A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Rat ASCs Isolation
2.3. In Vitro Culture of Rat ASCs
2.4. Evaluation of Morphology and Immunophenotypic Characterization of rat ASCs (3rd Passage)
2.5. Pluripotent Gene Expression of Cultured Rat ASCs
2.6. Rat ASCs Differentiation (Adipogenesis, Osteogenesis, and Chondrogenesis)
2.6.1. In Vitro Osteogenic Induction
2.6.2. In Vitro Chondrogenic Induction
2.6.3. In Vitro Adipogenic Induction
2.7. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statical Analysis
3. Results
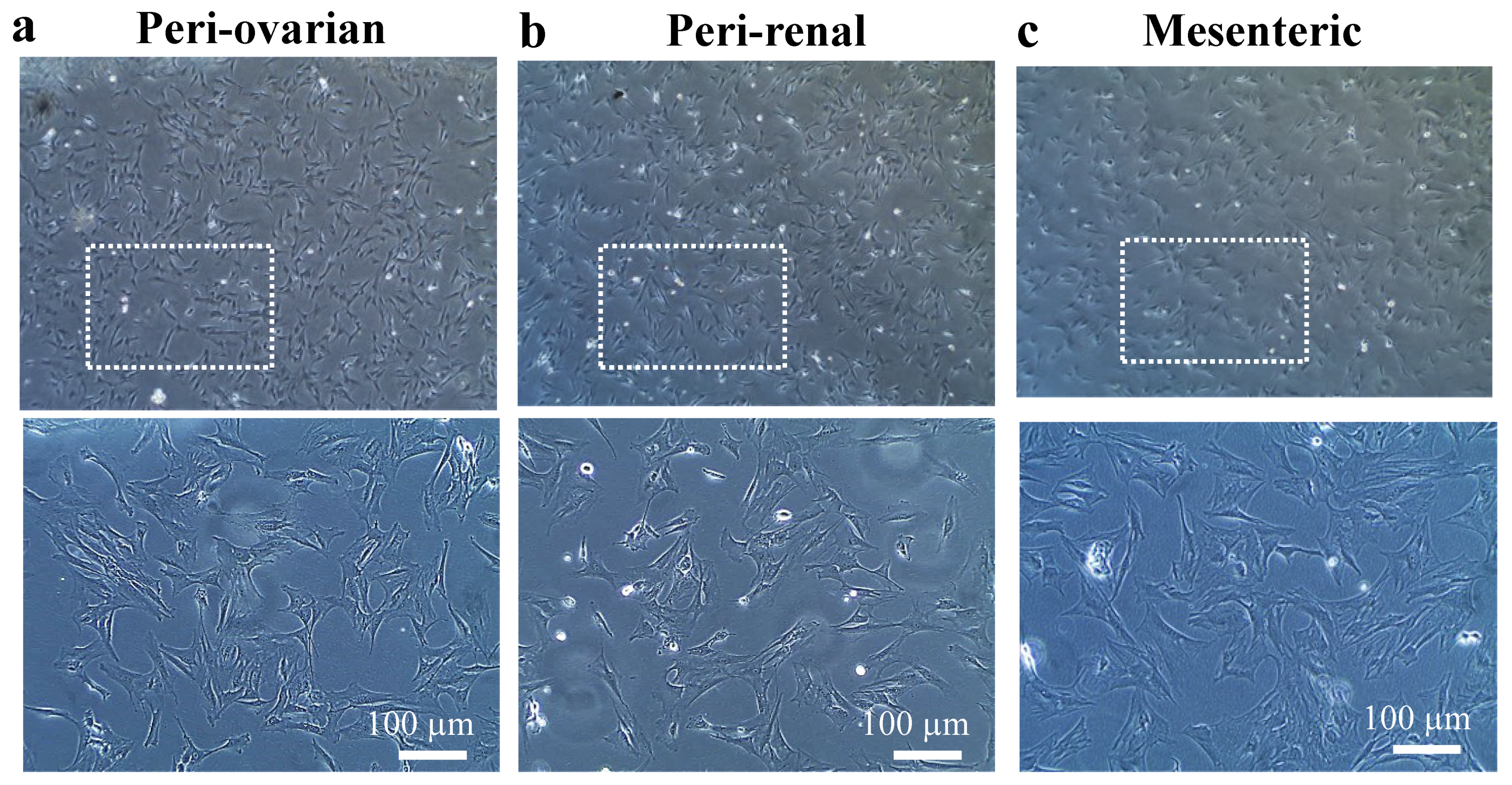
3.1. Cell Culture
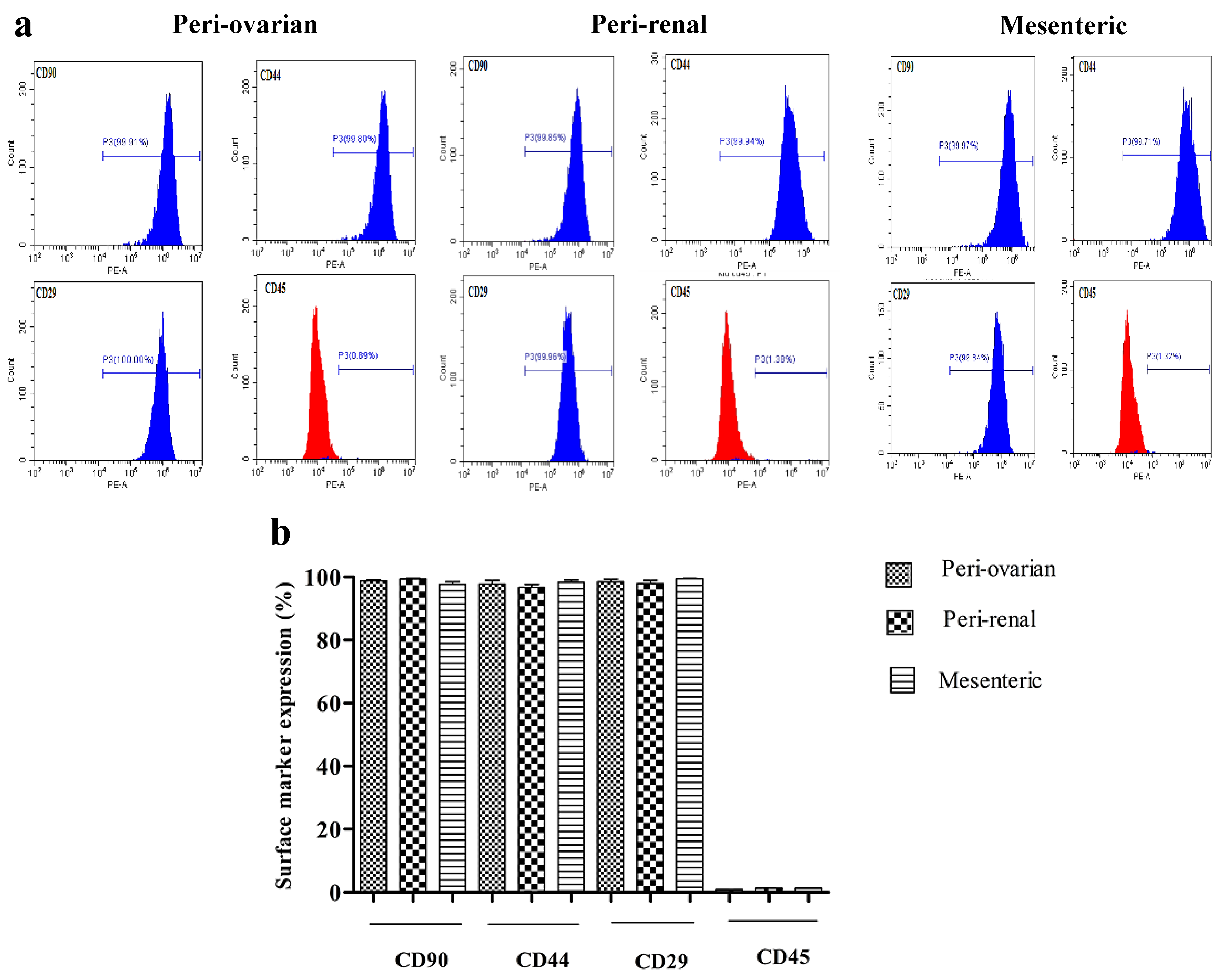
3.2. Immunophenotypic Characterization of Rat ASCs (3rd Passage)
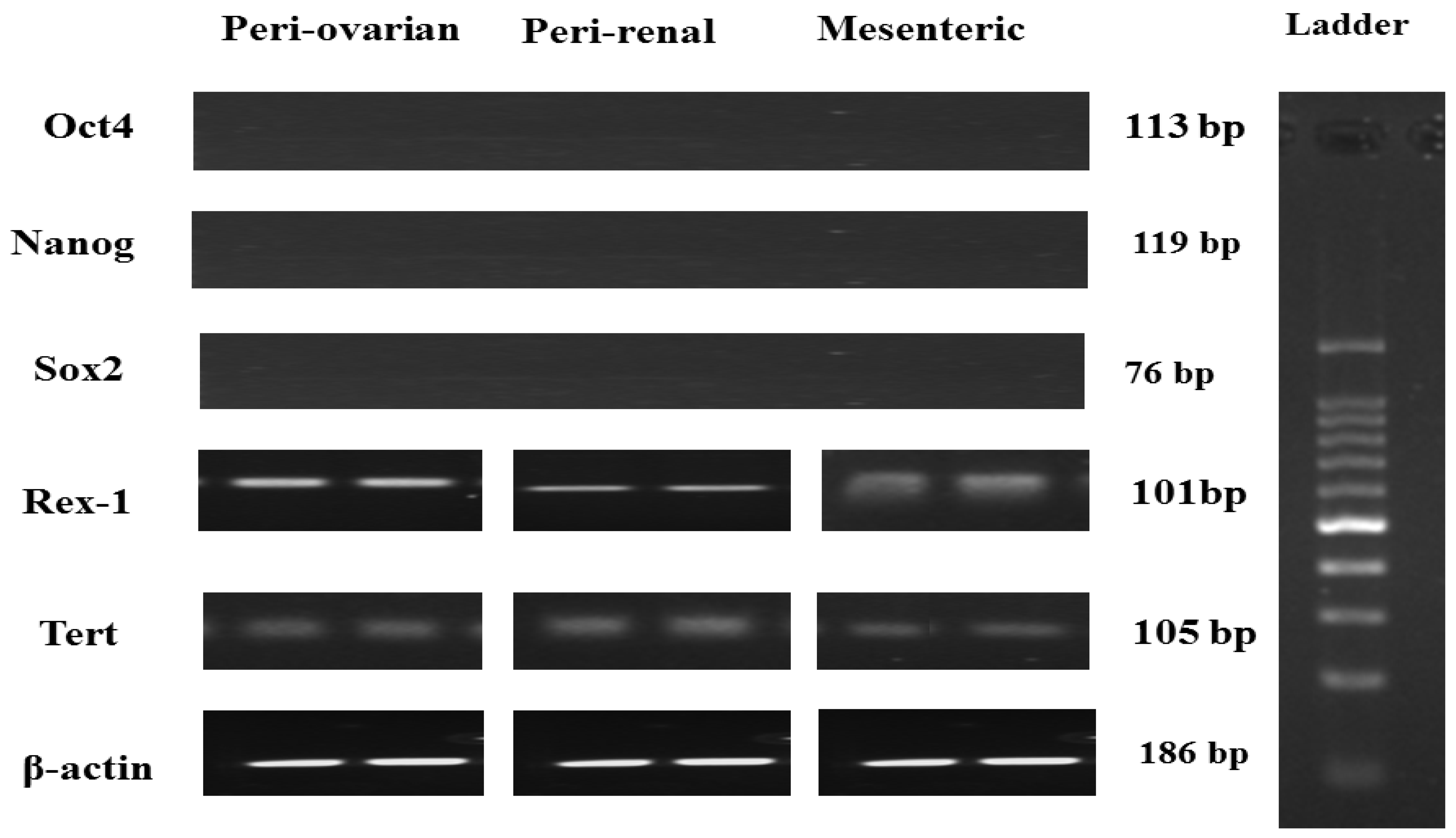
3.3. Pluripotent Gene Expression of Rat ASCs
3.4. Rat ASCs Differentiation Potential
3.4.1. Depot-Specific Differences in Adipogenic Potential
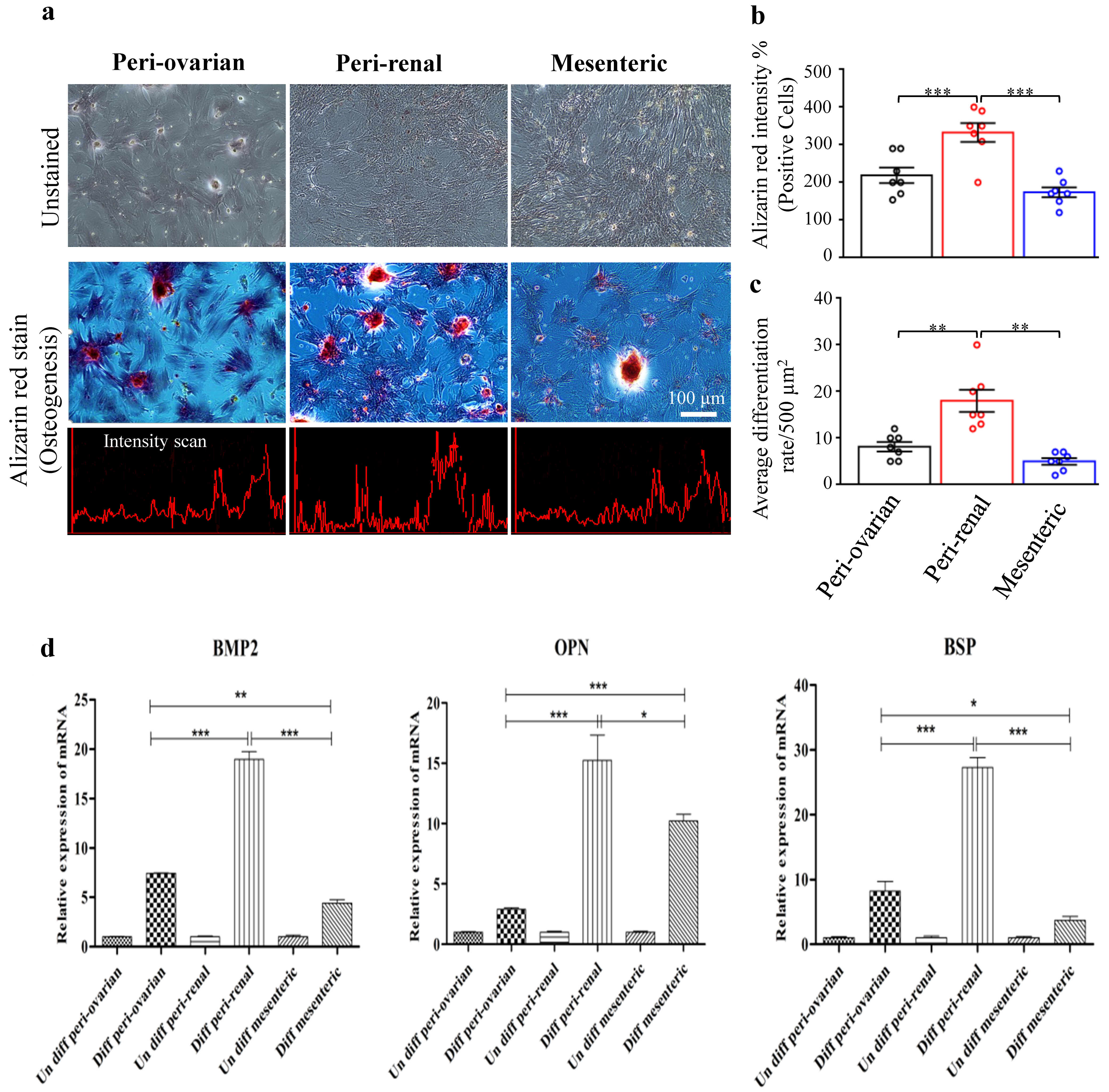
3.4.2. Depot-Specific Differences in Osteogenic Potential
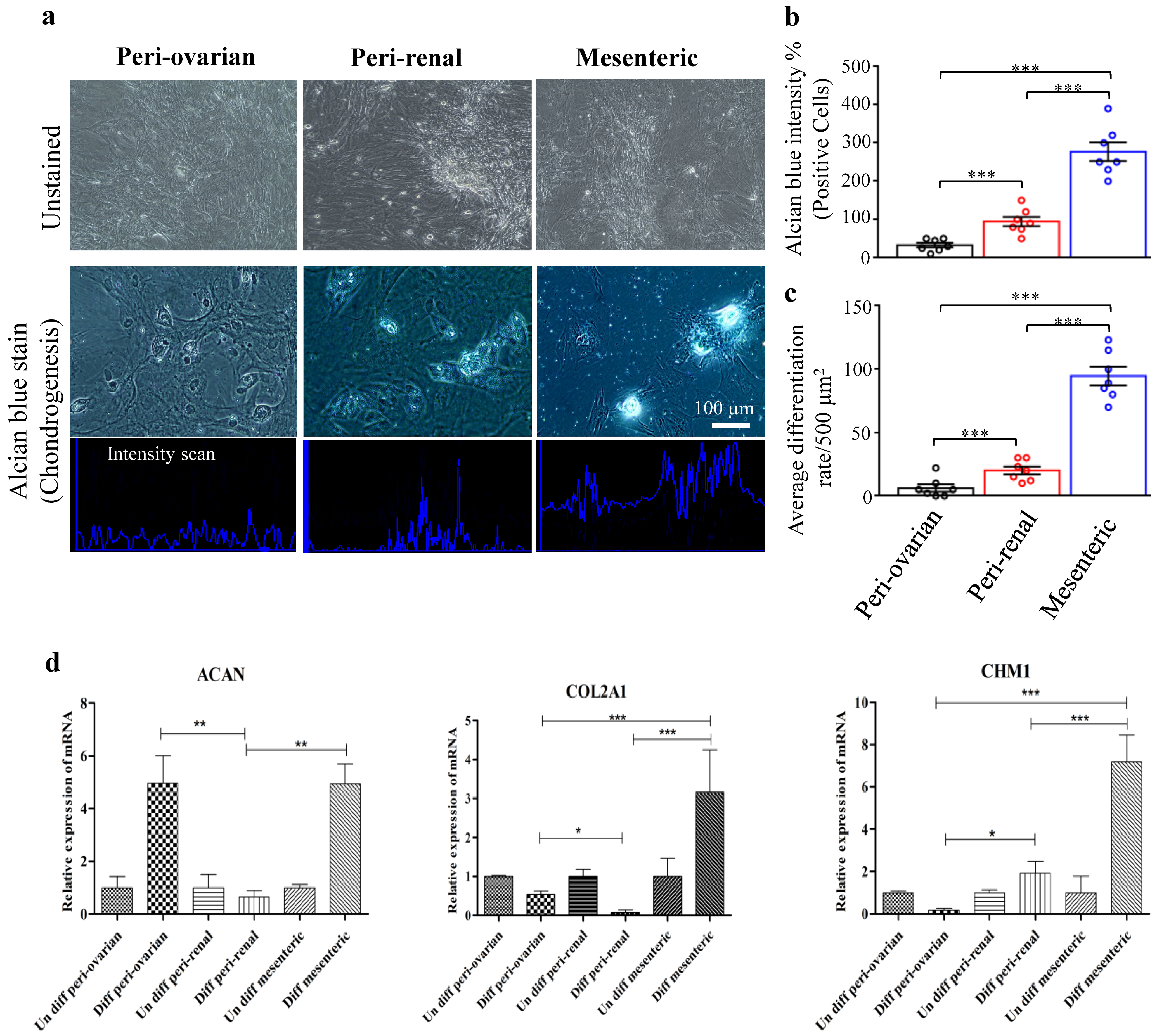
3.4.3. Depot-Specific Differences in Chondrogenic Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzsimmons, R.E.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F. Mesenchymal stem cells from adult bone marrow. In Mesenchymal Stem Cells; Springer’s Humana Press: Totowa, NJ, USA, 2008; pp. 27–44. [Google Scholar]
- Dicker, A.; Le Blanc, K.; Åström, G.; van Harmelen, V.; Götherström, C.; Blomqvist, L.; Arner, P.; Rydén, M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp. Cell Res. 2005, 308, 283–290. [Google Scholar] [CrossRef]
- Nakao, N.; Nakayama, T.; Yahata, T.; Muguruma, Y.; Saito, S.; Miyata, Y.; Yamamoto, K.; Naoe, T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: Advantages over bone marrow-derived mesenchymal stem cells. Am. J. Pathol. 2010, 177, 547–554. [Google Scholar] [CrossRef]
- Deasy, B.M.; Jankowski, R.J.; Huard, J. Muscle-derived stem cells: Characterization and potential for cell-mediated therapy. Blood Cells Mol. Dis. 2001, 27, 924–933. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Nakahara, H.; Goldberg, V.M.; Caplan, A.I. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J. Orthop. Res. 1991, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Lechner, A.; Habener, J.F. Stem/progenitor cells derived from adult tissues: Potential for the treatment of diabetes mellitus. Am. J. Physiol.-Endocrinol. Metab. 2003, 284, E259–E266. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Methods and strategies for procurement, isolation, characterization, and assessment of senescence of human mesenchymal stem cells from adipose tissue. In Stem Cells and Aging; Springer’s Humana Press: Totowa, NJ, USA, 2018; pp. 37–92. [Google Scholar]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Kazmierski, L.; Maj, M.; Drewa, T. Adipose-derived stem cells as a tool in cell-based therapies. Arch. Immunol. Ther. Exp. 2016, 64, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyllönen, L.; Haimi, S.; Mannerström, B.; Huhtala, H.; Rajala, K.M.; Skottman, H.; Sándor, G.K.; Miettinen, S. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: Updated status. Int. J. Mol. Sci. 2018, 19, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Huang, C.Y.C.; Candiotti, K.A.; Zeng, X.; Yuan, T.; Li, J.; Yu, H.; Abdi, S. Sox-9 facilitates differentiation of adipose tissue-derived stem cells into a chondrocyte-like phenotype in vitro. J. Orthop. Res. 2011, 29, 1291–1297. [Google Scholar] [CrossRef]
- De Girolamo, L.; Stanco, D.; Salvatori, L.; Coroniti, G.; Arrigoni, E.; Silecchia, G.; Russo, M.A.; Niada, S.; Petrangeli, E.; Brini, A. Stemness and osteogenic and adipogenic potential are differently impaired in subcutaneous and visceral adipose derived stem cells (ASCs) isolated from obese donors. Int. J. Immunopathol. Pharmacol. 2013, 26, 11–21. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Gentile, P.; Mazzaglia, D.; Cervelli, V.; Orlandi, A. The biomolecular basis of adipogenic differentiation of adipose-derived stem cells. Int. J. Mol. Sci. 2014, 15, 6517–6526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-C.C.; Sepulveda, J.L.; Rubin, J.P.; Marra, K.G. Cardiomyogenic differentiation potential of human adipose precursor cells. Int. J. Cardiol. 2009, 133, 399–401. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Arévalo-Turrubiarte, M.; Olmeo, C.; Accornero, P.; Baratta, M.; Martignani, E. Analysis of mesenchymal cells (MSCs) from bone marrow, synovial fluid and mesenteric, neck and tail adipose tissue sources from equines. Stem Cell Res. 2019, 37, 101442. [Google Scholar] [CrossRef] [PubMed]
- Reumann, M.K.; Linnemann, C.; Aspera-Werz, R.H.; Arnold, S.; Held, M.; Seeliger, C.; Nussler, A.K.; Ehnert, S. Donor site location is critical for proliferation, stem cell capacity, and osteogenic differentiation of adipose mesenchymal stem/stromal cells: Implications for bone tissue engineering. Int. J. Mol. Sci. 2018, 19, 1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnhold, S.; Elashry, M.I.; Klymiuk, M.C.; Geburek, F. Investigation of stemness and multipotency of equine adipose-derived mesenchymal stem cells (ASCs) from different fat sources in comparison with lipoma. Stem Cell Res. Ther. 2019, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Matsubara, Y.; Lin, K.; Sugimachi, K.; Furue, M. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem. Funct. Cell. Biochem. Modul. Act. Agents Dis. 2009, 27, 440–447. [Google Scholar] [CrossRef]
- Rebelatto, C.; Aguiar, A.; Moretao, M.; Senegaglia, A.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Barberini, D.J.; Freitas, N.P.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; da Cruz Landim-Alvarenga, F.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Boeuf, S.; Richter, W. Chondrogenesis of mesenchymal stem cells: Role of tissue source and inducing factors. Stem Cell Res. Ther. 2010, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siennicka, K.; Zołocińska, A.; Dębski, T.; Pojda, Z. Comparison of the Donor Age-Dependent and In Vitro Culture-Dependent Mesenchymal Stem Cell Aging in Rat Model. Stem Cells Int. 2021, 2021, 6665358. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/β-catenin signaling pathways. PLoS ONE 2017, 12, e0173877. [Google Scholar]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia 2011, 59, 734–749. [Google Scholar] [CrossRef]
- Lotfy, A.; Salama, M.; Zahran, F.; Jones, E.; Badawy, A.; Sobh, M. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: A comparative study. Int. J. Stem Cells 2014, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Luo, Y.; Mohsin, A.; Xu, C.; Wang, Q.; Hang, H.; Zhuang, Y.; Chu, J.; Guo, M. Co-culture with TM4 cells enhances the proliferation and migration of rat adipose-derived mesenchymal stem cells with high stemness. Cytotechnology 2018, 70, 1409–1422. [Google Scholar] [CrossRef]
- Parvaneh, M.; Karimi, G.; Jamaluddin, R.; Ng, M.H.; Zuriati, I.; Muhammad, S.I. Lactobacillus helveticus (ATCC 27558) upregulates Runx2 and Bmp2 and modulates bone mineral density in ovariectomy-induced bone loss rats. Clin. Interv. Aging 2018, 13, 1555. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao, Z.; Liu, J.; Huang, N.; Long, D.; Wang, J.; Li, X.; Liu, Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-β1/Smads pathway. Cell Prolif. 2010, 43, 333–343. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Q.; Liang, Q.-Q.; Li, C.-G.; Holz, J.; Tang, D.; Sheu, T.-J.; Li, T.-F.; Shi, Q.; Wang, Y.-J. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthr. Cartil. 2009, 17, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Khalilzadeh, M.; Panahi, G.; Rashidian, A.; Hadian, M.R.; Abdollahi, A.; Afshari, K.; Shakiba, S.; Norouzi-Javidan, A.; Rahimi, N.; Momeny, M. The protective effects of sumatriptan on vincristine-induced peripheral neuropathy in a rat model. Neurotoxicology 2018, 67, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Yu, C.; Belliveau, P.; Hamilton, A.; Flynn, L.E. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl. Med. 2014, 3, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Y.; Li, Z.; Guo, Z. Isolation and Multiple Differentiation of Rat Pericardial Fluid Cells. Front. Cell Dev. Biol. 2021, 9, 198. [Google Scholar]
- Li, C.; Wei, G.; Gu, Q.; Wen, G.; Qi, B.; Xu, L.; Tao, S. Donor age and cell passage affect osteogenic ability of rat bone marrow mesenchymal stem cells. Cell Biochem. Biophys. 2015, 72, 543–549. [Google Scholar] [CrossRef]
- Jia, L.-Y.; Zhang, Q.; Fang, N.; Chen, L.; Yu, L.-M.; Liu, J.-W.; Zhang, T. Enrichment and biological characteristics of peripheral blood-derived mesenchymal stem cells in rats. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015, 23, 506–511. [Google Scholar]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Schäffler, A.; Büchler, C. Concise review: Adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Taha, M.F.; Javeri, A.; Rohban, S.; Mowla, S.J. Upregulation of pluripotency markers in adipose tissue-derived stem cells by miR-302 and leukemia inhibitory factor. BioMed Res. Int. 2014, 2014, 941486. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, E.; Gava, B.; Manini, I.; Roviello, F.; Marotta, G.; Chiavarelli, M.; Sorrentino, V. Pluripotency regulators in human mesenchymal stem cells: Expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011, 20, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, L.; Song, Z.; Yang, G. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol. Biol. Rep. 2012, 39, 5791–5804. [Google Scholar] [CrossRef] [PubMed]
- Casella, S. Gene Expression Analysis of Rat Adi-pose Tissue-Derived Stem Cells. Int. J. Stem Cell Res. Transpl. 2015, 3, 120–124. [Google Scholar]
- Scotland, K.B.; Chen, S.; Sylvester, R.; Gudas, L.J. Analysis of Rex1 (zfp42) function in embryonic stem cell differentiation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 1863–1877. [Google Scholar] [CrossRef] [Green Version]
- Hidema, S.; Fukuda, T.; Date, S.; Tokitake, Y.; Matsui, Y.; Sasaki, H.; Nishimori, K. Transgenic expression of Telomerase reverse transcriptase (Tert) improves cell proliferation of primary cells and enhances reprogramming efficiency into the induced pluripotent stem cell. Biosci. Biotechnol. Biochem. 2016, 80, 1925–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tholpady, S.; Katz, A.; Ogle, R. Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. Off. Publ. Am. Assoc. Anat. 2003, 272, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Foissac, R.; Ladoux, A.; Chignon-Sicard, B. Autologous fat grafts: Can we match the donor fat site and the host environment for better postoperative outcomes and safety? Curr. Surg. Rep. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Lu, X.; Tan, A.; Chen, Y.; Li, Y.; Peng, X.; Yuan, S.; Cai, D.; Yu, Y. Peri-ovarian adipose tissue contributes to intraovarian control during folliculogenesis in mice. Reproduction 2018, 156, 133–144. [Google Scholar] [CrossRef]
- Foissac, R.; Villageois, P.; Chignon-Sicard, B.; Georgiou, C.; Camuzard, O.; Dani, C. Homeotic and embryonic gene expression in breast adipose tissue and in adipose tissues used as donor sites in plastic surgery. Plast. Reconstr. Surg. 2017, 139, 685e–692e. [Google Scholar] [CrossRef]
- Kouidhi, M.; Villageois, P.; Mounier, C.M.; Ménigot, C.; Rival, Y.; Piwnica, D.; Aubert, J.; Chignon-Sicard, B.; Dani, C. Characterization of human knee and chin adipose-derived stromal cells. Stem Cells Int. 2015, 2015, 592090. [Google Scholar] [CrossRef] [Green Version]
- Seifert, A.; Werheid, D.F.; Knapp, S.M.; Tobiasch, E. Role of Hox genes in stem cell differentiation. World J. Stem Cells 2015, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P.; Livne, E.; Gross, G.; Häupl, T.; Neves, N.M.; Marie, P.; Bianco, P.; Jorgensen, C. Human bone marrow mesenchymal stem cells: A systematic reappraisal via the genostem experience. Stem Cell Rev. Rep. 2011, 7, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satomura, K.; Krebsbach, P.; Bianco, P.; Gehron Robey, P. Osteogenic imprinting upstream of marrow stromal cell differentiation. J. Cell. Biochem. 2000, 78, 391–403. [Google Scholar] [CrossRef]
- Hanna, C.W. Placental imprinting: Emerging mechanisms and functions. PLoS Genet. 2020, 16, e1008709. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.H.; Perie, L.; Swart, E.; Gerlach, C.; van Rooij, N.; de Boer, R.J.; Schumacher, T.N. Diverse and heritable lineage imprinting of early haematopoietic progenitors. Nature 2013, 496, 229–232. [Google Scholar] [CrossRef]
- Bogutz, A.B.; Brind’Amour, J.; Kobayashi, H.; Jensen, K.N.; Nakabayashi, K.; Imai, H.; Lorincz, M.C.; Lefebvre, L. Evolution of imprinting via lineage-specific insertion of retroviral promoters. Nat. Commun. 2019, 10, 5674. [Google Scholar] [CrossRef] [Green Version]
- Gavin, K.M.; Sullivan, T.M.; Kohrt, W.M.; Majka, S.M.; Klemm, D.J. Ovarian hormones regulate the production of adipocytes from bone marrow-derived cells. Front. Endocrinol. 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, P.C.; Koch, B.; Hickmann, E.; Schubert, R.; Cinatl, J.; Hauser, I.A.; Geiger, H. Isolation, characterization, differentiation and immunomodulatory capacity of mesenchymal stromal/stem cells from human perirenal adipose tissue. Cells 2019, 8, 1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassis, L.A.; Police, S.B.; Yiannikouris, F.; Thatcher, S.E. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 2008, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Yin, Z.; Xie, Y. Roles of the kidney in the formation, remodeling and repair of bone. J. Nephrol. 2016, 29, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Name | Direction | Primer Sequence (5′—3′) | Refrence |
|---|---|---|---|
| ADIPOQ | Forward Reverse | TAATTCAGAGCAGCCCGTAGTGGGGATAACACTCAGAACC | [36] |
| CFD | Forward Reverse | GGAGTGACCAAGGATGAGG ACCCAGTGAGGCATTGTG | [36] |
| BMP2 | Forward Reverse | CAGGTCTTTGCACCAAGATG GCTGGACTTAAGACGCTTCC | [37] |
| OPN | Forward Reverse | GAAGAGCCAGGAGTCCGATG CTTCCCGTTGCTGTCCTGAT | This study (M14656.1) |
| BSP | Forward Reverse | AGGCTACGAGGGTCAGGATT GCACCTTCCTGAGTTGAGCT | This study (XM_017599076.2) |
| ACAN | Forward Reverse | CTCTGCCTCCCGTGAAAC TGAAGTGCCTGCATCTATGT | [38] |
| COL2A1 | Forward Reverse | TCCTAAGGGTGCCAATGGTGA AGGACCAACTTTGCCTTGAGGAC | [39] |
| CHM1 | Forward Reverse | GAGAACTGTGAGGGCTGTCA GATACCTCGGGCCAGAAGTG | This study (NM_030854.1) |
| β-actin | Forward Reverse | GCAGGAGTACGATGAGTCCG ACGCAGCTCAGTAACAGTCC | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendawy, H.; Kaneda, M.; Metwally, E.; Shimada, K.; Tanaka, T.; Tanaka, R. A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats. Cells 2021, 10, 2469. https://doi.org/10.3390/cells10092469
Hendawy H, Kaneda M, Metwally E, Shimada K, Tanaka T, Tanaka R. A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats. Cells. 2021; 10(9):2469. https://doi.org/10.3390/cells10092469
Chicago/Turabian StyleHendawy, Hanan, Masahiro Kaneda, Elsayed Metwally, Kazumi Shimada, Takashi Tanaka, and Ryou Tanaka. 2021. "A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats" Cells 10, no. 9: 2469. https://doi.org/10.3390/cells10092469
APA StyleHendawy, H., Kaneda, M., Metwally, E., Shimada, K., Tanaka, T., & Tanaka, R. (2021). A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats. Cells, 10(9), 2469. https://doi.org/10.3390/cells10092469








