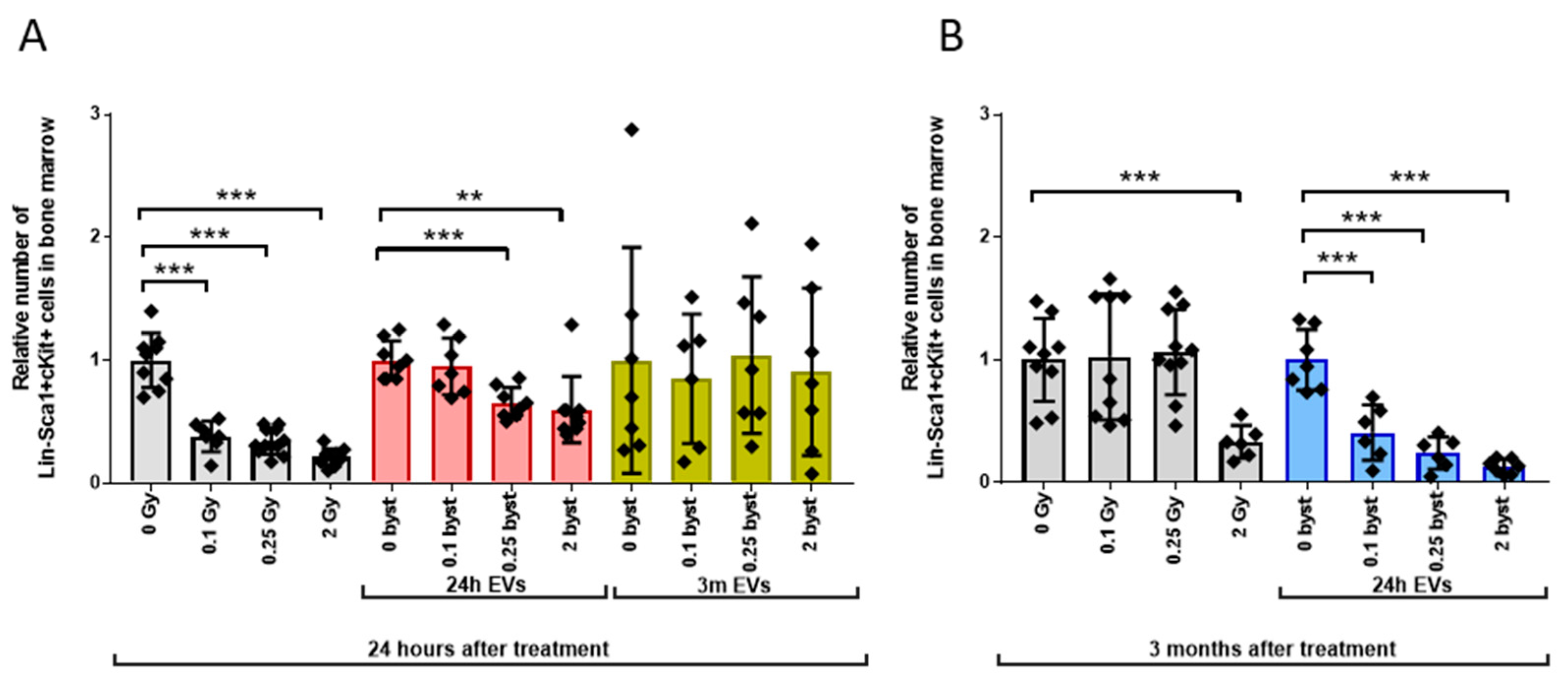
3.2.1. Alterations in the HSPC Pool
3.2.2. Alterations in the LP Pool
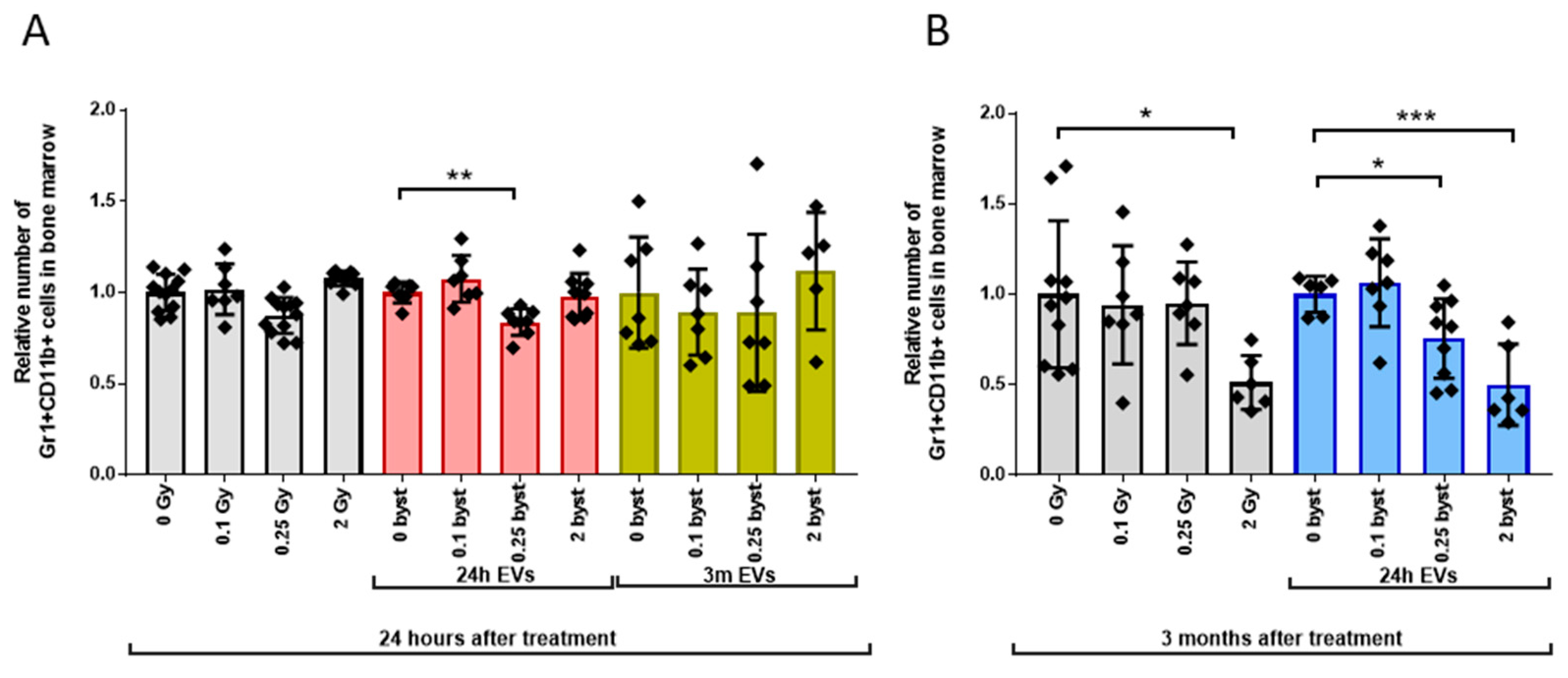
3.2.3. Alterations in the GM Pool
3.2.4. Alterations in the MSC Pool
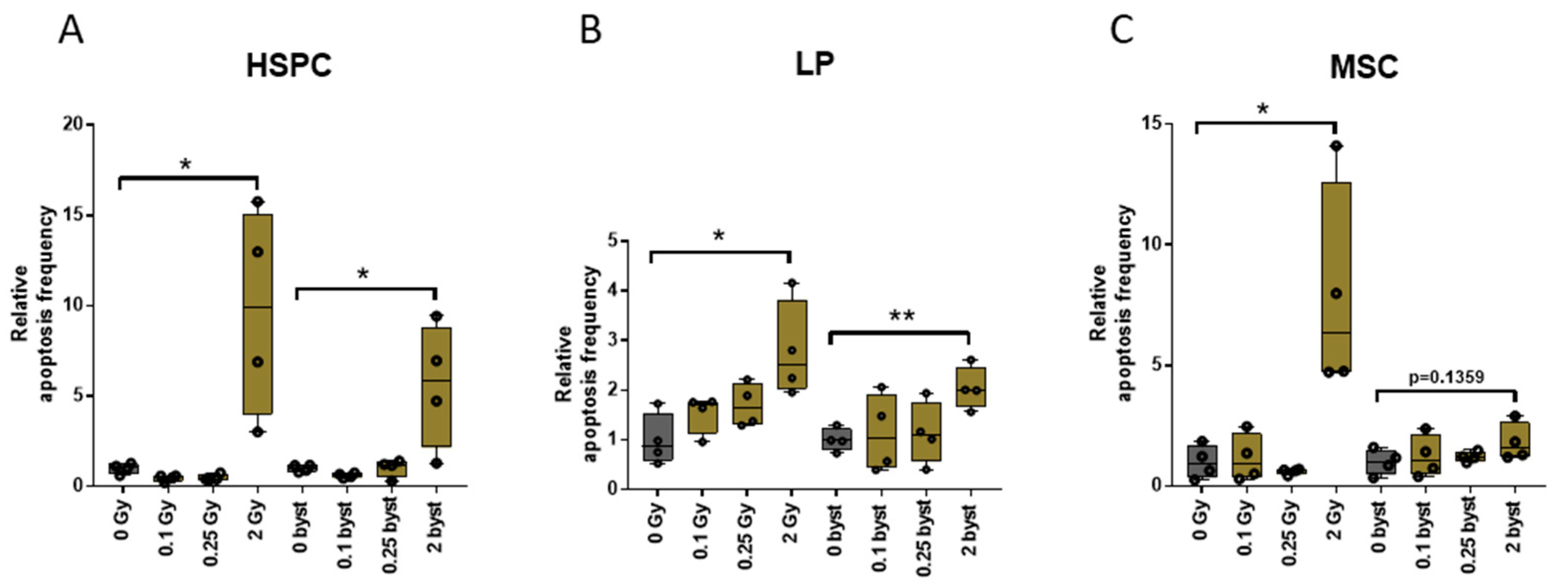
3.3. EVs Can Mediate IR-Induced Apoptosis in the BM in a Bystander Manner
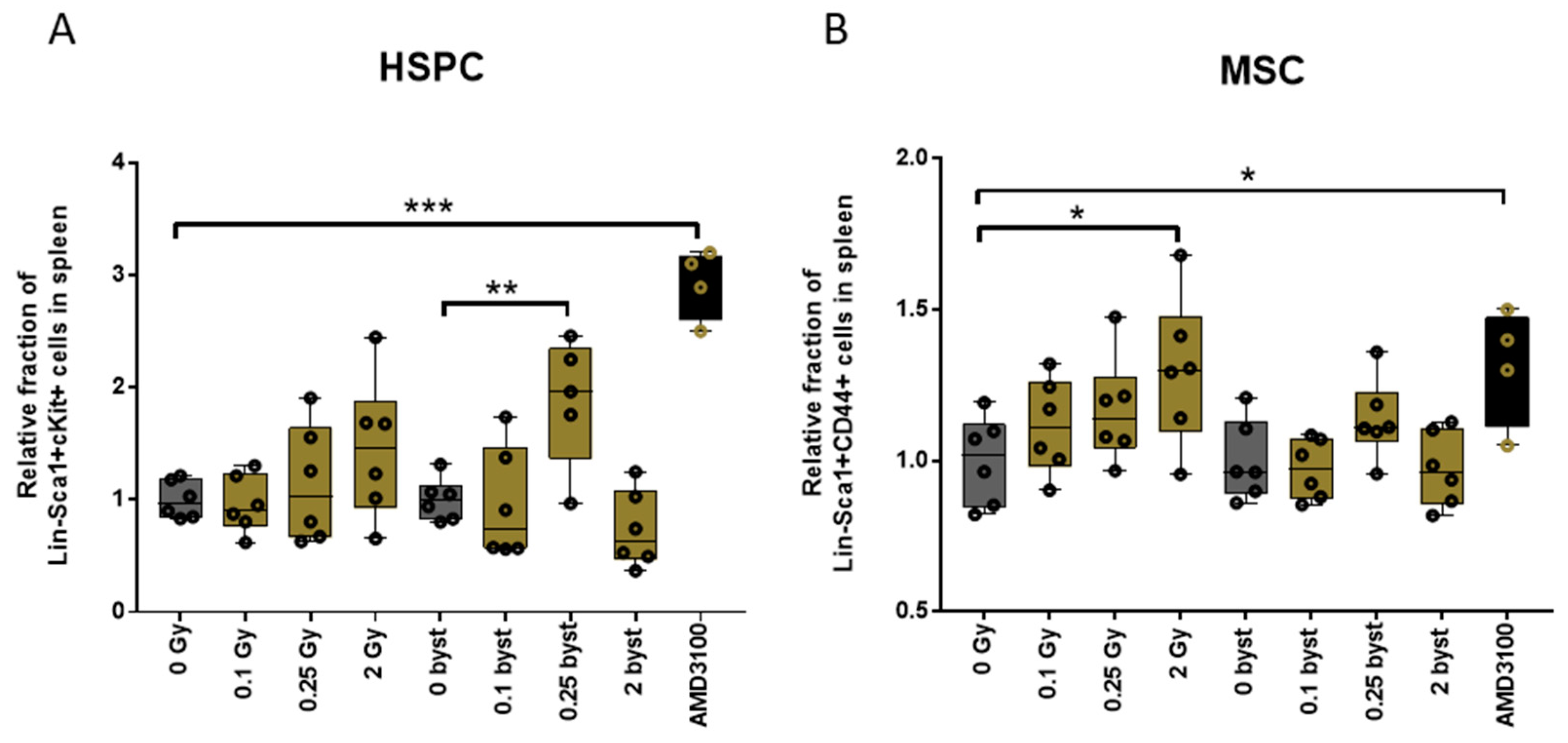
3.4. IR and EV Transfer from Irradiated to Bystander Mice Induce Stem Cell Migration from Bone Marrow to the Spleen
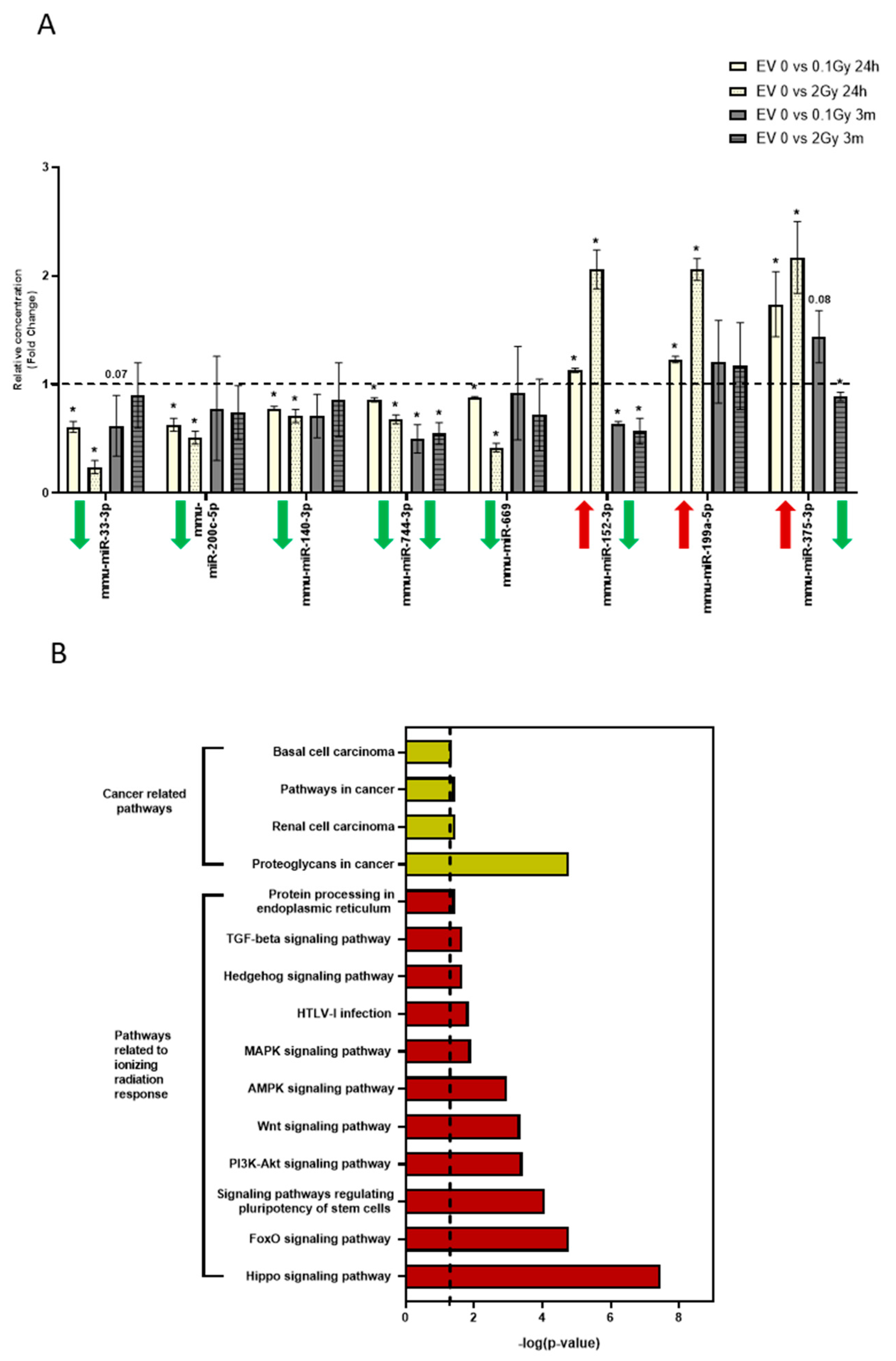
3.5. The miRNA Profile of EVs Isolated 24 Hours and 3 Months after Irradiation Are Different
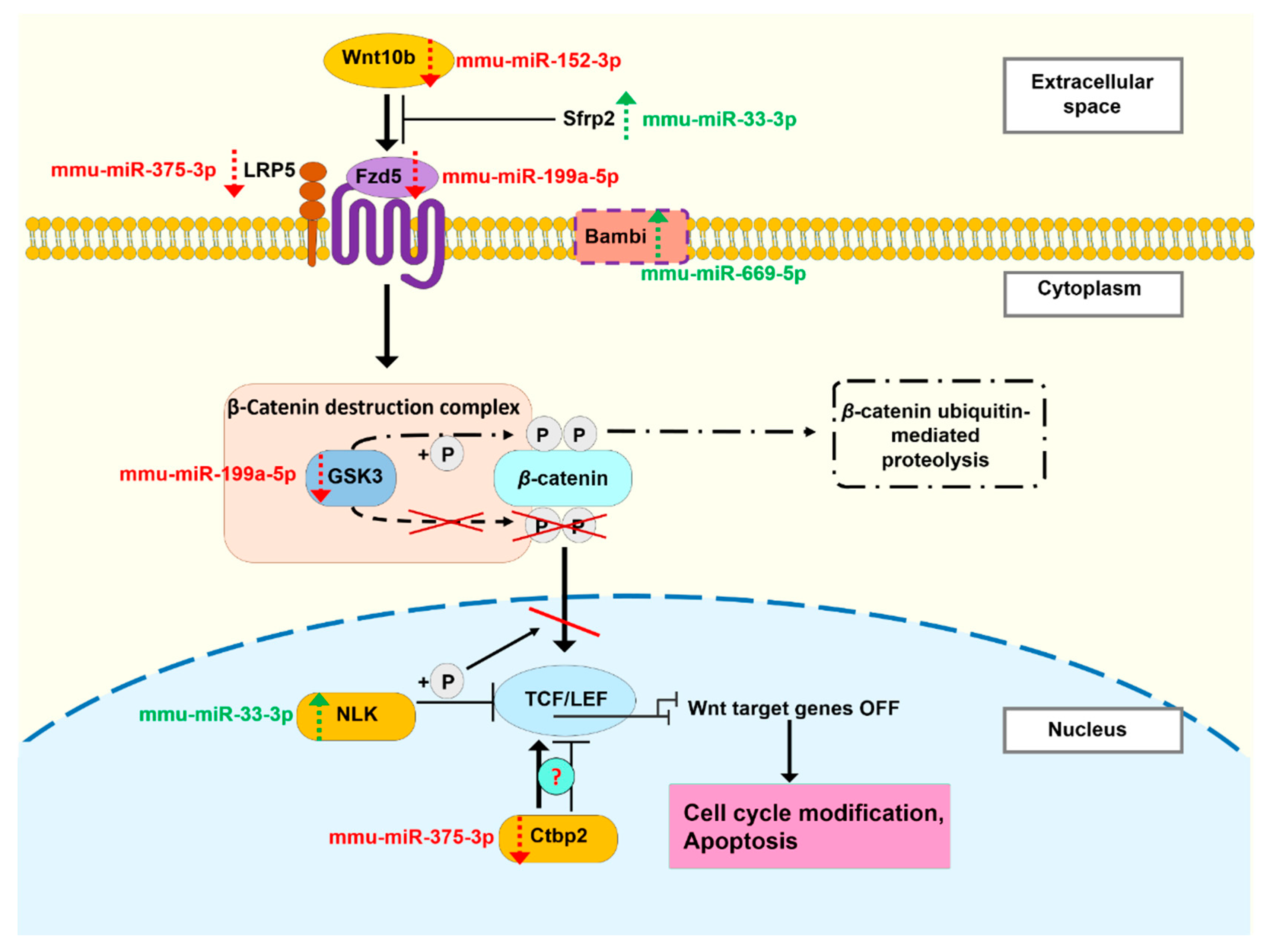
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, H.; Randers-Pehrson, G.; Waldren, C.A.; Vannais, D.; Hall, E.J.; Hei, T.K. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc. Natl. Acad. Sci. USA 2000, 97, 2099–2104. [Google Scholar] [CrossRef] [Green Version]
- Lorimore, A.S.; Coates, P.J.; Scobie, E.G.; Milne, G.; Wright, E.G. Inflammatory-type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects? Oncogene 2001, 20, 7085–7095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mothersill, C.; Rea, D.; Wright, E.G.; Lorimore, S.A.; Murphy, D.; Seymour, C.B.; O’Malley, K. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinog 2001, 22, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Randers-Pehrson, G.; Suzuki, M.; Waldren, C.A.; Hei, T.K. Genotoxic damage in non-irradiated cells: Con-tribution from the bystander effect. Radiat. Prot. Dosim. 2002, 99, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Lyng, F.M.; Maguire, P.; McClean, B.; Seymour, C.; Mothersill, C. The Involvement of Calcium and MAP Kinase Signaling Pathways in the Production of Radiation-Induced Bystander Effects. Radiat. Res. 2006, 165, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Albanese, J.; Dainiak, N. Ionizing Radiation Alters Fas Antigen Ligand at the Cell Surface and on Exfoliated Plasma Mem-brane-Derived Vesicles: Implications for Apoptosis and Intercellular Signaling. Radiat. Res. 2000, 153, 49–61. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Res. 2012, 64, 676–705. [Google Scholar] [CrossRef] [Green Version]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Aatonen, M.; Öhman, T.; Nyman, T.; Laitinen, S.; Grönholm, M.; Siljander, P.R.-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 2014, 3, 24692. [Google Scholar] [CrossRef]
- Valencia, K.; Luis-Ravelo, D.; Bovy, N.; Antón, I.; Martínez-Canarias, S.; Zandueta, C.; Ormazábal, C.; Struman, I.; Tabruyn, S.; Rebmann, V.; et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol. 2014, 8, 689–703. [Google Scholar] [CrossRef]
- Schey, K.L.; Luther, J.; Rose, K.L. Proteomics characterization of exosome cargo. Methods 2015, 87, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lydic, T.A.; Townsend, S.; Adda, C.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au-Lässer, C.; Au-Eldh, M.; Au-Lötvall, J. Isolation and Characterization of RNA-Containing Exosomes. JoVE 2012, 59, e3037. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Zhu, Y.-L.; Hu, F.-H.; Wang, Y.-Y.; Huang, N.-P.; Xiao, Z.-D. Dynamics of exosome internalization and traf-ficking. J. Cell. Physiol. 2013, 228, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Gil, N.M.; Barcia, J.M.; Aparicio, S.; Pérez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef]
- Hedlund, M.; Nagaeva, O.; Kargl, D.; Baranov, V.; Mincheva-Nilsson, L. Thermal- and Oxidative Stress Causes Enhanced Release of NKG2D Ligand-Bearing Immunosuppressive Exosomes in Leukemia/Lymphoma T and B Cells. PLoS ONE 2011, 6, e16899. [Google Scholar] [CrossRef]
- Al-Mayah, A.H.J.; Irons, S.; Pink, R.; Carter, D.R.F.; Kadhim, M.A. Possible Role of Exosomes Containing RNA in Mediating Nontargeted Effect of Ionizing Radiation. Radiat. Res. 2012, 177, 539–545. [Google Scholar] [CrossRef]
- Al-Mayah, A.; Bright, S.; Chapman, K.; Irons, S.; Luo, P.; Carter, D.; Goodwin, E.; Kadhim, M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. Mol. Mech. Mutagen. 2015, 772, 38–45. [Google Scholar] [CrossRef]
- Jella, K.K.; Rani, S.; O’Driscoll, L.; McClean, B.; Byrne, H.J.; Lyng, F.M. Exosomes Are Involved in Mediating Radiation Induced Bystander Signaling in Human Keratinocyte Cells. Radiat. Res. 2014, 181, 138–145. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Ding, N.; Hu, W.; Zhang, X.; Wang, B.; Hua, J.; Wei, W.; Zhu, Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015, 12, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Muller, L.; Hong, C.-S.; Stolz, D.B.; Watkins, S.C.; Whiteside, T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 2014, 411, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Mehdiani, A.; Maier, A.; Pinto, A.; Barth, M.; Akhyari, P.; Lichtenberg, A. An innovative method for exosome quantification and size measurement. JoVE 2015, 95, e50974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranyai, T.; Herczeg, K.; Onódi, Z.; Voszka, I.; Módos, K.; Marton, N.; Nagy, G.; Mäger, I.; Wood, M.J.; El Andaloussi, S.; et al. Isolation of Exo-somes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatog-raphy Methods. PLoS ONE 2015, 10, e0145686. [Google Scholar] [CrossRef] [Green Version]
- Szatmári, T.; Persa, E.; Kis, E.; Benedek, A.; Hargitai, R.; Sáfrány, G.; Lumniczky, K. Extracellular vesicles mediate low dose ionizing radiation-induced immune and inflammatory responses in the blood. Int. J. Radiat. Biol. 2019, 95, 12–22. [Google Scholar] [CrossRef]
- Hargitai, R.; Kis, D.; Persa, E.; Szatmári, T.; Sáfrány, G.; Lumniczky, K. Oxidative Stress and Gene Expression Modifi-cations Mediated by Extracellular Vesicles: An In vivo Study of the Radiation-Induced Bystander Effect. Antioxidants 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, T.; Kis, D.; Bogdándi, E.N.; Benedek, A.; Bright, S.; Bowler, D.; Persa, E.; Kis, E.; Balogh, A.; Naszályi, L.N.; et al. Extracellular Vesicles Mediate Radiation-Induced Systemic Bystander Sig-nals in the Bone Marrow and Spleen. Front. Immunol. 2017, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Broxmeyer, H.E.; Orschell, C.M.; Clapp, D.W.; Hangoc, G.; Cooper, S.; Plett, P.A.; Liles, W.C.; Li, X.; Graham-Evans, B.; Campbell, T.B.; et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005, 201, 1307–1318. [Google Scholar] [CrossRef] [Green Version]
- Vogel, R.; Willmott, G.; Kozak, D.; Roberts, G.S.; Anderson, W.; Groenewegen, L.; Glossop, B.; Barnett, A.; Turner, A.; Trau, M. Quantitative Sizing of Nano/Microparticles with a Tunable Elastomeric Pore Sensor. Anal. Chem. 2011, 83, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.S.; Yu, S.; Zeng, Q.; Chan, L.C.; Anderson, W.; Colby, A.H.; Grinstaff, M.W.; Reid, S.; Vogel, R. Tunable pores for measuring concentrations of synthetic and biological nanoparticle dispersions. Biosens. Bioelectron. 2012, 31, 17–25. [Google Scholar] [CrossRef]
- Kis, D.; Persa, E.; Szatmári, T.; Antal, L.; Bóta, A.; Csordás, I.B.; Hargitai, R.; Jezsó, B.; Kis, E.; Mihály, J.; et al. The effect of ionising radiation on the phenotype of bone marrow-derived extracellular vesicles. Br. J. Radiol. 2020, 93, 20200319. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for sta-tistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hu, M.; Zhang, Z.; Qi, Y.; Wang, J. The regulation of hematopoietic stem cell fate in the context of radiation. Radiat. Med. Prot. 2020, 1, 31–34. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Snow, J.W.; Kim, J.; Orkin, S.H. DNA methyltransferase 1 is essential for and uniquely regulates hem-atopoietic stem and progenitor cells. Cell 2009, 5, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Gan, B.; Hu, J.; Jiang, S.; Liu, Y.; Sahin, E.; Zhuang, L.; Fletcher-Sananikone, E.; Colla, S.; Wang, Y.A.; Chin, L.; et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nat. Cell Biol. 2010, 468, 701–704. [Google Scholar] [CrossRef]
- Pietras, E.M.; Reynaud, D.; Kang, Y.-A.; Carlin, D.; Calero-Nieto, F.J.; Leavitt, A.D.; Stuart, J.M.; Göttgens, B.; Passegué, E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Re-generative Conditions. Cell Stem Cell 2015, 17, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Shi, H.; Mao, F.; Wang, J.; Yan, Y.; Zhang, X.; Qian, H.; Xu, W. A novel method to isolate mesenchymal stem cells from mouse umbilical cord. Mol. Med. Rep. 2017, 17, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Yang, H.-M.; Park, J.; Choi, G.-S.; Joh, J.-W.; Kwon, C.D.; Chun, J.; Lee, S.-K.; Kim, S.-J. Isolation and Characterization of Mouse Mesenchymal Stem Cells. Transplant. Proc. 2008, 40, 2649–2654. [Google Scholar] [CrossRef]
- Baddoo, M.; Hill, K.; Wilkinson, R.; Gaupp, D.; Hughes, C.; Kopen, G.C.; Phinney, D.G. Characterization of mes-enchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 2003, 89, 1235–1249. [Google Scholar] [CrossRef]
- Bogdándi, E.N.; Balogh, A.; Felgyinszki, N.; Szatmári, T.; Persa, E.; Hildebrandt, G.; Sáfrány, G.; Lumniczky, K. Effects of Low-Dose Radiation on the Immune System of Mice after Total-Body Irradiation. Radiat. Res. 2010, 174, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Persa, E.; Bogdándi, E.N.; Benedek, A.; Hegyesi, H.; Sáfrány, G.; Lumniczky, K. The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm. Res. 2013, 62, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Widlak, P.; Pietrowska, M. The Influence of Ionizing Radiation on Exosome Composition, Secretion and Intercellular Communication. Protein Pept. Lett. 2016, 23, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arscott, W.; Tandle, A.T.; Zhao, S.; Shabason, J.E.; Gordon, I.K.; Schlaff, C.D.; Zhang, G.; Tofilon, P.J.; Camphausen, K.A. Ionizing Radiation and Glioblastoma Exosomes: Implications in Tumor Biology and Cell Migration. Transl. Oncol. 2013, 6, 638–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.; Paine, M.S.; Brooks, A.M.; McCubrey, J.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jella, K.K.; Jaafar, L.; Moreno, C.S.; Dynan, W.S. Characterization of exosome release and extracellular ve-si-cle-associated miRNAs for human bronchial epithelial cells irradiated with high charge and energy ions. Life Sci. Space Res. 2021, 28, 11–17. [Google Scholar] [CrossRef]
- Elbakrawy, E.; Kaur Bains, S.; Bright, S.; Al-Abedi, R.; Mayah, A.; Goodwin, E.; Kadhim, M. Radiation-Induced Se-nescence Bystander Effect: The Role of Exosomes. Biology 2020, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Tuncay Cagatay, S.; Mayah, A.; Mancuso, M.; Giardullo, P.; Pazzaglia, S.; Saran, A.; Daniel, A.; Traynor, D.; Meade, A.D.; Lyng, F.; et al. Phenotypic and Functional Characteristics of Exosomes Derived from Irra-diated Mouse Organs and Their Role in the Mechanisms Driving Non-Targeted Effects. Int. J. Mol. Sci. 2020, 21, 8389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, W.; Hull, L.; Smith, J.T.; Kiang, J.G.; Xiao, M. Effects of Low-to-Moderate Doses of Gamma Radiation on Mouse Hematopoietic System. Radiat. Res. 2018, 190, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Mauch, P.; Constine, L.; Greenberger, J.; Knospe, W.; Sullivan, J.; Liesveld, J.L.; Deeg, H. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. 1995, 31, 1319–1339. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Pazhanisamy, S.K.; Li, H.; Meng, A.; Zhou, D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic. Biol. Med. 2010, 48, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberger, J.S.; Epperly, M. Bone Marrow–Derived Stem Cells and Radiation Response. Semin. Radiat. Oncol. 2009, 19, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, M.; Zhao, S.-Y.; Zhang, S.-Q.; Lu, L.; He, X.; Feng, G.-X.; Wu, X.; Fan, S.-J. Exosomes are involved in total body irradiation-induced intestinal injury in mice. Acta Pharmacol. Sin. 2021, 42, 1111–1123. [Google Scholar] [CrossRef]
- Cao, X.; Wu, X.; Frassica, D.; Yu, B.; Pang, L.; Xian, L.; Wan, M.; Lei, W.; Armour, M.; Tryggestad, E.; et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Mutschelknaus, L.; Azimzadeh, O.; Heider, T.; Winkler, K.; Vetter, M.; Kell, R.; Tapio, S.; Merl-Pham, J.; Huber, S.M.; Edalat, L.; et al. Radiation alters the cargo of exosomes released from squamous head and neck cancer cells to promote migration of recipient cells. Sci. Rep. 2017, 7, 12423. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Dukes, A.; Drewry, M.; Helwa, I.; Johnson, M.H.; Isales, C.M.; Hill, W.D.; Liu, Y.; Shi, X.; Fulzele, S.; et al. MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng. Part A 2017, 23, 1231–1240. [Google Scholar] [CrossRef]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.; Zonderman, A.B.; Biragyn, A.; et al. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [Green Version]
- Yentrapalli, R.; Merl-Pham, J.; Azimzadeh, O.; Mutschelknaus, L.; Peters, C.; Hauck, S.; Atkinson, M.; Tapio, S.; Moertl, S. Quantitative changes in the protein and miRNA cargo of plasma exosome-like vesicles after exposure to ionizing radiation. Int. J. Radiat. Biol. 2017, 93, 569–580. [Google Scholar] [CrossRef]
- Duale, N.; Eide, D.M.; Amberger, M.L.; Graupner, A.; Brede, D.A.; Olsen, A.K. Using prediction models to identify miRNA-based markers of low dose rate chronic stress. Sci. Total Environ. 2020, 717, 137068. [Google Scholar] [CrossRef]
- Małachowska, B.; Tomasik, B.; Stawiski, K.; Kulkarni, S.; Guha, C.; Chowdhury, D.; Fendler, W. Circulating mi-croRNAs as Biomarkers of Radiation Exposure: A Systematic Review and Meta-AnalysisInt. J. Radiat. Oncol. Biol. Phys. 2020, 106, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wang, X.; Zhang, H.; Wang, Z.; Nan, G.; Li, Y.; Zhang, F.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: Implications in targeted cancer therapies. Lab. Investig. 2016, 96, 116–136. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhang, Y.; Yang, L.-H.; Mi, X.-Y.; Dai, S.-D.; Li, Q.-C.; Xu, H.-T.; Yu, J.-H.; Li, G.; Zhao, J.; et al. X-radiation inhibits histone deacetylase 1 and 2, upregulates Axin expression and induces apoptosis in non-small cell lung cancer. Radiat. Oncol. 2012, 7, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.; Lee, S.; Lorang-Leins, D.; Trepel, J.; Smart, D.K. SIRT2 Interacts with β-Catenin to Inhibit Wnt Signaling Output in Response to Radiation-Induced Stress. Mol. Cancer Res. 2014, 12, 1244–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, C.Y.; Nusse, R. THE WNT SIGNALING PATHWAY IN DEVELOPMENT AND DISEASE. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef] [Green Version]
- Pinson, K.I.; Brennan, J.; Monkley, S.; Avery, B.J.; Skarnes, W.C. An LDL-receptor-related protein mediates Wnt sig-nalling in mice. Nature 2000, 407, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Arensman, M.D.; Nguyen, P.; Kershaw, K.M.; Lay, A.R.; Ostertag-Hill, C.A.; Sherman, M.H.; Downes, M.; Liddle, C.; Evans, R.; Dawson, D.W. Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic Cancer. Mol. Cancer Res. 2015, 13, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Daniels, D.L.; Eklof Spink, K.; Weis, W.I. Beta-catenin: Molecular plasticity and drug design. Trends Biochem. Sci. 2001, 26, 672–678. [Google Scholar] [CrossRef]
- van de Wetering, M.; Cavallo, R.; Dooijes, D.; van Beest, M.; van Es, J.; Loureiro, J.; Ypma, A.; Hursh, D.; Jones, T.; Bejsovec, A.; et al. Armadillo Coactivates Transcription Driven by the Product of the Drosophila Segment Polarity Gene dTCF. Cell 1997, 88, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Daniels, D.L.; Weis, W.I. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated tran-scription activation. Nat. Struct. Mol. Biol. 2005, 12, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Peter, O.; Schweizer, L.; Basler, K. Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 1997, 385, 829–833. [Google Scholar] [CrossRef]
- Gordon, M.; Nusse, R. Wnt Signaling: Multiple Pathways, Multiple Receptors, and Multiple Transcription Factors. J. Biol. Chem. 2006, 281, 22429–22433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallo, R.A.; Cox, R.T.; Moline, M.M.; Roose, J.; Polevoy, G.A.; Clevers, H.; Peifer, M.; Bejsovec, A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nat. Cell Biol. 1998, 395, 604–608. [Google Scholar] [CrossRef]
- Chen, G.; Fernandez, J.; Mische, S.; Courey, A.J. A functional interaction between the histone deacetylase Rpd3 and the corepressor Groucho in Drosophila development. Genes Dev. 1999, 13, 2218–2230. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int. J. Mol. Med. 2006, 17, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak-Celińska, A.; Słocińska, M.; Barciszewska, A.-M.; Nowak, S.; Baer-Dubowska, W. Wnt pathway antagonists, SFRP1, SFRP2, SOX17, and PPP2R2B, are methylated in gliomas and SFRP1 methylation predicts shorter survival. J. Appl. Genet. 2015, 57, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Ishitani, T.; Ninomiya-Tsuji, J.; Nagai, S.; Nishita, M.; Meneghini, M.; Barker, N.; Waterman, M.; Bowerman, B.; Clevers, H.; Shibuya, H.; et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between be-ta-catenin and transcription factor TCF. Nature 1999, 399, 798–802. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.-J.; Zhang, J.; Yu, G.-R.; Kim, D.-G. C-terminal-truncated HBV X promotes hepato-oncogenesis through inhibition of tumor-suppressive β-catenin/BAMBI signaling. Exp. Mol. Med. 2016, 48, e275. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.-J.; Yan, J.; Jin, P.-Y.; Zheng, G.-H.; Zhang, H.-L.; Bai, M.; Wu, D.-M.; Lu, J.; Zheng, Y.-L. Mechanism of MicroRNA-708 Targeting BAMBI in Cell Proliferation, Migration, and Apoptosis in Mice with Melanoma via the wnt and TGF-β Signaling Pathways. Technol. Cancer Res. Treat. 2018, 17, 1533034618756784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, X.-E.; Song, C.; Sun, S.; Yang, G.; Li, X. BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/β-Catenin Signaling. Int. J. Mol. Sci. 2015, 16, 17734–17745. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.S.; Lane, T.F.; Kuo, A.; Shackleford, G.M.; Leder, P. Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. PNAS 1995, 92, 2268–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.; Baranwal, S.; Love, I.M.; Patel, N.J.; Grossman, S.R.; Patel, B.B. Inhibition of C-terminal binding protein attenuates transcription factor 4 signaling to selectively target colon cancer stem cells. Funct. A Membr. -Embed. Domain Evol. Mult. GPI Lipid Anchor Pathw. Proteins PIG-B PIG-M PIG-U PIG-W PIG-V PIG-Z 2014, 13, 3506–3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.; Li, J.; Blauwkamp, T.; Bhambhani, C.; Campbell, N.; Cadigan, K.M. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J. 2006, 25, 2735–2745. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, D.; Csordás, I.B.; Persa, E.; Jezsó, B.; Hargitai, R.; Szatmári, T.; Sándor, N.; Kis, E.; Balázs, K.; Sáfrány, G.; et al. Extracellular Vesicles Derived from Bone Marrow in an Early Stage of Ionizing Radiation Damage Are Able to Induce Bystander Responses in the Bone Marrow. Cells 2022, 11, 155. https://doi.org/10.3390/cells11010155
Kis D, Csordás IB, Persa E, Jezsó B, Hargitai R, Szatmári T, Sándor N, Kis E, Balázs K, Sáfrány G, et al. Extracellular Vesicles Derived from Bone Marrow in an Early Stage of Ionizing Radiation Damage Are Able to Induce Bystander Responses in the Bone Marrow. Cells. 2022; 11(1):155. https://doi.org/10.3390/cells11010155
Chicago/Turabian StyleKis, Dávid, Ilona Barbara Csordás, Eszter Persa, Bálint Jezsó, Rita Hargitai, Tünde Szatmári, Nikolett Sándor, Enikő Kis, Katalin Balázs, Géza Sáfrány, and et al. 2022. "Extracellular Vesicles Derived from Bone Marrow in an Early Stage of Ionizing Radiation Damage Are Able to Induce Bystander Responses in the Bone Marrow" Cells 11, no. 1: 155. https://doi.org/10.3390/cells11010155
APA StyleKis, D., Csordás, I. B., Persa, E., Jezsó, B., Hargitai, R., Szatmári, T., Sándor, N., Kis, E., Balázs, K., Sáfrány, G., & Lumniczky, K. (2022). Extracellular Vesicles Derived from Bone Marrow in an Early Stage of Ionizing Radiation Damage Are Able to Induce Bystander Responses in the Bone Marrow. Cells, 11(1), 155. https://doi.org/10.3390/cells11010155