The Cross-Talk between Myeloid and Mesenchymal Stem Cells of Human Bone Marrow Represents a Biomarker of Aging That Regulates Immune Response and Bone Reabsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Donors
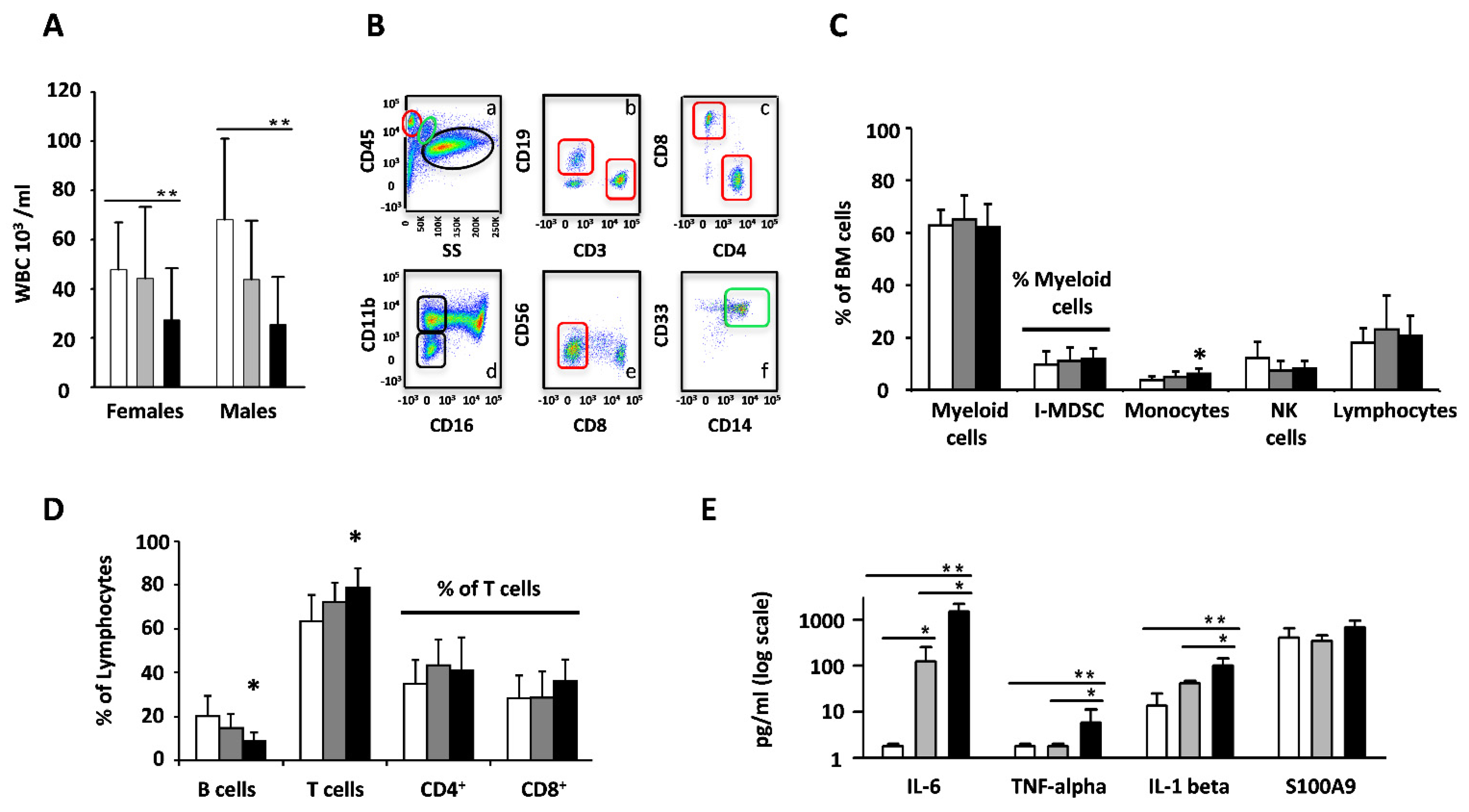
2.2. Bone Marrow Hematocytometric and Flow Cytometric Analysis
2.3. Plasma Recovery and Cytokine Detection
2.4. Immunomagnetic Sorting
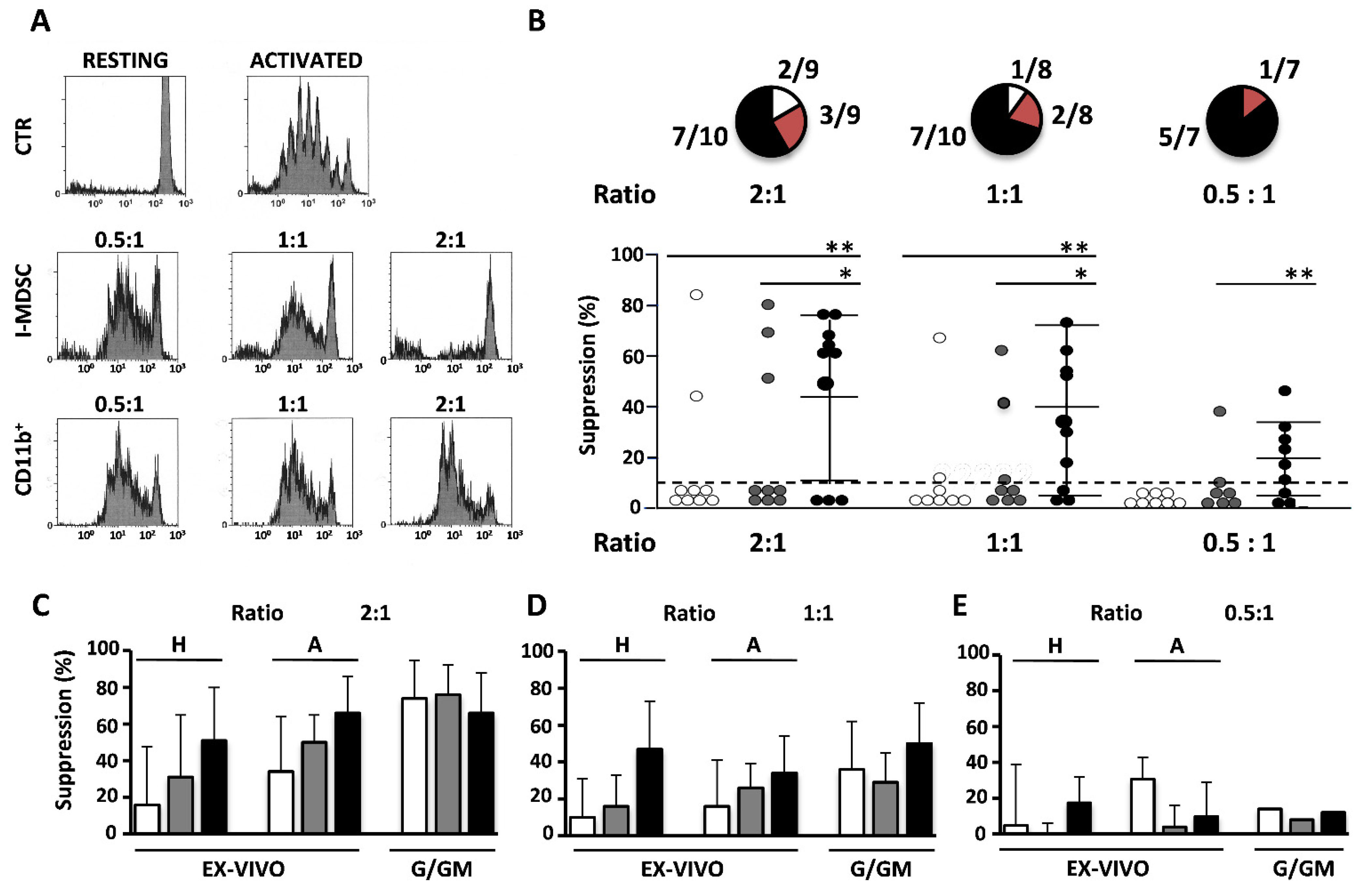
2.5. Induction of Suppression Activity and Suppression Assay
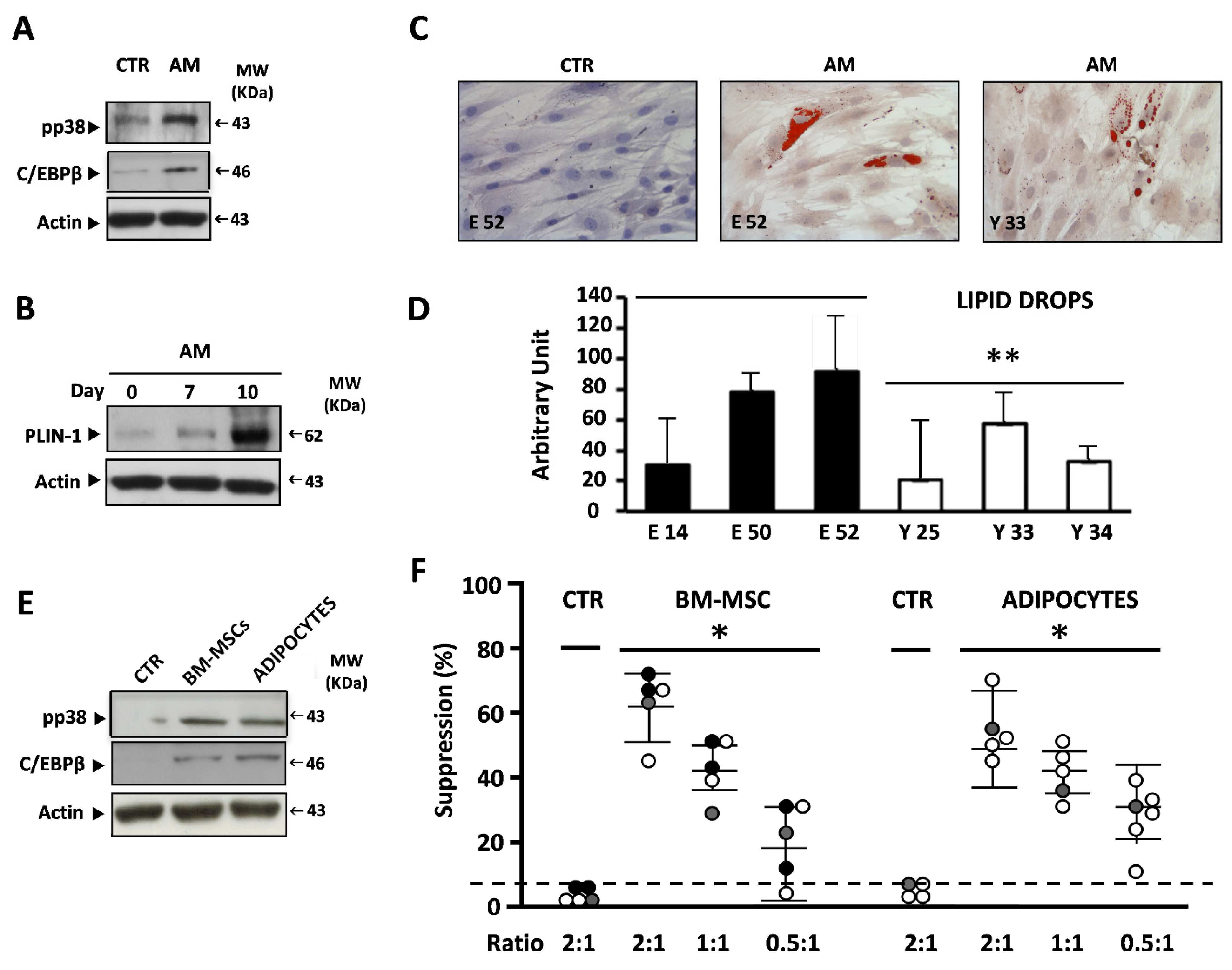
2.6. BM-Derived MSC (BM-MSC) Culture, Characterization and Adipogenic Differentiation
2.7. Red Oil Staining
2.8. Immunoblotting (IB)
2.9. BM-MSC Cloning
2.10. Statistical Analysis
3. Results
3.1. Cell Subset and Cytokine Levels Are Remodeled during Aging of the Bone Marrow
3.2. Ex Vivo Suppressive Activity of I-MDSCs Is Primarily Found in Elders
3.3. BM-MSCs and BM-MSC-Derived Adipocytes Efficiently Induce the Suppressive Activity of I-MDSCs
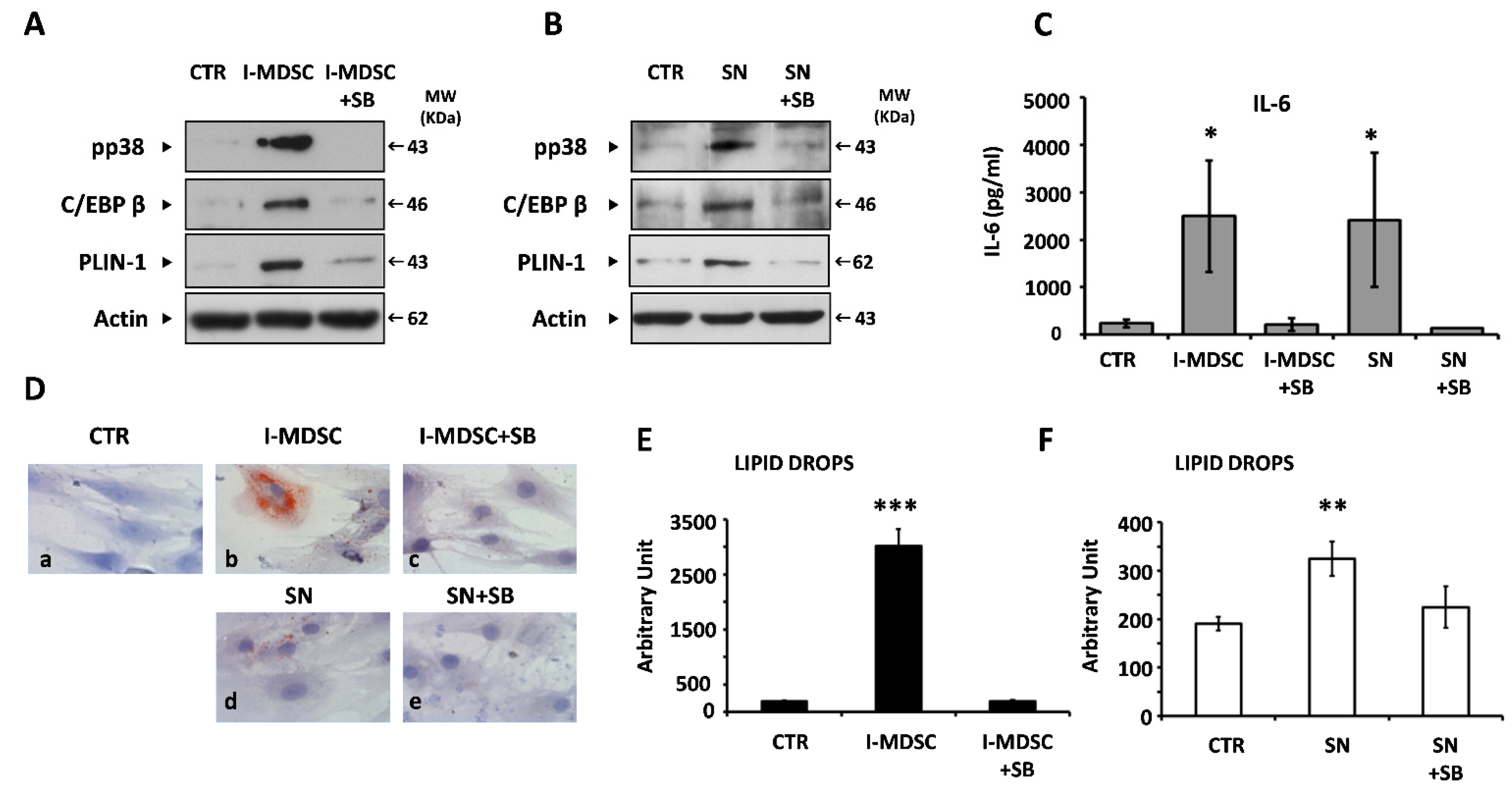
3.4. Myeloid Cells and Soluble Factors Secreted during Co-Culture Induce IL-6 Gene Expression and Adipogenesis in BM-MSCs
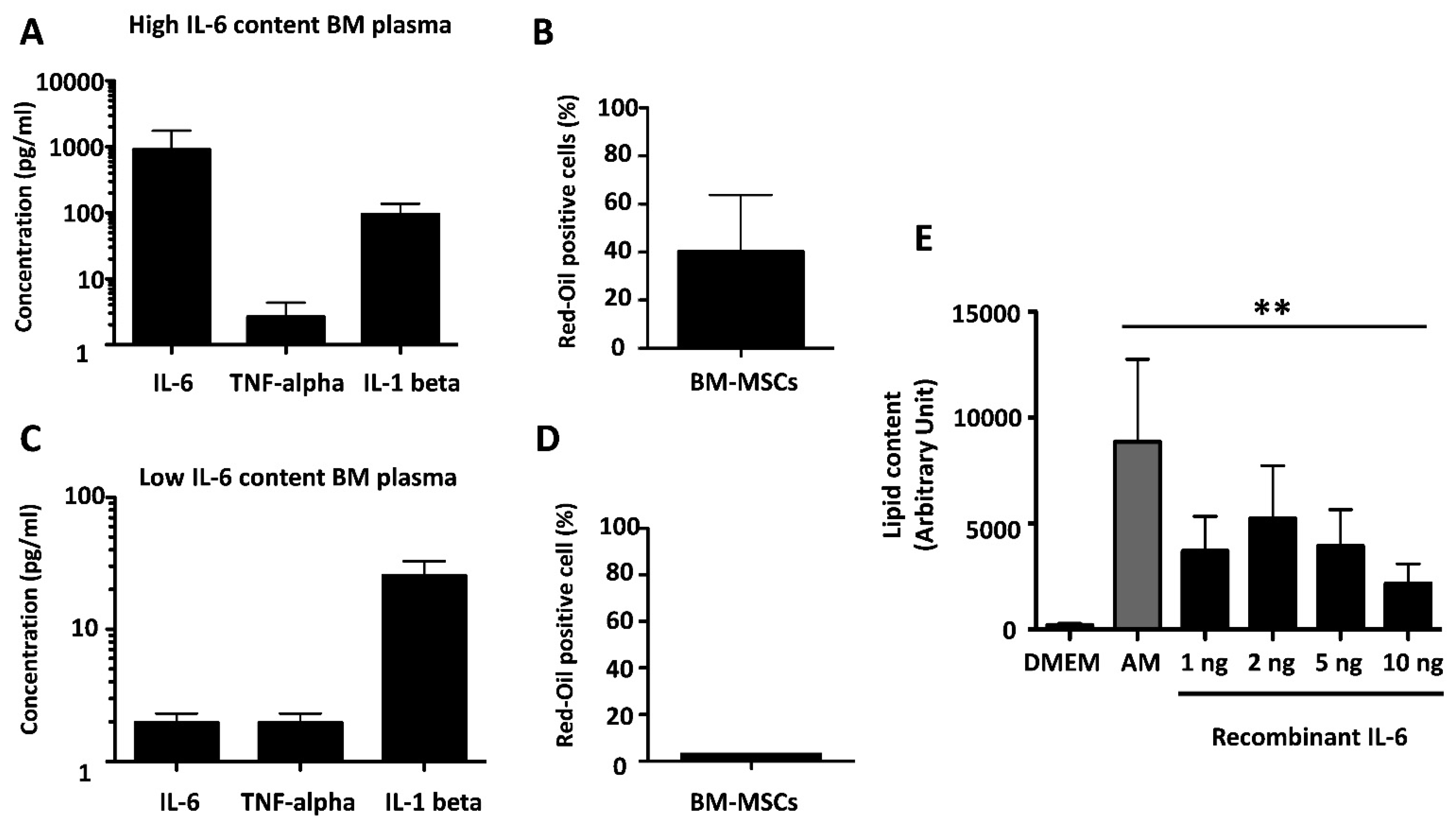
3.5. IL-6 Is an Adipogenic Factor in BM-MSCs
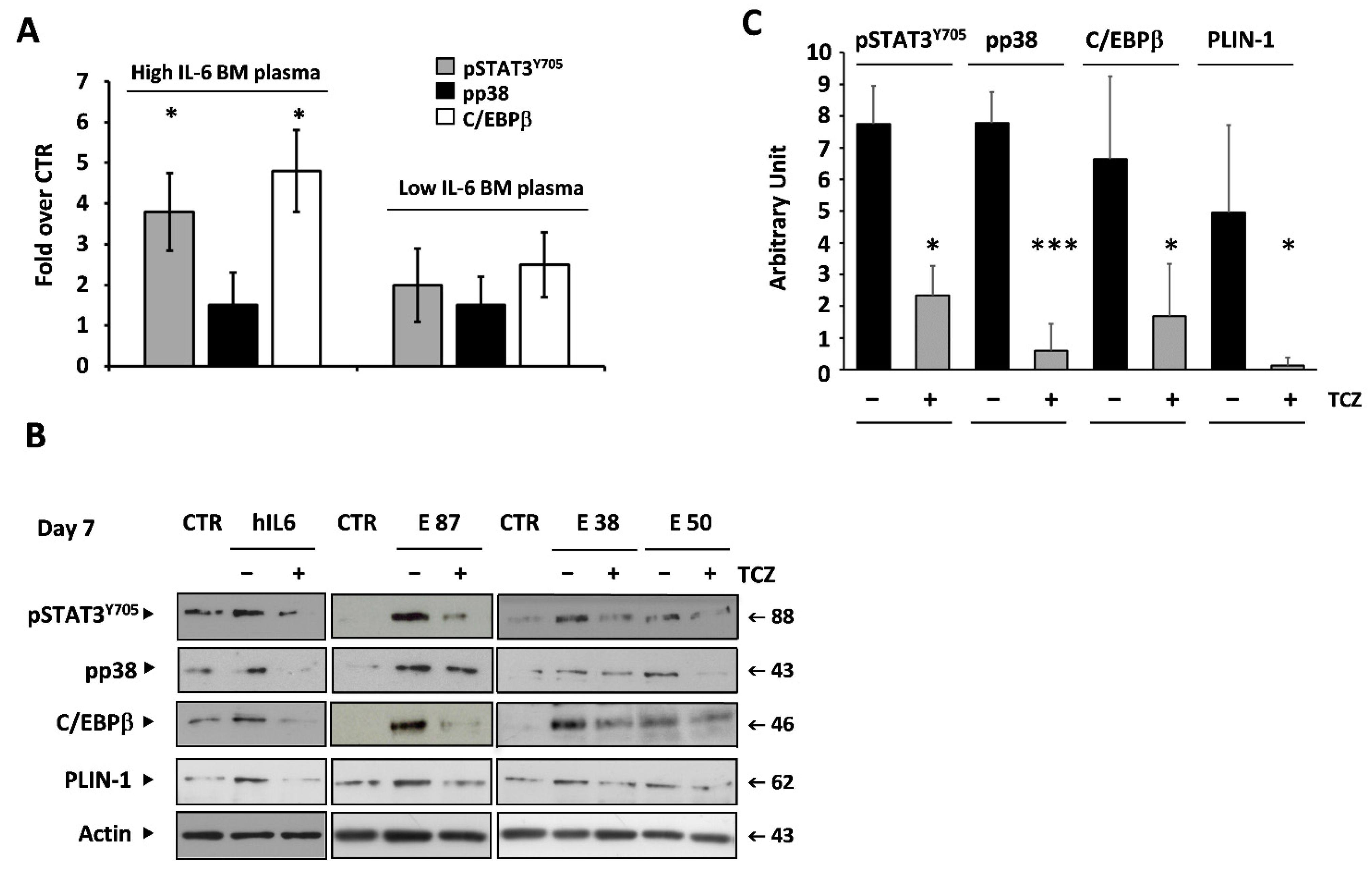
3.6. IL-6 Mediates the Adipogenic Effect of BM Plasma on BM-MSCs
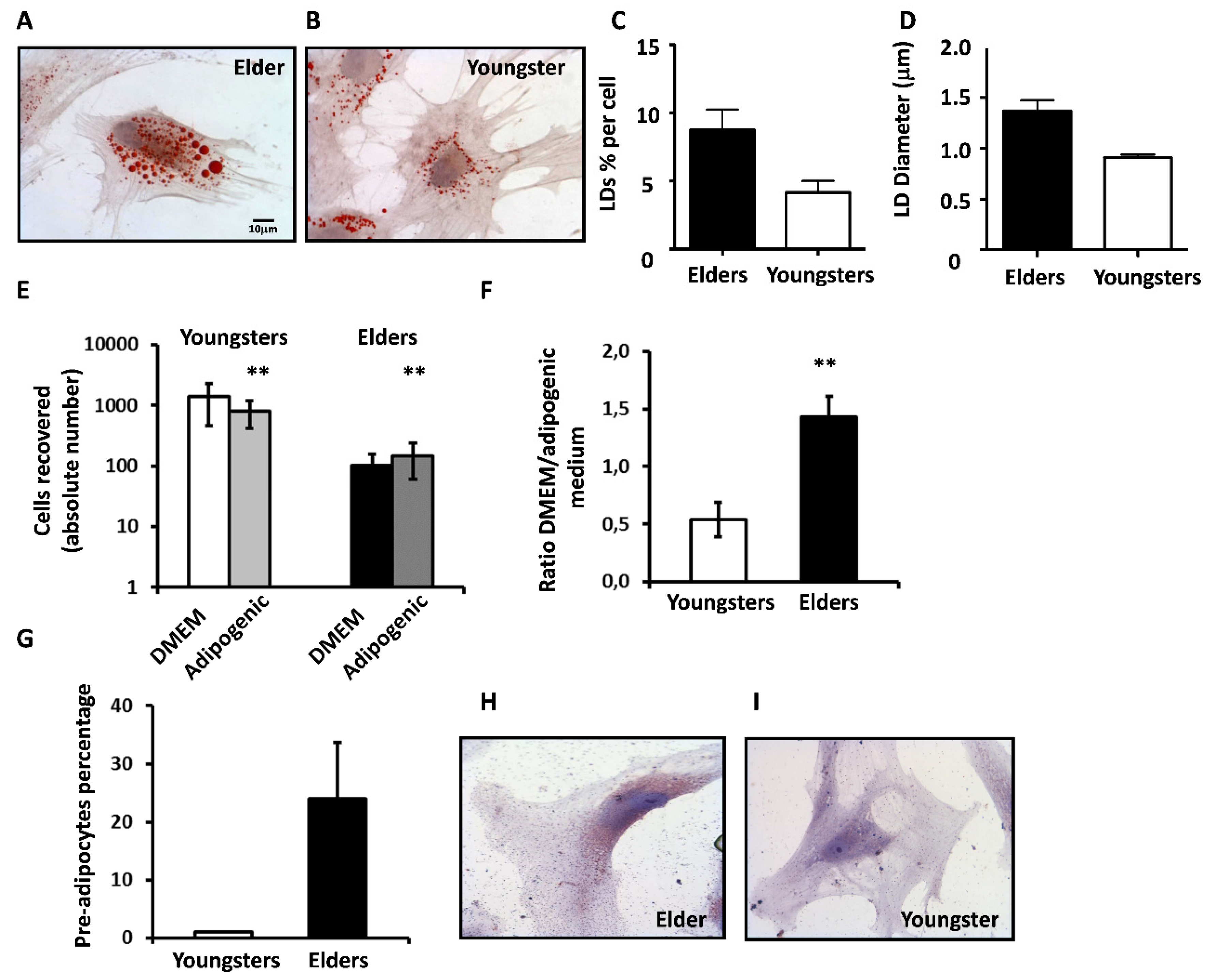
3.7. BM-MSCs from Elders Are Prone to Adipocyte Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BM | Bone marrow |
| BM-MSC | Bone marrow mesenchymal stem cell |
| CD | Cluster of differentiation |
| C/EBP beta | CAAT/enhancer-binding protein β |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECL | Enhanced chemiluminescence |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FACS | Fluorescence activated cell sorting |
| FCS | Fetal calf serum |
| G-CSF | Granulocyte colony-stimulating factors |
| GM-CSF | Granulocyte macrophage colony-stimulating factors |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HP | Horseradish peroxidase |
| HSC | Hematopoietic stem cell |
| IBMX | 3-isobutyl-1-methylxanthine |
| IL | Interleukin |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| Lin | Lineage |
| mAb | Monoclonal antibody |
| MDSC | Myeloid-derived suppressor cell |
| I-MDSC | Immature myeloid-derived suppressor cell |
| MAPK | Mitogen-activated protein kinase |
| MSC | Mesenchymal stem cell |
| PBMC | Peripheral blood mononuclear cell |
| PBS | Phosphate-buffered saline |
| PPAR-γ | Peroxisome proliferator-activated receptor-g |
| RBC | Red blood cell |
| SDS-PAGE | Sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| STAT | Signal transducers and activators of transcription |
| TNF-alpha | Tumor necrosis factor alpha |
| WBC | White blood cell |
References
- Zamboni, M.; Rossi, A.P.; Fantin, F.; Zamboni, G.; Chirumbolo, S.; Zoico, E.; Mazzali, G. Adipose tissue, diet and aging. Mech. Ageing Dev. 2014, 136–137, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Coder, B.; Su, D.-M. Thymic involution beyond T-cell insufficiency. Oncotarget 2015, 6, 21777–21778. [Google Scholar] [CrossRef]
- Chinn, I.K.; Blackburn, C.; Manley, N.R.; Sempowski, G.D. Changes in primary lymphoid organs with aging. Semin. Immunol. 2012, 24, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardouin, P.; Pansini, V.; Cortet, B. Bone marrow fat. Jt. Bone Spine 2014, 81, 313–319. [Google Scholar] [CrossRef]
- Mercier, F.E.; Ragu, C.; Scadden, D.T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2011, 12, 49–60. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Tarantino, U.; Piccirilli, E.; Fantini, M.; Baldi, J.; Gasbarra, E.; Bei, R. Sarcopenia and Fragility Fractures: Molecular and Clinical Evidence of the Bone-Muscle Interaction. J. Bone Jt. Surg. Am. 2015, 97, 429–437. [Google Scholar] [CrossRef]
- Abdelmagid, S.M.; Barbe, M.; Safadi, F.F. Role of inflammation in the aging bones. Life Sci. 2015, 123, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Zuo, B.; Zhang, L. The role of bone marrow microenvironment in governing the balance between os-te-oblatogenesis and adipogenesis. Aging Dis. 2016, 7, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Anthony, B.A.; Link, D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014, 35, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Takada, I.; Kouzmenko, A.P.; KatO, S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; MacDougald, O. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, M.; Jager, J.; Laurent, K.; Gonzalez, T.; Cormont, M.; Binétruy, B.; Brustel, Y.L.M.; Tanti, J.-F.; Bost, F. p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett. 2007, 581, 5591–5596. [Google Scholar] [CrossRef]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipo-genesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Chen, H.; Su, H.; Wu, X.; Xie, Z.; Wu, Y.; Shen, H. IL6 Receptor Facilitates Adipogenesis Differentiation of Human Mesenchymal Stem Cells through Activating P38 Pathway. Int. J. Stem Cells 2020, 13, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Kuranda, K.; Vargaftig, J.; de la Rochere, P.; Dosquet, C.; Charron, D.; Bardin, F.; Tonnelle, C.; Bonnet, D.; Goodhardt, M. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell. 2011, 10, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, W.W.; Schrier, S.L.; Weissman, I.L. Age-associated changes in human hematopoietic stem cells. Semin. Hematol. 2017, 54, 39–42. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan-Wong, K.; Nadeau, S.; Carrier-Leclerc, A.; Apablaza, F.; Hamdy, R.; Shum-Tim, D.; Rodier, F.; Colmegna, I. In-creased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells’ homeostasis. Oncotarget 2016, 7, 13285–13296. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Condamine, T.; Mastio, J.; Gabrilovich, D.I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Solito, S.; Falisi, E.; Diaz-Montero, C.M.; Doni, A.; Pinton, L.; Rosato, A.; Francescato, S.; Basso, G.; Zanovello, P.; Onicescu, G.; et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 2011, 118, 2254–2265. [Google Scholar] [CrossRef] [Green Version]
- Solito, S.; Marigo, I.; Pinton, L.; Damuzzo, V.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann. N. Y. Acad. Sci. 2014, 1319, 47–65. [Google Scholar] [CrossRef]
- Bowdish, D.M. Myeloid-derived suppressor cells, age and cancer. OncoImmunology 2013, 2, e24754. [Google Scholar] [CrossRef] [Green Version]
- Salminem, A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020, 57, 100998. [Google Scholar] [CrossRef]
- Nagaraj, S.; Schrum, A.; Cho, H.-I.; Celis, E.; Gabrilovich, D.I. Mechanism of T Cell Tolerance Induced by Myeloid-Derived Suppressor Cells. J. Immunol. 2010, 184, 3106–3116. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Bareyan, D.; Daynes, R.A. A Role for Immature Myeloid Cells in Immune Senescence. J. Immunol. 2011, 186, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Hsieh, K.; Lee, Y.K.; Londos, C.; Raaka, B.M.; Dalen, K.T.; Kimmel, A.R. Perilipin family members preferentially sequester to either triacylglycerol- or cholesteryl ester-specific intracellular lipid storage droplets. J. Cell Sci. 2012, 125, 4067–4076. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Benavides, T.; Velez-Delvalle, C.; Marsch-Moreno, M.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Lipogenic Enzymes Complexes and Cytoplasmic Lipid Droplet Formation During Adipogenesis. J. Cell. Biochem. 2016, 117, 2315–2326. [Google Scholar] [CrossRef]
- Sakai, A.; Oda, M.; Itagaki, M.; Yoshida, N.; Arihiro, K.; Kimura, A. Establishment of an HS23 stromal cell-dependent myeloma cell line: Fibronectin and IL-6 are critical. Int. J. Hematol. 2010, 92, 598–608. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheu-matoid arthritis: Past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef]
- Hardouin, P.; Rharass, T.; Lucas, S. Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue? Front. Endocrinol. 2016, 7, 85. [Google Scholar] [CrossRef]
- Mattiucci, D.; Maurizi, G.; Izzi, V.; Cenci, L.; Ciarlantini, M.; Mancini, S.; Mensà, E.; Pascarella, R.; Vivarelli, M.; Olivieri, A.; et al. Bone marrow adipocytes support hematopoietic stem cell survival. J. Cell. Physiol. 2018, 233, 1500–1511. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Schulz, T.J. The emerging role of bone marrow adipose tissue in bone health and dysfunction. J. Mol. Med. 2017, 95, 1291–1301. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schurmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 2017, 20, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.R.; Bloom, D.E. Towards a comprehensive public health response to population ageing. Lancet 2015, 385, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.J.; Wu, F.; Guo, Y.; Robledo, L.M.G.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
| Volunteers | Total Number | Age (Range) | Mean Age ± SD | BM Donors | Cardiac Surgery | Femur Fracture | |||
|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | ||||
| Young people | 18 | 18–35 | 26 ± 4.8 | 8 | 10 | 0 | 0 | 0 | 0 |
| Adults | 17 | 36–65 | 53 ± 9.6 | 4 | 2 | 0 | 4 | 5 | 2 |
| Elders | 22 | 66–92 | 80 ± 8.6 | 0 | 0 | 0 | 4 | 14 | 4 |
| Site of BM sampling | Iliac crest | Iliac crest | Sternum | Sternum | Femur | Femur | |||
| Total number | 57 | 12 | 12 | 0 | 8 | 19 | 6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perico, M.E.; Maluta, T.; Conti, G.; Vella, A.; Provezza, L.; Cestari, T.; De Cao, G.; Segalla, L.; Tecchio, C.; Benedetti, F.; et al. The Cross-Talk between Myeloid and Mesenchymal Stem Cells of Human Bone Marrow Represents a Biomarker of Aging That Regulates Immune Response and Bone Reabsorption. Cells 2022, 11, 1. https://doi.org/10.3390/cells11010001
Perico ME, Maluta T, Conti G, Vella A, Provezza L, Cestari T, De Cao G, Segalla L, Tecchio C, Benedetti F, et al. The Cross-Talk between Myeloid and Mesenchymal Stem Cells of Human Bone Marrow Represents a Biomarker of Aging That Regulates Immune Response and Bone Reabsorption. Cells. 2022; 11(1):1. https://doi.org/10.3390/cells11010001
Chicago/Turabian StylePerico, Maria Elisa, Tommaso Maluta, Giamaica Conti, Antonio Vella, Lisa Provezza, Tiziana Cestari, Giulia De Cao, Lydia Segalla, Cristina Tecchio, Fabio Benedetti, and et al. 2022. "The Cross-Talk between Myeloid and Mesenchymal Stem Cells of Human Bone Marrow Represents a Biomarker of Aging That Regulates Immune Response and Bone Reabsorption" Cells 11, no. 1: 1. https://doi.org/10.3390/cells11010001
APA StylePerico, M. E., Maluta, T., Conti, G., Vella, A., Provezza, L., Cestari, T., De Cao, G., Segalla, L., Tecchio, C., Benedetti, F., Santini, F., Bronte, V., Magnan, B., Sbarbati, A., & Ramarli, D. (2022). The Cross-Talk between Myeloid and Mesenchymal Stem Cells of Human Bone Marrow Represents a Biomarker of Aging That Regulates Immune Response and Bone Reabsorption. Cells, 11(1), 1. https://doi.org/10.3390/cells11010001






