The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Stimulation with Xenobiotics
2.3. Cell Viability Assay
2.4. Endotoxin Assay
2.5. Measurement of Cytokine Concentrations
2.6. Quantification of Reactive Oxygen Species (ROS)
2.7. Statistical Analysis
2.8. Proteomics Sample Preparation
2.9. Proteomics Using LC-MS/MS
2.10. Analysis of Proteomics Data
3. Results
3.1. DINCH and MINCH Have No Cytotoxic Effect in Macrophages at Physiologically Relevant Concentrations
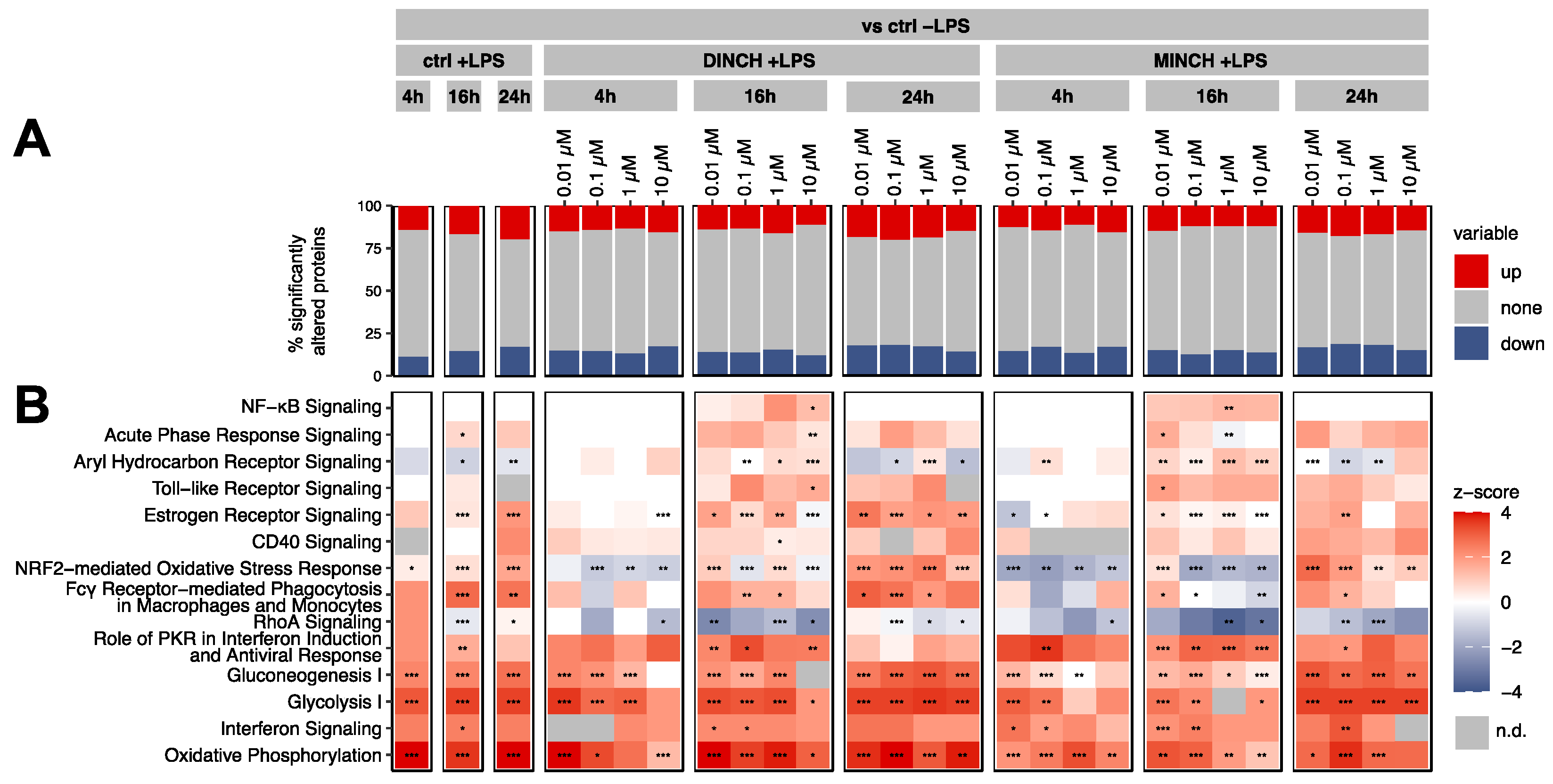
3.2. Proteomic Analysis of the Cellular Effects of DINCH and MINCH Indicate Activation of the NF-κB Signaling Pathway
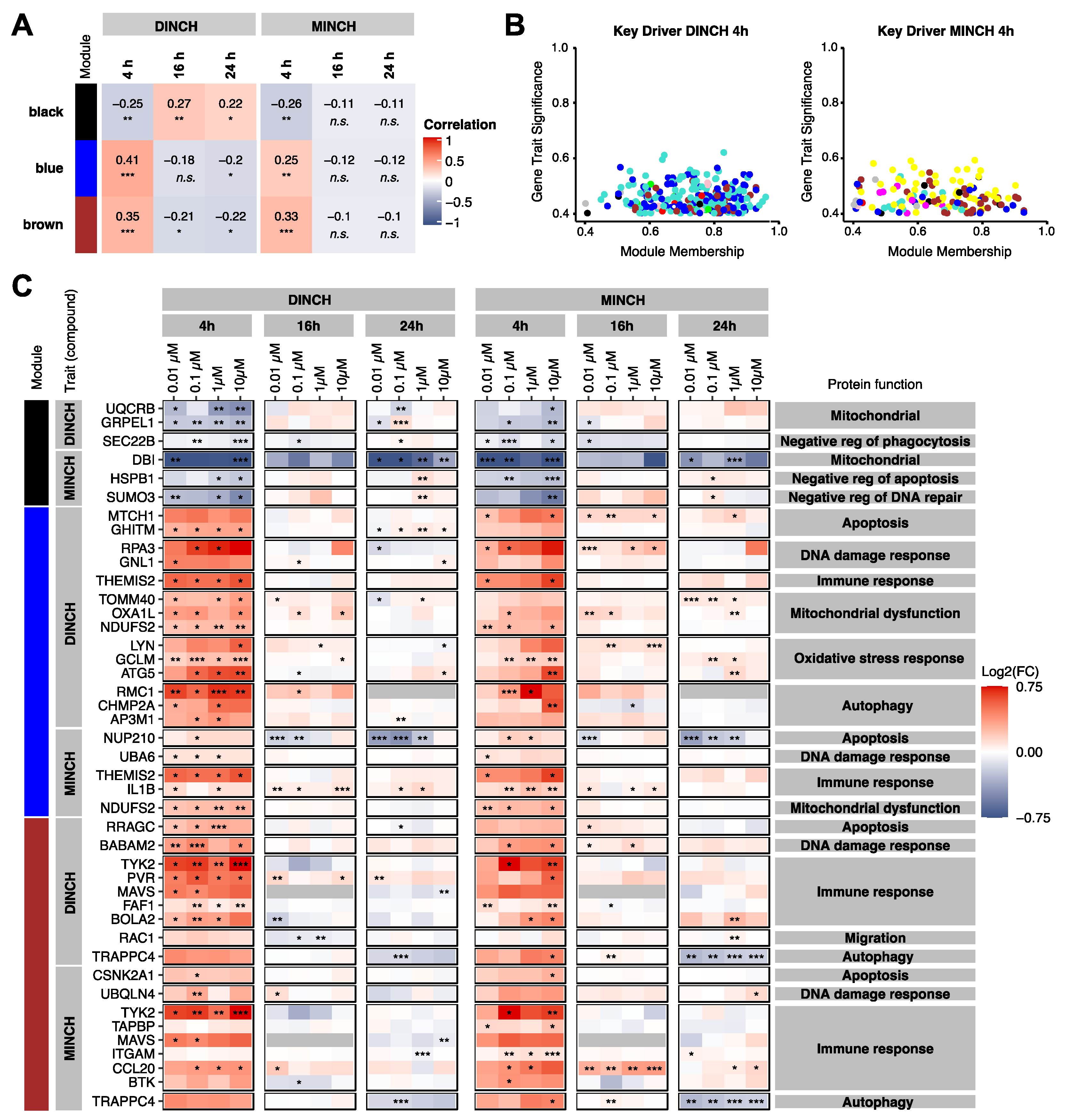
3.3. Keydriver Analysis Reveals Cellular Stress in Response to DINCH and MINCH
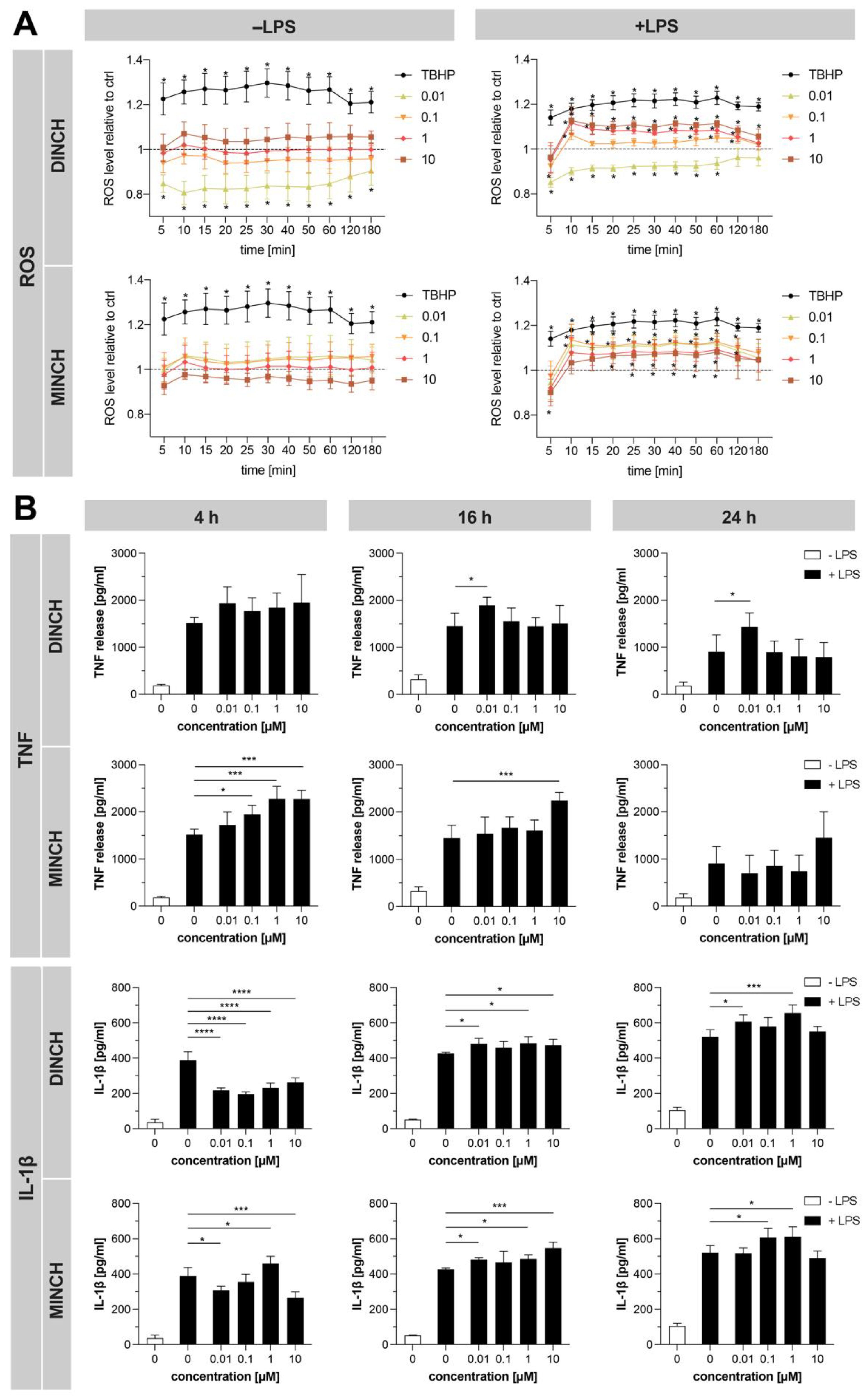
3.4. Validation of Proteomics Results Confirms Increased ROS and Cytokine Levels
4. Discussion
4.1. DINCH and MINCH Induce a Pro-Inflammatory Immune Response
4.2. DINCH and MINCH Induce Respiratory Burst and Oxidative Stress in Macrophages
4.3. Induction of Autophagy for Cell Recovery
4.4. DINCH and MINCH Inhibit Phagocytotic Pathways
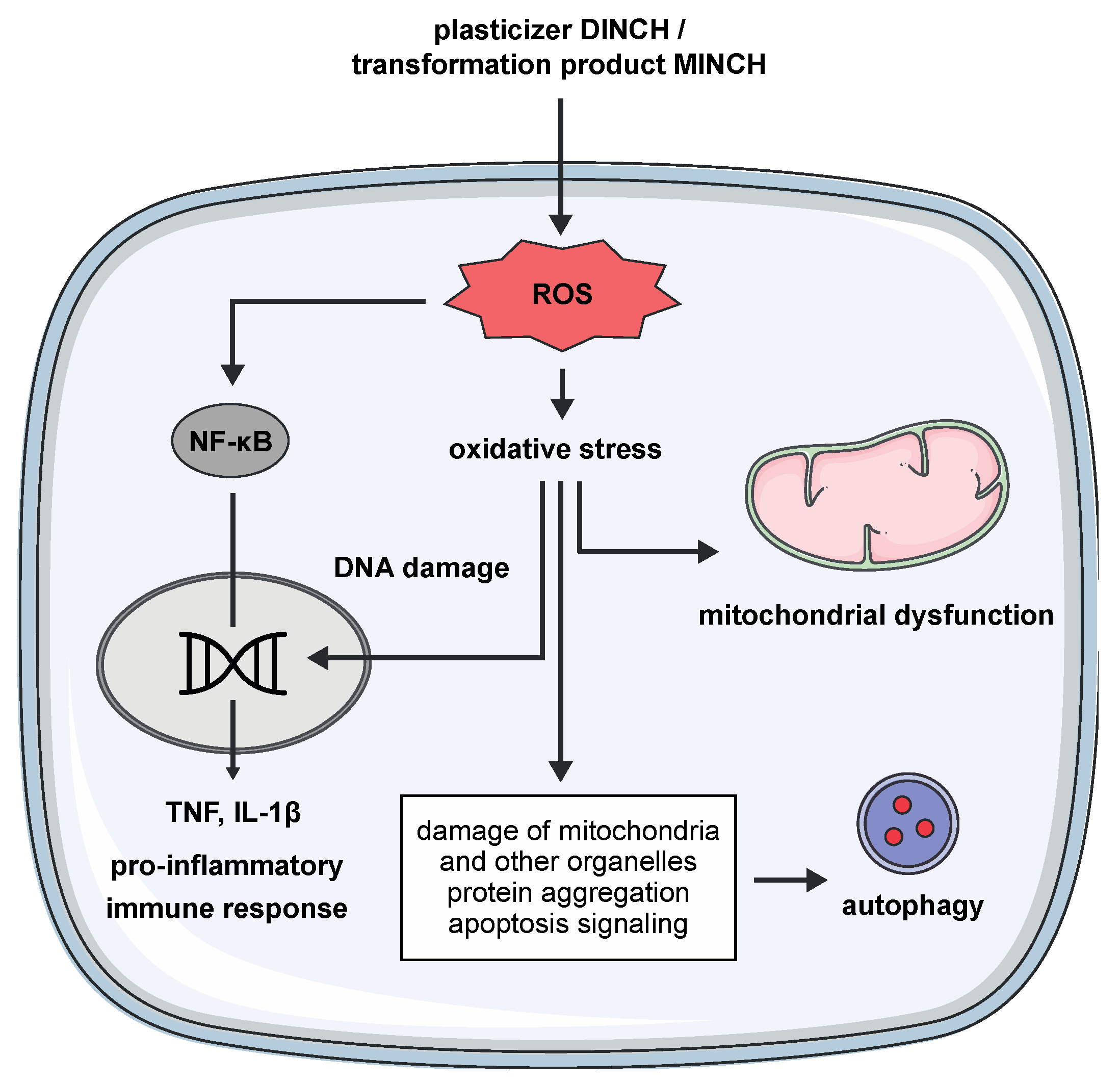
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, S.; Pluddemann, A. Tissue macrophages: Heterogeneity and functions. BMC. Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Nowak, K.; Jablonska, E.; Ratajczak-Wrona, W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019, 125, 350–364. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 2010, 271, 73–82. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food. Res. 2007, 51, 899–911. [Google Scholar] [CrossRef]
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune System: An Emerging Player in Mediating Effects of Endocrine Disruptors on Metabolic Health. Endocrinology 2018, 159, 32–45. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World. J. Biol. Chem. 2017, 8, 108–119. [Google Scholar] [CrossRef]
- Csaba, G. The Immuno-Endocrine System: Hormones, Receptors and Endocrine Function of Immune Cells. The Packed-Transport Theory. Adv. Neuroimmune Biol. 2011, 1, 71–85. [Google Scholar] [CrossRef]
- Csaba, G. Hormones in the immune system and their possible role. A critical review. Acta. Microbiol. Immunol. Hung. 2014, 61, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Batlle, D.; Pena, O.M.; Huff, R.D.; Randhawa, A.; Carlsten, C.; Bolling, A.K. Dibutyl phthalate modulates phenotype of granulocytes in human blood in response to inflammatory stimuli. Toxicol. Lett. 2018, 296, 23–30. [Google Scholar] [CrossRef]
- Couleau, N.; Falla, J.; Beillerot, A.; Battaglia, E.; D’Innocenzo, M.; Plancon, S.; Laval-Gilly, P.; Bennasroune, A. Effects of Endocrine Disruptor Compounds, Alone or in Combination, on Human Macrophage-Like THP-1 Cell Response. PLoS ONE 2015, 10, e0131428. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.F.; Nielsen, C.H.; Brorson, M.M.; Frederiksen, H.; Hartoft-Nielsen, M.L.; Rasmussen, A.K.; Bendtzen, K.; Feldt-Rasmussen, U. Influence of phthalates on in vitro innate and adaptive immune responses. PLoS ONE 2015, 10, e0131168. [Google Scholar] [CrossRef]
- Nishioka, J.; Iwahara, C.; Kawasaki, M.; Yoshizaki, F.; Nakayama, H.; Takamori, K.; Ogawa, H.; Iwabuchi, K. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflamm. Res. 2012, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.F.; Bendtzen, K.; Boas, M.; Frederiksen, H.; Nielsen, C.H.; Rasmussen, A.K.; Feldt-Rasmussen, U. Influence of phthalates on cytokine production in monocytes and macrophages: A systematic review of experimental trials. PLoS ONE 2015, 10, e0120083. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Lee, S.; Lau, M.; Marques, L.; Papadopoulos, V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Substance Infocard for 1,2-Cyclohexanedicarboxylic acid, 1,2-diisononyl ester; Helsinki, Finland. 2021. Available online: https://europa.eu/de/substance-information/-/substanceinfo/100.103.017 (accessed on 6 August 2021).
- Kasper-Sonnenberg, M.; Koch, H.M.; Apel, P.; Ruther, M.; Palmke, C.; Bruning, T.; Kolossa-Gehring, M. Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: Biomonitoring data on young adults from the Environmental Specimen Bank (ESB). Int. J. Hyg. Environ. Health 2019, 222, 1084–1092. [Google Scholar] [CrossRef]
- Campioli, E.; Lau, M.; Papadopoulos, V. Effect of subacute and prenatal DINCH plasticizer exposure on rat dams and male offspring hepatic function: The role of PPAR-α. Environ. Res. 2019, 179, 108773. [Google Scholar] [CrossRef]
- Kratochvil, I.; Hofmann, T.; Rother, S.; Schlichting, R.; Moretti, R.; Scharnweber, D.; Hintze, V.; Escher, B.I.; Meiler, J.; Kalkhof, S.; et al. Mono(2-ethylhexyl) phthalate (MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) but not di(2-ethylhexyl) phthalate (DEHP) bind productively to the peroxisome proliferator-activated receptor gamma. Rapid Commun. Mass Spectrom. 2018. [Google Scholar] [CrossRef]
- Koch, H.M.; Schutze, A.; Palmke, C.; Angerer, J.; Bruning, T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH((R))) in humans after single oral doses. Arch. Toxicol. 2013, 87, 799–806. [Google Scholar] [CrossRef]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef]
- Bennasroune, A.; Rojas, L.; Foucaud, L.; Goulaouic, S.; Laval-Gilly, P.; Fickova, M.; Couleau, N.; Durandet, C.; Henry, S.; Falla, J. Effects of 4-nonylphenol and/or diisononylphthalate on THP-1 cells: Impact of endocrine disruptors on human immune system parameters. Int. J. Immunopathol. Pharmacol. 2012, 25, 365–376. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Campioli, E.; Papadopoulos, V. Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue (2015) Environmental Research 140: 145-156 Reply to the letter by Otter, R. Environ. Res. 2016, 144, 167–169. [Google Scholar] [CrossRef]
- Schaffert, A.; Krieg, L.; Weiner, J.; Schlichting, R.; Ueberham, E.; Karkossa, I.; Bauer, M.; Landgraf, K.; Junge, K.M.; Wabitsch, M.; et al. Alternatives for the worse: Molecular insights into adverse effects of bisphenol a and substitutes during human adipocyte differentiation. Environ. Int. 2021, 156, 106730. [Google Scholar] [CrossRef] [PubMed]
- Bannuscher, A.; Karkossa, I.; Buhs, S.; Nollau, P.; Kettler, K.; Balas, M.; Dinischiotu, A.; Hellack, B.; Wiemann, M.; Luch, A.; et al. A multi-omics approach reveals mechanisms of nanomaterial toxicity and structure-activity relationships in alveolar macrophages. Nanotoxicology 2020, 14, 181–195. [Google Scholar] [CrossRef]
- Hughes, C.S.; Foehr, S.; Garfield, D.A.; Furlong, E.E.; Steinmetz, L.M.; Krijgsveld, J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Wang, Z.; Karkossa, I.; Grosskopf, H.; Rolle-Kampczyk, U.; Hackermuller, J.; von Bergen, M.; Schubert, K. Comparison of quantitation methods in proteomics to define relevant toxicological information on AhR activation of HepG2 cells by BaP. Toxicology 2020, 152652. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic. Acids. Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40. [Google Scholar] [CrossRef]
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21. [Google Scholar] [CrossRef]
- Dragulescu, A.; Arendt, C. xlsx: Read, Write, Format Excel 2007 and Excel 97/2000/XP/2003 Files. R Package Version 0.6.5. 2020. Available online: https://CRAN.R-project.org/package=xlsx. (accessed on 6 August 2021).
- Xiao, N. ggsci: Scientific Journal and Sci-Fi Themed Color Palettes for ‘ggplot2’. R Package Version 2.9. 2018. Available online: https://CRAN.R-project.org/package=ggsci (accessed on 6 August 2021).
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Graffelman, J. and van Eeuwijk, F. Calibration of multivariate scatter plots for exploratory analysis of relations within and between sets of variables in genomic research. Biom. J. 2005, 47, 863–879. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.3.1. 2019. Available online: https://CRAN.R-project.org/package=readxl. (accessed on 6 August 2021).
- Spiess A., N. qpcR: Modelling and Analysis of Real-Time PCR Data. R Package Version 1.4-1. 2018. Available online: https://CRAN.R-project.org/package=qpcR (accessed on 6 August 2021).
- Mahto, A. Splitstackshape: Stack and Reshape Datasets After Splitting Concatenated Values. R Package Version 1.4.8. 2019. Available online: https://CRAN.R-project.org/package=splitstackshape (accessed on 6 August 2021).
- Wickham, H. Tidyr: Tidy Messy Data. R Package Version 1.0.0. 2019. Available online: https://www.tidyverse.org/blog/2019/09/tidyr-1-0-0/ (accessed on 6 August 2021).
- Turner, S. Tmisc: Turner Miscellaneous. R Package Version 0.1.22. 2019. Available online: https://CRAN.R-project.org/package=Tmisc (accessed on 6 August 2021).
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome. Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. FastRFunctions for Robust Correlations and Hierarchical Clustering. J. Stat. Softw. 2012, 46, 1–17. [Google Scholar] [CrossRef]
- Toporova, L.; Balaguer, P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2020, 502, 110665. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Karkossa, I.; Schor, J.; Engelmann, B.; Steinheuer, L.M.; Bruns, T.; Rolle-Kampczyk, U.; Hackermuller, J.; von Bergen, M. A Multi-Omics Analysis of Mucosal-Associated-Invariant T Cells Reveals Key Drivers of Distinct Modes of Activation. Front. Immunol. 2021, 12, 616967. [Google Scholar] [CrossRef] [PubMed]
- Karkossa, I.; Bannuscher, A.; Hellack, B.; Bahl, A.; Buhs, S.; Nollau, P.; Luch, A.; Schubert, K.; von Bergen, M.; Haase, A. An in-depth multi-omics analysis in RLE-6TN rat alveolar epithelial cells allows for nanomaterial categorization. Part. Fibre Toxicol. 2019, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and metabolic approach of plastic additive effects: Immune cells responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar] [CrossRef]
- Chiu, K.; Bashir, S.T.; Nowak, R.A.; Mei, W.; Flaws, J.A. Subacute exposure to di-isononyl phthalate alters the morphology, endocrine function, and immune system in the colon of adult female mice. Sci. Rep. 2020, 10, 18788. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gomez de Las Heras, M.M.; Gabande-Rodriguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Marques, C.; Pestana, D.; Faria, A.; Norberto, S.; Calhau, C.; Monteiro, R. Effects of xenoestrogens in human M1 and M2 macrophage migration, cytokine release, and estrogen-related signaling pathways. Environ. Toxicol. 2016, 31, 1496–1509. [Google Scholar] [CrossRef]
- Yamashita, U.; Sugiura, T.; Kuroda, E. Effect of endocrine disrupters on immune responses in vitro. J. UOEH 2002, 24, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamashita, U.; Sugiura, T.; Yoshida, Y.; Kuroda, E. Effect of endocrine disrupters on macrophage functions in vitro. J. UOEH 2005, 27, 1–10. [Google Scholar] [CrossRef]
- Zheng, S.J.; Tian, H.J.; Cao, J.; Gao, Y.Q. Exposure to di(n-butyl)phthalate and benzo(a)pyrene alters IL-1beta secretion and subset expression of testicular macrophages, resulting in decreased testosterone production in rats. Toxicol. Appl. Pharmacol. 2010, 248, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Duong, T.B.; Deschamps, F.; Papadopoulos, V. Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue. Environ. Res. 2015, 140, 145–156. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, J.; Yang, K.; Yang, W.; Shen, G.; Li, X.; Lei, Y.; Pang, S.; Wang, C.; et al. New insights into the mechanism of phthalate-induced developmental effects. Environ. Pollut. 2018, 241, 674–683. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Kasper, S.; Behr, A.C.; Braeuning, A.; Jessel, S.; Seidel, A.; Volkel, W.; Lampen, A. The urinary metabolites of DINCH((R)) have an impact on the activities of the human nuclear receptors ERalpha, ERbeta, AR, PPARalpha and PPARgamma. Toxicol. Lett. 2018, 287, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ovrevik, J.; Lag, M.; Lecureur, V.; Gilot, D.; Lagadic-Gossmann, D.; Refsnes, M.; Schwarze, P.E.; Skuland, T.; Becher, R.; Holme, J.A. AhR and Arnt differentially regulate NF-kappaB signaling and chemokine responses in human bronchial epithelial cells. Cell. Commun. Signal. 2014, 12, 48. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009, 206, 2027–2035. [Google Scholar] [CrossRef]
- Vogel, C.F.; Khan, E.M.; Leung, P.S.; Gershwin, M.E.; Chang, W.L.; Wu, D.; Haarmann-Stemmann, T.; Hoffmann, A.; Denison, M.S. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: A role for nuclear factor-kappaB. J. Biol. Chem. 2014, 289, 1866–1875. [Google Scholar] [CrossRef]
- Laskin, D.L. Macrophages and inflammatory mediators in chemical toxicity: A battle of forces. Chem. Res. Toxicol. 2009, 22, 1376–1385. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose Response 2014, 12, 259–276. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Song, S.; Zhang, L.; Zhang, H.; Wei, W.; Jia, L. Perinatal BPA exposure induces hyperglycemia, oxidative stress and decreased adiponectin production in later life of male rat offspring. Int. J. Environ. Res. Public. Health 2014, 11, 3728–3742. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Rosado-Berrios, C.A.; Velez, C.; Zayas, B. Mitochondrial permeability and toxicity of diethylhexyl and monoethylhexyl phthalates on TK6 human lymphoblasts cells. Toxicol. In Vitro 2011, 25, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Luo, J.; Liu, Y.; Yang, X. The oxidative stress responses caused by phthalate acid esters increases mRNA abundance of base excision repair (BER) genes in vivo and in vitro. Ecotoxicol. Environ. Saf. 2021, 208, 111525. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Das, D.N.; Panda, P.K. Role of Xenobiotic in Autophagy Inflection in Cell Death and Carcinogenesis. In Autophagy in Tumor and Tumor Microenvironment; Bhutia, S.K., Ed.; Springer: Singapore, 2020; pp. 1–34. [Google Scholar]
- Bolt, A.M.; Klimecki, W.T. Autophagy in toxicology: Self-consumption in times of stress and plenty. J. Appl. Toxicol. 2012, 32, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Bolt, A.M.; Byrd, R.M.; Klimecki, W.T. Autophagy is the predominant process induced by arsenite in human lymphoblastoid cell lines. Toxicol Appl Pharm. 2010, 244, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Leon, P.; Dobrinski, I. Exposure to phthalate esters induces an autophagic response in male germ cells. Environ. Epigenet. 2017, 3, dvx010. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, J.; Zeng, L.; Yang, D.; Shao, S.; Wang, J.; Wei, J.; Xiong, J.; Chen, J. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ. Pollut. 2018, 243, 563–572. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, L.; Zhang, X.; Yang, G.; Zhang, C.; Yao, X.; Wu, X.; Fu, M.; Sun, X.; Liu, X. Pyrroloquinoline quinone protected autophagy-dependent apoptosis induced by mono(2-ethylhexyl) phthalate in INS-1 cells. Hum. Exp. Toxicol. 2020, 39, 194–211. [Google Scholar] [CrossRef]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Criollo, A.; Chereau, F.; Malik, S.A.; Niso-Santano, M.; Marino, G.; Galluzzi, L.; Maiuri, M.C.; Baud, V.; Kroemer, G. Autophagy is required for the activation of NFkappaB. Cell Cycle 2012, 11, 194–199. [Google Scholar] [CrossRef]
- Harris, J.; Hartman, M.; Roche, C.; Zeng, S.G.; O’Shea, A.; Sharp, F.A.; Lambe, E.M.; Creagh, E.M.; Golenbock, D.T.; Tschopp, J.; et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J. Biol. Chem. 2011, 286, 9587–9597. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Haas, K.; Moll, L.; Grabbe, S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 2019, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, G.B.; Cunningham, P.S.; Poolman, T.M.; Iqbal, M.; Maidstone, R.; Baxter, M.; Bagnall, J.; Begley, N.; Saer, B.; Hussell, T.; et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. USA 2020, 117, 1543–1551. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaffert, A.; Arnold, J.; Karkossa, I.; Blüher, M.; von Bergen, M.; Schubert, K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells 2021, 10, 2367. https://doi.org/10.3390/cells10092367
Schaffert A, Arnold J, Karkossa I, Blüher M, von Bergen M, Schubert K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells. 2021; 10(9):2367. https://doi.org/10.3390/cells10092367
Chicago/Turabian StyleSchaffert, Alexandra, Josi Arnold, Isabel Karkossa, Matthias Blüher, Martin von Bergen, and Kristin Schubert. 2021. "The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages" Cells 10, no. 9: 2367. https://doi.org/10.3390/cells10092367
APA StyleSchaffert, A., Arnold, J., Karkossa, I., Blüher, M., von Bergen, M., & Schubert, K. (2021). The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells, 10(9), 2367. https://doi.org/10.3390/cells10092367






