Preconditioned Mesenchymal Stromal Cells to Improve Allotransplantation Outcome
Abstract
:1. Introduction
2. MSCs in Allotransplantation and Limitations
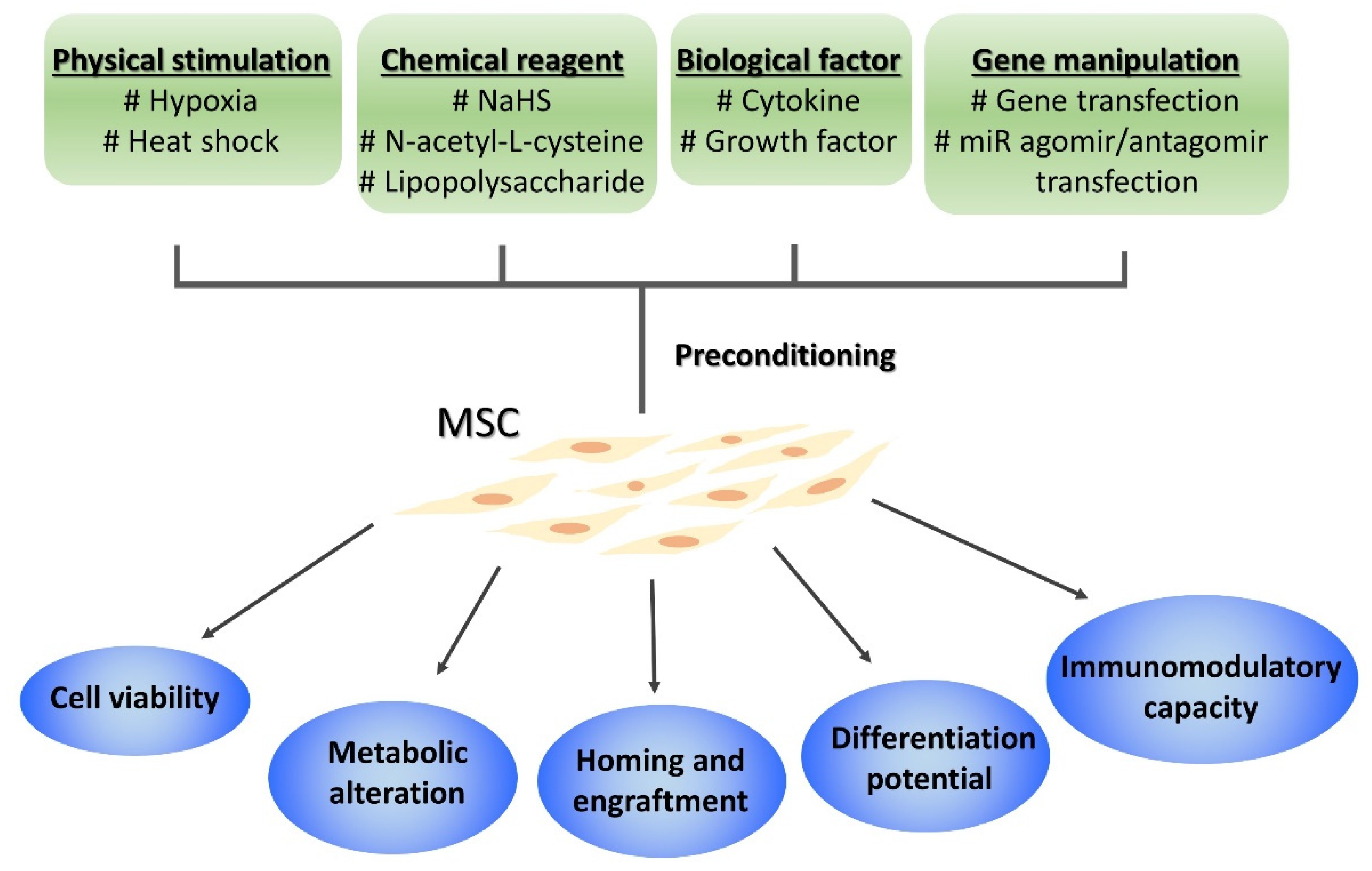
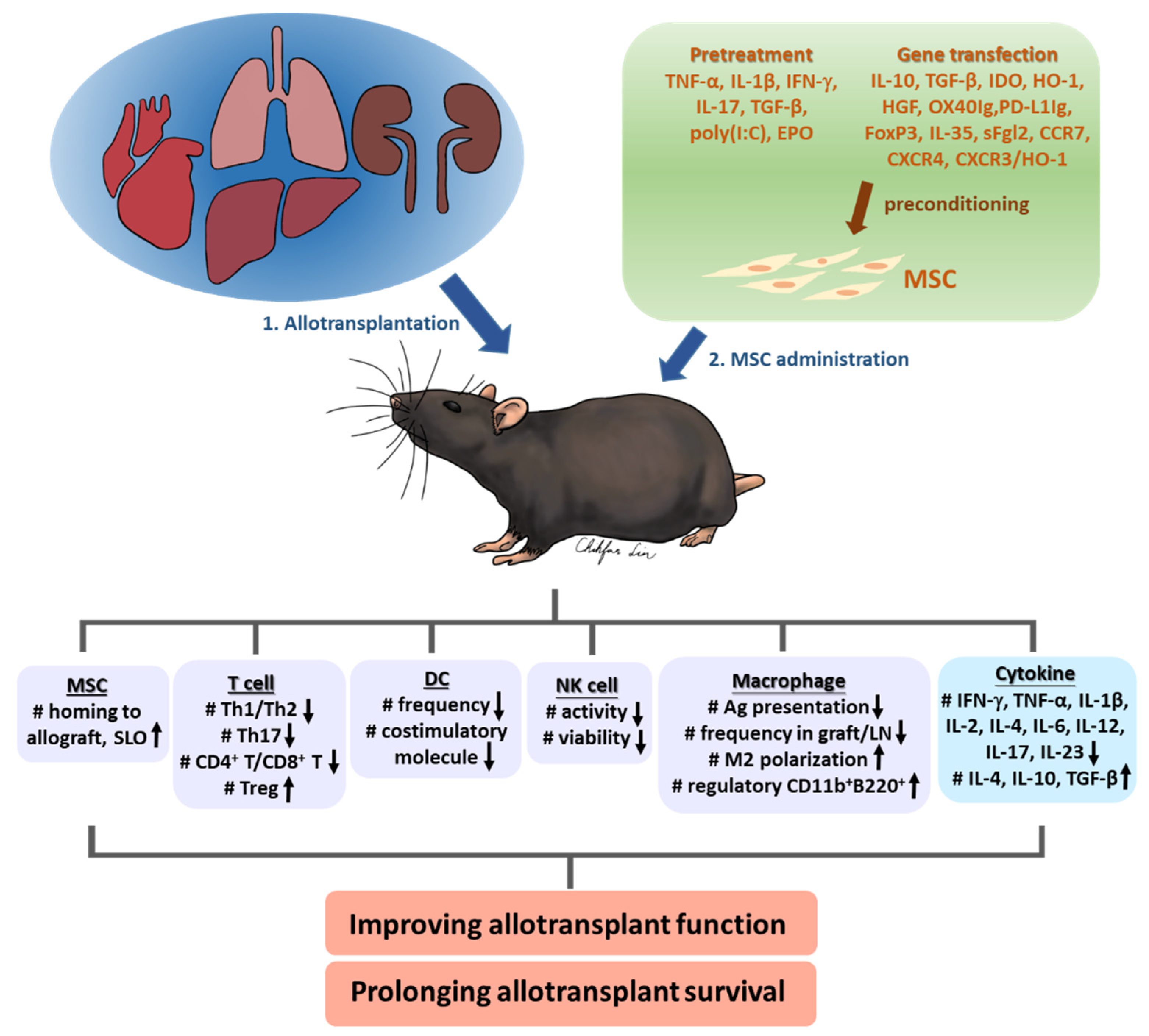
3. Improving MSC Efficacy by Preconditioning
3.1. Pretreatment
3.1.1. Cytokines IFN-γ, TNF-α, IL-1β, IL-17, TGF-β
3.1.2. Toll-Like Receptor, TLR3 Agonist Poly(I:C)
3.1.3. Erythropoietin
3.2. Gene Transfection
3.2.1. Anti-inflammatory Cytokine: TGF-β1, IL-10
3.2.2. Anti-inflammatory Mediator: IDO, HO-1, HGF
3.2.3. Signal 2 for T Cell Activation: OX40Ig, PD-L1Ig
3.2.4. Treg and Treg Effector: FoxP3, sFgl2, IL-35
3.2.5. Chemokine Receptor: CCR7, CXCR4, CXCR3
| Year [Ref] | Species | Donor_Recipient | Allotransplant_Specifics | MSC Origin_Tissue Souce @ | Preconditioning Reagent_Duration | MSC Dose (Timing, POD) & | Administration Route | Allotransplant Survival (Preconditioned MSCs vs. MSCs) (Days) # |
|---|---|---|---|---|---|---|---|---|
| Pretreatment | ||||||||
| 2019 [36] | Rat | DA_LEW | corneal | Syn_BM | TNF-α and IL-1β_72 hr | 1 × 106 (1, 7) | IV_tail | no SD |
| 2019 [37] | Rat | BN_LEW | VCA_hindlimb | Allo_BM | IFN-γ_24 hr | 2 × 106 (0) | IV_tail | 15 vs. 10 |
| 2018 [38] | Mouse | Balb/c_C57BL/6 | skin | Allo_BM | IL-17_5 days | NS (0) | IV_tail | 19.2 vs. 15.8 |
| 2020 [40] | Mouse | C57BL/6_Balb/c | corneal | Syn_BM | TGF-β_72 hr | 1 × 106 (1, 7) | IV_tail | 34.3 vs. 20.4 |
| 2020 [42] | Mouse | Balb/c_C57BL/6 | heart | Syn_AD | poly(I:C)_1 hr | 5 × 105 (1) | IV_caudal | 12.3 vs. 10.2 |
| 2018 [44] | Rat | Wistar_SD | kidney | Syn_BM | EPO_48 hr | 1 × 106 (0) | IV_caudal | no SD |
| Gene Transfection | ||||||||
| 2014 [45] | Rat | DA_LEW | liver | Allo_BM | IL-10 | 2.5 × 105 (0) | IV_jagular | 76 vs. 66 |
| 2020 [46] | Rat | Wistar_LEW | corneal | Allo_BM | IL-10 | 2 × 106 (0) | subconjunctival | 28 vs. 12 |
| 2016 [47] | Rat | DA_LEW | liver | Syn_BM | hTGF-β1 | 5 × 106 (0) | IV_protal | 110 vs. 56.3 |
| 2020 [48] | Rat | Wistar_SD | heart | Syn_BM | IDO | 1 × 106 (2) | IV | SD |
| 2015 [49] | Rabbit | New Zealand_Japanese White | kidney | Allo_BM | IDO | 2 × 106/Kg (0) | IV | 62.8 vs. 16 |
| 2017 [52] | Rat | LEW_BN | liver_50% reduced size | Syn_BM | HO-1 | 1 × 107 (0) | IV_penile | 38 vs. 25 |
| 2016 [53] | Rat | LEW_BN | liver | Syn_BM | HO-1 | 5 × 106 (0) | IV_penile | 77 vs. 61 |
| 2016 [54] | Rat | BN_LEW | intestine | Syn_BM | HO-1 | 1 × 106 (0) | IV_penile | 24 vs. 15 |
| 2009 [59] | Mouse | C57BL/6_Balb/c | skin | Allo_BM | hHGF | 1 × 106 (0) | IV | 16.73 vs. 14.27 |
| 2017 [61] | Rat | BN_LEW | kidney | Syn_AD | OX40Ig | 2 × 106 (−4) | IV_penile | 14.2 vs. 10.2 |
| 2018 [62] | Rat | Wistar_SD | liver | Syn_BM | PD-L1Ig | NS (0) | IV_protal | 100 vs. 23.4 |
| 2015 [63] | Rat | LEW_ACI | liver | Allo_BM | FoxP3 | 2.5 × 106 (0) | IV_portal | >100 vs. 21 |
| 2019 [66] | Mouse | Balb/c_C57BL/6 | heart | Syn_AD | IL-35 | 1 × 106 (1) | IV_caudal | 17.5 vs. 10.67 |
| 2020 [68] | Mouse | Balb/c_C57BL/6 | heart | Syn_AD | sFgl2 | 1 × 106 (1) | IV | 52 vs. 15.3 |
| 2019 [69] | Rat | BN_LEW | VCA_ inferior epigastric flap | Xeno(human)_AD | CCR7 | 2 × 106 (−1) | IV_tail | 14.38 vs. 7.75 |
| 2013 [70] | Rat | Wistar_SD | kidney | Syn_BM | CXCR4 | 2 × 106 (1) | IV | NR |
| 2017 [71] | Rat | BN_LEW | intestine | Syn_BM | CXCR3/HO-1 | 5 × 106 (−7) | IV | 53 vs. 26 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gorantla, V.S.; Barker, J.H.; Jones, J.W., Jr.; Prabhune, K.; Maldonado, C.; Granger, D.K. Immunosuppressive agents in transplantation: Mechanisms of action and current anti-rejection strategies. Microsurgery 2000, 20, 420–429. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Issa, F.; Casiraghi, F.; Reinders, M.E.J. Cellular therapies in organ transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 34, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.Y.; Ghetu, N.; Wallace, C.G.; Wei, F.C.; Liao, S.K. The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. J. Stem Cell Res. Ther. 2014, 4, 237. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.M.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Yen, B.L. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J. Biomed. Sci. 2011, 18, 49. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Khubutiya, M.S.; Vagabov, A.V.; Temnov, A.A.; Sklifas, A.N. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy 2014, 16, 579–585. [Google Scholar] [CrossRef]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front. Cell Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef]
- Do, J.S.; Zwick, D.; Kenyon, J.D.; Zhong, F.; Askew, D.; Huang, A.Y.; Van’t Hof, W.; Finney, M.; Laughlin, M.J. Mesenchymal stromal cell mitochondrial transfer to human induced T-regulatory cells mediates FOXP3 stability. Sci. Rep. 2021, 11, 10676. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Ghetu, N.; Huang, W.C.; Wang, Y.L.; Wallace, C.G.; Wen, C.J.; Chen, H.C.; Shih, L.Y.; Lin, C.F.; Hwang, S.M.; et al. Syngeneic adipose-derived stem cells with short-term immunosuppression induce vascularized composite allotransplantation tolerance in rats. Cytotherapy 2014, 16, 369–380. [Google Scholar] [CrossRef]
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010, 90, 1312–1320. [Google Scholar] [CrossRef]
- Ge, W.; Jiang, J.; Baroja, M.L.; Arp, J.; Zassoko, R.; Liu, W.; Bartholomew, A.; Garcia, B.; Wang, H. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am. J. Transplant. 2009, 9, 1760–1772. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Z.; Wang, Y.; Xu, R.; Sun, Y.; Zhang, M.; Yu, X.; Wang, H.; Meng, L.; Su, H.; et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl. Med. 2017, 6, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.A.; Gonwa, T.A.; Hodge, D.O.; Hei, D.J.; Centanni, J.M.; Zubair, A.C. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Transl. Med. 2018, 7, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Erpicum, P.; Weekers, L.; Detry, O.; Bonvoisin, C.; Delbouille, M.H.; Grégoire, C.; Baudoux, E.; Briquet, A.; Lechanteur, C.; Maggipinto, G.; et al. Infusion of third-party mesenchymal stromal cells after kidney transplantation: A phase I-II, open-label, clinical study. Kidney Int. 2019, 95, 693–707. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Introna, M.; Gotti, E.; Todeschini, M.; Cavinato, R.A.; Capelli, C.; Rambaldi, A.; Cassis, P.; Rizzo, P.; et al. Autologous mesenchymal stromal cells and kidney transplantation: A pilot study of safety and clinical feasibility. Clin. J. Am. Soc. Nephrol. 2011, 6, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Reinders, M.E.; de Fijter, J.W.; Roelofs, H.; Bajema, I.M.; de Vries, D.K.; Schaapherder, A.F.; Claas, F.H.; van Miert, P.P.; Roelen, D.L.; van Kooten, C.; et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Transl. Med. 2013, 2, 107–111. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, Z.; Han, F.; Zhao, M.; Cao, R.; Zhao, D.; Hong, L.; Na, N.; Li, H.; Miao, B.; et al. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: Pilot results of a multicenter randomized controlled trial. J. Transl. Med. 2018, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Reinders, M.E.J.; Groeneweg, K.E.; Hendriks, S.H.; Bank, J.R.; Dreyer, G.J.; de Vries, A.P.J.; van Pel, M.; Roelofs, H.; Huurman, V.A.L.; Meij, P.; et al. Autologous bone marrow-derived mesenchymal stromal cell therapy with early tacrolimus withdrawal: The randomized prospective, single-center, open-label TRITON study. Am. J. Transplant. 2021. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Logan, T.M.; Ma, T. Metabolism in Human Mesenchymal Stromal Cells: A Missing Link Between hMSC Biomanufacturing and Therapy? Front. Immunol. 2019, 10, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Sun, A.; Zhu, W.; Huang, Z.; Hu, X.; Jia, J.; Zou, Y.; Ge, J. Transplantation of Mesenchymal Stem Cells Preconditioned with Hydrogen Sulfide Enhances Repair of Myocardial Infarction in Rats. Tohoku J. Exp. Med. 2012, 226, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.W.; Qiu, Y.R.; Fu, Y.; Liu, J.; He, Z.J.; Huang, Z.T. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. J. Neurosci. Res. 2017, 95, 2059–2070. [Google Scholar] [CrossRef]
- Nan, Z.; Fan, H.; Tang, Q.; Zhang, M.; Xu, M.; Chen, Q.; Liu, Y.; Dong, Y.; Wu, H.; Deng, S. Dual expression of CXCR4 and IL-35 enhances the therapeutic effects of BMSCs on TNBS-induced colitis in rats through expansion of Tregs and suppression of Th17 cells. Biochem. Biophys. Res. Commun. 2018, 499, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, X.; Liu, Y.; Chen, X.; Li, X.; Chu, Y.; Zhu, H.; Liu, W.; Xu, F.; Zhou, F.; et al. The Therapeutic Effect of ICAM-1-Overexpressing Mesenchymal Stem Cells on Acute Graft-Versus-Host Disease. Cell. Physiol. Biochem. 2018, 46, 2624–2635. [Google Scholar] [CrossRef] [Green Version]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Murphy, N.; Treacy, O.; Lynch, K.; Morcos, M.; Lohan, P.; Howard, L.; Fahy, G.; Griffin, M.D.; Ryan, A.E.; Ritter, T. TNF-α/IL-1β—licensed mesenchymal stromal cells promote corneal allograft survival via myeloid cell-mediated induction of Foxp3+ regulatory T cells in the lung. FASEB J. 2019, 33, 9404–9421. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xi, Y.; Han, F.; Liu, Y.; Li, N.; Ren, Z.; Xue, J.; Guo, L.; Hu, D. Vascularized composite allograft rejection is delayed by infusion of IFN-γ-conditioned BMSCs through upregulating PD-L1. Cell Tissue Res. 2019, 376, 211–220. [Google Scholar] [CrossRef]
- Ma, T.; Wang, X.; Jiao, Y.; Wang, H.; Qi, Y.; Gong, H.; Zhang, L.; Jiang, D. Interleukin 17 (IL-17)-Induced Mesenchymal Stem Cells Prolong the Survival of Allogeneic Skin Grafts. Ann. Transplant. 2018, 23, 615–621. [Google Scholar] [CrossRef]
- Rodríguez, T.M.; Saldías, A.; Irigo, M.; Zamora, J.V.; Perone, M.J.; Dewey, R.A. Effect of TGF-β1 Stimulation on the Secretome of Human Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2015, 4, 894–898. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.; Treacy, O.; Chen, X.; Murphy, N.; Lohan, P.; Islam, M.N.; Donohoe, E.; Griffin, M.D.; Watson, L.; McLoughlin, S.; et al. TGF-β1-Licensed Murine MSCs Show Superior Therapeutic Efficacy in Modulating Corneal Allograft Immune Rejection In Vivo. Mol. Ther. 2020, 28, 2023–2043. [Google Scholar] [CrossRef]
- Shoshina, O.O.; Kozhin, P.M.; Shadrin, V.S.; Romashin, D.D.; Rusanov, A.L.; Luzgina, N.G. Phenotypic Features of Mesenchymal Stem Cell Subpopulations Obtained under the Influence of Various Toll-Like Receptors Ligands. Bull. Exp. Biol. Med. 2021, 170, 555–559. [Google Scholar] [CrossRef]
- Bao, Z.; Li, J.; Zhang, P.; Pan, Q.; Liu, B.; Zhu, J.; Jian, Q.; Jia, D.; Yi, C.; Moeller, C.J.; et al. Toll-Like Receptor 3 Activator Preconditioning Enhances Modulatory Function of Adipose—Derived Mesenchymal Stem Cells in a Fully MHC-Mismatched Murine Model of Heterotopic Heart Transplantation. Ann. Transplant. 2020, 25, e921287. [Google Scholar] [CrossRef]
- Purroy, C.; Fairchild, R.L.; Tanaka, T.; Baldwin, W.M., III; Manrique, J.; Madsen, J.C.; Colvin, R.B.; Alessandrini, A.; Blazar, B.R.; Fribourg, M.; et al. Erythropoietin receptor-mediated molecular crosstalk promotes T cell immunoregulation and transplant survival. J. Am. Soc. Nephrol. 2017, 28, 2377–2392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, S.; Hu, J.M.; Chen, H.; Liu, D.; Li, M.; Guo, Y.; Fan, L.P.; Li, L.Y.; Liu, Y.G.; et al. Preliminary Study of Bone Marrow-Derived Mesenchymal Stem Cells Pretreatment with Erythropoietin in Preventing Acute Rejection after Rat Renal Transplantation. Transplant. Proc. 2018, 50, 3873–3880. [Google Scholar] [CrossRef]
- Niu, J.; Yue, W.; Song, Y.; Zhang, Y.; Qi, X.; Wang, Z.; Liu, B.; Shen, H.; Hu, X. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin. Exp. Immunol. 2014, 176, 473–484. [Google Scholar] [CrossRef]
- Lu, X.; Ru, Y.; Chu, C.; Lv, Y.; Gao, Y.; Jia, Z.; Huang, Y.; Zhang, Y.; Zhao, S. Lentivirus-mediated IL-10-expressing Bone Marrow Mesenchymal Stem Cells promote corneal allograft survival via upregulating lncRNA 003946 in a rat model of corneal allograft rejection. Theranostics 2020, 10, 8446–8467. [Google Scholar] [CrossRef]
- Tang, J.; Yang, R.; Lv, L.; Yao, A.; Pu, L.; Yin, A.; Li, X.; Yu, Y.; Nyberg, S.L.; Wang, X. Transforming growth factor-β-Expressing Mesenchymal Stem Cells Induce Local Tolerance in a Rat Liver Transplantation Model of Acute Rejection. Stem Cells 2016, 34, 2681–2692. [Google Scholar] [CrossRef]
- He, J.G.; Li, B.B.; Zhou, L.; Yan, D.; Xie, Q.L.; Zhao, W. Indoleamine 2,3-dioxgenase-transfected mesenchymal stem cells suppress heart allograft rejection by increasing the production and activity of dendritic cells and regulatory T cells. J. Investig. Med. 2020, 68, 728–737. [Google Scholar] [CrossRef]
- He, Y.; Zhou, S.; Liu, H.; Shen, B.; Zhao, H.; Peng, K.; Wu, X. Indoleamine 2, 3-Dioxgenase Transfected Mesenchymal Stem Cells Induce Kidney Allograft Tolerance by Increasing the Production and Function of Regulatory T Cells. Transplantation 2015, 99, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.; Zeerleder, S.; Voermans, C. Heme oxygenase-1: Equally important in allogeneic hematopoietic stem cell transplantation and organ transplantation? Transpl. Immunol. 2021, 68, 101419. [Google Scholar] [CrossRef]
- Chabannes, D.; Hill, M.; Merieau, E.; Rossignol, J.; Brion, R.; Soulillou, J.P.; Anegon, I.; Cuturi, M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007, 110, 3691–3694. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.Y.; Wu, B.; Liu, T.; Yang, Y.; Yin, M.L.; Zheng, W.P.; Zhang, B.Y.; Song, H.L. Immunomodulatory effects of bone marrow mesenchymal stem cells overexpressing heme oxygenase-1: Protective effects on acute rejection following reduced-size liver transplantation in a rat model. Cell. Immunol. 2017, 313, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Song, H.L.; Yang, Y.; Yin, M.L.; Zhang, B.Y.; Cao, Y.; Dong, C.; Shen, Z.Y. Improvement of Liver Transplantation Outcome by Heme Oxygenase-1-Transduced Bone Marrow Mesenchymal Stem Cells in Rats. Stem Cells Int. 2016, 2016, 9235073. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, H.L.; Zhang, W.; Wu, B.J.; Fu, N.N.; Dong, C.; Shen, Z.Y. Heme oxygenase-1-transduced bone marrow mesenchymal stem cells in reducing acute rejection and improving small bowel transplantation outcomes in rats. Stem Cell Res. Ther. 2016, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Okunishi, K.; Dohi, M.; Nakagome, K.; Tanaka, R.; Mizuno, S.; Matsumoto, K.; Miyazaki, J.; Nakamura, T.; Yamamoto, K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 2005, 175, 4745–4753. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.-H.; Wu, F.; Liu, L.; Chen, H.-b.; Zheng, R.-Q.; Wang, H.-L.; Yu, L.-N. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res. Ther. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res. Ther. 2019, 10, 372. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.F.; Wu, C.T.; Wu, D.L.; Lu, Y.; Liu, H.J.; Ha, X.Q.; Zhang, Q.W.; Wang, H.; Jia, X.X.; Wang, L.S. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol. Ther. 2003, 8, 467–474. [Google Scholar] [CrossRef]
- Bian, L.; Guo, Z.K.; Wang, H.X.; Wang, J.S.; Wang, H.; Li, Q.F.; Yang, Y.F.; Xiao, F.J.; Wu, C.T.; Wang, L.S. In vitro and in vivo immunosuppressive characteristics of hepatocyte growth factor-modified murine mesenchymal stem cells. In Vivo 2009, 23, 21–27. [Google Scholar]
- Lin, C.H.; Wang, Y.L.; Anggelia, M.R.; Chuang, W.Y.; Cheng, H.Y.; Mao, Q.; Zelken, J.A.; Lin, C.H.; Zheng, X.X.; Lee, W.P.; et al. Combined Anti-CD154/CTLA4Ig Costimulation Blockade-Based Therapy Induces Donor-Specific Tolerance to Vascularized Osteomyocutaneous Allografts. Am. J. Transplant. 2016, 16, 2030–2041. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, Y.; Shen, Z.; Zou, X.; Chen, X.; Chen, L.; Wang, Y. Immunomodulatory effects of OX40Ig gene-modified adipose tissue-derived mesenchymal stem cells on rat kidney transplantation. Int. J. Mol. Med. 2017, 39, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhang, Y.Y.; Deng, J. PDL1Ig gene-loaded BMSCs Induce liver transplantation immune tolerance. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 3214–3223. [Google Scholar] [CrossRef]
- Qi, H.; Chen, G.; Huang, Y.; Si, Z.; Li, J. Foxp3-modified bone marrow mesenchymal stem cells promotes liver allograft tolerance through the generation of regulatory T cells in rats. J. Transl. Med. 2015, 13, 274. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.G.; Liu, Y.; Chen, F. Soluble fibrinogen like protein 2 (sFGL2), the novel effector molecule for immunoregulation. Oncotarget 2017, 8, 3711–3723. [Google Scholar] [CrossRef] [Green Version]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhao, N.; Li, B.; Gao, H.; Yan, Y.; Guo, H. Inhibition of cardiac allograft rejection in mice using interleukin-35-modified mesenchymal stem cells. Scand. J. Immunol. 2019, 89, e12750. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhao, Z.; Tang, C.; Ding, L.; Li, Z.; Zheng, D.; Zong, L.; Wu, Z. Soluble fibrinogen-like protein 2 ameliorates acute rejection of liver transplantation in rat via inducing Kupffer cells M2 polarization. Cancer Med. 2018, 7, 3168–3177. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.; Lu, J.; Li, Z.; Jia, H.; Chen, M.; Chang, Y.; Liu, Y.; Li, P.; Zhang, B.; et al. Mesenchymal stem cells transfected with sFgl2 inhibit the acute rejection of heart transplantation in mice by regulating macrophage activation. Stem Cell Res. Ther. 2020, 11, 241. [Google Scholar] [CrossRef]
- Ma, T.; Luan, S.; Tao, R.; Lu, D.; Guo, L.; Liu, J.; Shu, J.; Zhou, X.; Han, Y.; Jia, Y.; et al. Targeted Migration of Human Adipose-Derived Stem Cells to Secondary Lymphoid Organs Enhances Their Immunomodulatory Effect and Prolongs the Survival of Allografted Vascularized Composites. Stem Cells 2019, 37, 1581–1594. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, G.; Wang, F.; Liu, H.; Liu, L.; Han, Y.; Zhang, J.; Yuan, J. Protective effects of mesenchymal stem cells with CXCR4 up-regulation in a rat renal transplantation model. PLoS ONE 2013, 8, e82949. [Google Scholar] [CrossRef] [Green Version]
- Yin, M.L.; Song, H.L.; Yang, Y.; Zheng, W.P.; Liu, T.; Shen, Z.Y. Effect of CXCR3/HO-1 genes modified bone marrow mesenchymal stem cells on small bowel transplant rejection. World J. Gastroenterol. 2017, 23, 4016–4038. [Google Scholar] [CrossRef]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tögel, F.; Yang, Y.; Zhang, P.; Hu, Z.; Westenfelder, C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 295, F315–F321. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Zhang, J.; Gilad, A.A.; Kedziorek, D.A.; Ruiz-Cabello, J.; Young, R.G.; Pittenger, M.F.; van Zijl, P.C.; Huang, J.; Bulte, J.W. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 2008, 39, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Freyman, T.; Polin, G.; Osman, H.; Crary, J.; Lu, M.; Cheng, L.; Palasis, M.; Wilensky, R.L. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006, 27, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Chen, J.; Alahdal, M.; Chang, C.; Duan, L.; Zhu, W.; Mou, L.; Xiong, J.; Wang, M.; Wang, D. Intra-articular injection of hUC-MSCs expressing miR-140-5p induces cartilage self-repairing in the rat osteoarthritis. J. Bone Miner. Metab. 2020, 38, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Mohme, M.; Maire, C.L.; Geumann, U.; Schliffke, S.; Dührsen, L.; Fita, K.; Akyüz, N.; Binder, M.; Westphal, M.; Guenther, C.; et al. Local Intracerebral Immunomodulation Using Interleukin-Expressing Mesenchymal Stem Cells in Glioblastoma. Clin. Cancer Res. 2020, 26, 2626–2639. [Google Scholar] [CrossRef] [Green Version]
- McGinley, L.M.; McMahon, J.; Stocca, A.; Duffy, A.; Flynn, A.; O’Toole, D.; O’Brien, T. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum. Gene Ther. 2013, 24, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, S.; Pool, M.B.F.; Rozenberg, K.M.; Keller, A.K.; Moers, C.; Møldrup, U.; Møller, B.K.; Lignell, S.J.M.; Krag, S.; Sierra-Parraga, J.M.; et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: A porcine renal autotransplantation study. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Pieróg, J.; Tamo, L.; Fakin, R.; Kocher, G.; Gugger, M.; Grodzki, T.; Geiser, T.; Gazdhar, A.; Schmid, R.A. Bone marrow stem cells modified with human interleukin 10 attenuate acute rejection in rat lung allotransplantation. Eur. J. Cardio-Thorac. Surg. 2018, 53, 194–200. [Google Scholar] [CrossRef]
- Soares, M.A.; Massie, J.P.; Rifkin, W.J.; Rao, N.; Duckworth, A.M.; Park, C.; Kadle, R.L.; David, J.A.; Rabbani, P.S.; Ceradini, D.J. Ex vivo allotransplantation engineering: Delivery of mesenchymal stem cells prolongs rejection-free allograft survival. Am. J. Transplant. 2018, 18, 1657–1667. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, S.; Gu, C.; Xiong, Y.; Shen, H.; Liu, F.; Yang, J. Ex-vivo treatment of allografts using adipose-derived stem cells induced prolonged rejection-free survival in an allogenic hind-limb transplantation model. Ann. Transl. Med. 2020, 8, 867. [Google Scholar] [CrossRef]
- Gao, W.; Lu, Y.; El Essawy, B.; Oukka, M.; Kuchroo, V.K.; Strom, T.B. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am. J. Transplant. 2007, 7, 1722–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.U.; Kim, L.K.; Choi, J.M. Revisiting the Concept of Targeting NFAT to Control T Cell Immunity and Autoimmune Diseases. Front. Immunol. 2018, 9, 2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steines, L.; Poth, H.; Schuster, A.; Amann, K.; Banas, B.; Bergler, T. Disruption of Tfh:B Cell Interactions Prevents Antibody-Mediated Rejection in a Kidney Transplant Model in Rats: Impact of Calcineurin Inhibitor Dose. Front. Immunol. 2021, 12, 657894. [Google Scholar] [CrossRef]
- Hajkova, M.; Jaburek, F.; Porubska, B.; Bohacova, P.; Holan, V.; Krulova, M. Cyclosporine A promotes the therapeutic effect of mesenchymal stem cells on transplantation reaction. Clin. Sci. 2019, 133, 2143–2157. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Crop, M.J.; Korevaar, S.S.; Peeters, A.M.; Eijken, M.; Maat, L.P.; Balk, A.H.; Weimar, W.; Baan, C.C. Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin. Transplantation 2008, 86, 1283–1291. [Google Scholar] [CrossRef]
- Hajkova, M.; Hermankova, B.; Javorkova, E.; Bohacova, P.; Zajicova, A.; Holan, V.; Krulova, M. Mesenchymal Stem Cells Attenuate the Adverse Effects of Immunosuppressive Drugs on Distinct T Cell Subopulations. Stem Cell Rev. Rep. 2017, 13, 104–115. [Google Scholar] [CrossRef]
- Schweizer, R.; Taddeo, A.; Waldner, M.; Klein, H.J.; Fuchs, N.; Kamat, P.; Targosinski, S.; Barth, A.A.; Drach, M.C.; Gorantla, V.S.; et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am. J. Transplant. 2020, 20, 1272–1284. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Renner, P.; Soeder, Y.; Popp, F.C.; Hoogduijn, M.J.; Geissler, E.K.; Schlitt, H.J.; Dahlke, M.H. Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transpl. Immunol. 2011, 25, 141–147. [Google Scholar] [CrossRef]
| Year [Ref] | Species | Allotransplant_Specifics | MSC Origin_Tissue Souce @ | Preconditioning Reagent_Duration | MSC Dose (Timing, POD) & | Administration Route | Allotransplant Survival (Preconditioned MSCs vs. MSCs) (Days) # |
|---|---|---|---|---|---|---|---|
| 2017 [52] | Rat | liver_50% reduced size | Syn_BM | HO-1 | 1 × 107 (0) | IV_penile | 38 vs. 25 |
| 2016 [53] | Rat | liver | Syn_BM | HO-1 | 5 × 106 (0) | IV_penile | 77 vs. 61 |
| 2014 [45] | Rat | liver | Allo_BM | IL-10 | 2.5 × 105 (0) | IV_jagular | 76 vs. 66 |
| 2016 [47] | Rat | liver | Syn_BM | hTGF-β1 | 5 × 106 (0) | IV_portal | 110 vs. 56.3 |
| 2018 [62] | Rat | liver | Syn_BM | PD-L1Ig | NS (0) | IV_portal | 100 vs. 23.4 |
| 2015 [63] | Rat | liver | Allo_BM | FoxP3 | 2.5 × 106 (0) | IV_portal | >100 vs. 21 |
| 2020 [42] | Mouse | heart | Syn_AD | poly(I:C)_1 hr | 5 × 105 (1) | IV_caudal | 12.3 vs. 10.2 |
| 2020 [48] | Rat | heart | Syn_BM | IDO | 1 × 106 (2) | IV | SD |
| 2019 [66] | Mouse | heart | Syn_AD | IL-35 | 1 × 106 (1) | IV_caudal | 17.5 vs. 10.67 |
| 2020 [68] | Mouse | heart | Syn_AD | sFgl2 | 1 × 106 (1) | IV | 52 vs. 15.3 |
| 2018 [44] | Rat | kidney | Syn_BM | EPO_48 hr | 1 × 106 (0) | IV_caudal | no SD |
| 2015 [49] | Rabbit | kidney | Allo_BM | IDO | 2 × 106/Kg (0) | IV | 62.8 vs. 16 |
| 2017 [61] | Rat | kidney | Syn_AD | OX40Ig | 2 × 106 (−4) | IV_penile | 14.2 vs. 10.2 |
| 2013 [70] | Rat | kidney | Syn_BM | CXCR4 | 2 × 106 (1) | IV | NR |
| 2016 [54] | Rat | intestine | Syn_BM | HO-1 | 1 × 106 (0) | IV_penile | 24 vs. 15 |
| 2017 [71] | Rat | intestine | Syn_BM | CXCR3 and HO-1 | 5 × 106 (−7) | IV | 53 vs. 26 |
| 2019 [36] | Rat | corneal | Syn_BM | TNF-α and IL-1β_72 hr | 1 × 106 (1, 7) | IV_tail | no SD |
| 2020 [40] | Mouse | corneal | Syn_BM | TGF-β_72 hr | 1 × 106 (1, 7) | IV_tail | 34.3 vs. 20.4 |
| 2020 [46] | Rat | corneal | Allo_BM | IL-10 | 2 × 106 (0) | subconjunctival | 28 vs. 12 |
| 2019 [37] | Rat | VCA_hindlimb | Allo_BM | IFN-γ_24 hr | 2 × 106 (0) | IV_tail | 15 vs. 10 |
| 2019 [69] | Rat | VCA_inferior epigastric flap | Xeno(human)_AD | CCR7 | 2 × 106 (−1) | IV_tail | 14.38 vs. 7.75 |
| 2018 [38] | Mouse | skin | Allo_BM | IL-17_5 days | NS (0) | IV_tail | 19.2 vs. 15.8 |
| 2009 [59] | Mouse | skin | Allo_BM | hHGF | 1 × 106 (0) | IV | 16.73 vs. 14.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-Y.; Anggelia, M.R.; Lin, C.-H.; Lin, C.-F. Preconditioned Mesenchymal Stromal Cells to Improve Allotransplantation Outcome. Cells 2021, 10, 2325. https://doi.org/10.3390/cells10092325
Cheng H-Y, Anggelia MR, Lin C-H, Lin C-F. Preconditioned Mesenchymal Stromal Cells to Improve Allotransplantation Outcome. Cells. 2021; 10(9):2325. https://doi.org/10.3390/cells10092325
Chicago/Turabian StyleCheng, Hui-Yun, Madonna Rica Anggelia, Cheng-Hung Lin, and Chih-Fan Lin. 2021. "Preconditioned Mesenchymal Stromal Cells to Improve Allotransplantation Outcome" Cells 10, no. 9: 2325. https://doi.org/10.3390/cells10092325
APA StyleCheng, H.-Y., Anggelia, M. R., Lin, C.-H., & Lin, C.-F. (2021). Preconditioned Mesenchymal Stromal Cells to Improve Allotransplantation Outcome. Cells, 10(9), 2325. https://doi.org/10.3390/cells10092325






