Abstract
Lichen sclerosus (LS) is a chronic inflammatory skin disorder with unknown pathogenesis. The aberrant expression of microRNAs (miRNAs) is considered to exert a crucial role in LS. We used the next-generation sequencing technology (RNASeq) for miRNA profiling and Ingenuity Pathway Analysis (IPA) for molecular network analysis. We performed qRT-PCR, miRNA transfection and Matrigel assays for functional studies. We identified a total of 170 differentially expressed miRNAs between female LS and matched adjacent normal tissue using RNASeq, with 119 upregulated and 51 downregulated. Bioinformatics analysis revealed molecular networks that may shed light on the pathogenesis of LS. We verified the expression of a set of miRNAs that are related to autoimmunity, such as upregulated miR-326, miR-142-5p, miR-155 and downregulated miR-664a-3p and miR-181a-3p in LS tissue compared to the matched adjacent normal tissue. The differentially expressed miRNAs were also verified in blood samples from LS patients compared to healthy female volunteers. Functional studies demonstrated that a forced expression of miR-142-5p in human dermal fibroblast PCS-201-010 cells resulted in decreased cell proliferation and migration. These findings suggest that differentially expressed miRNAs may play an important role in LS pathogenesis; therefore, they could serve as biomarkers for LS management.
1. Introduction
Lichen sclerosus (LS) is the condition of chronic, lymphocyte-mediated inflammatory dermatitis that has a negative impact on quality of life and may proceed to malignant disease [1,2]. LS can affect any part of the skin, but it most frequently occurs in the anogenital area, with a higher incidence of onset among premenarchal and post-menopausal females [3,4]. The typical lesions of vulvar LS are white plaques and papules, often with areas of ecchymosis, excoriation, and ulceration. Presenting symptoms may include intense pruritus, pain, burning and dyspareunia. Between 4 and 7% of women with LS develop vulvar squamous carcinoma [5]. LS is among one of the most common referrals for vulvar pruritus and is the most common cause of inflammatory structural changes to the vulvar region [6,7,8]. Histologically, LS is characterized by a band-like lymphocytic infiltrate and a thinned epidermis with vacuolar changes in the basal layer. In the long-standing, classic LS, the lymphocytic infiltrate is located under a band of homogenized collagen below the dermoepidermal junction [9]. Although there is increasing evidence that autoimmune mechanisms play a pathogenic role, the etiology of LS has not yet been adequately explained [10,11,12]. Currently, LS diagnosis is based on the physical examination and dermatopathological analysis of skin biopsy. However, there is a lack of unanimity, especially in the early stages of disease. In addition, the histological evaluation of LS and other immunologically mediated diseases, such as erosive lichen planus (ELP), can be difficult, as the first phase of LS can show histological overlap with ELP [13]. The therapeutic options for LS, namely ultrapotent topical corticosteroids, require long-term or life-long regimens and are not curative [14,15]. Therefore, the development of non-invasive and reliable biomarkers could potentially impact LS screening, diagnosis and treatment.
MicroRNAs (miRNAs) are small, endogenous RNA molecules that act as regulators of gene expression by binding to the 3′-untranslated region (3′ UTR) of target mRNAs [16,17]. They play critical roles in cellular homeostasis and biological processes, and are therefore involved in the development of various diseases in broad pathological conditions [18]. Accumulating evidence suggests that dysregulation of miRNAs is associated with inflammation and autoimmunity [19], suggesting their roles in LS development. For example, miR-142 overexpression might be involved in the autoimmune neuroinflammation and pathogenesis of multiple sclerosis through changing the pattern of T cell differentiation [20]. The downregulation of miR-142-3p inhibits keratinocyte proliferation in oral lichen planus (OLP) [21]. Although the aberrant expression of miRNAs has been implicated in LS pathogenesis [12,22], there are very limited studies to date.
The aim of this study was to explore the role of miRNAs in vulvar LS pathogenesis by identifying differentially expressed miRNAs and their associated molecular networks and decipher the functional role of selected candidate miRNAs for their potential clinical utility in the diagnosis and treatment of LS.
2. Materials and Methods
2.1. Clinical Samples
With The George Washington University’s IRB approval and patients’ consent, 4 mm vulvar punch biopsies of active LS along with matched normal adjacent tissues, and 2 mL of peripheral blood samples were collected at the time of clinical consultation (Table 1). Subjects with vulvar LS were diagnosed by both visual observation and dermatopathological confirmation, and recruited from a gynecology practice specializing in vulvar dermatoses. Tissue and blood samples were immediately placed into RNAlater Storage Solution (Invitrogen AM7020) before being transported to the research lab for storage in a −80 °C freezer or immediate RNA isolation. An additional 23 peripheral blood samples from healthy female volunteers were used as controls.

Table 1.
Clinical samples of tissue and blood from patients with active lichen sclerosus.
2.2. Cell Lines
Normal human dermal fibroblast, PCS-201-010, was purchased from ATCC and cultured in Fibroblast Growth Kit—Serum-Free (ATCC® PCS-201-040) with 3.75% L-glutamine, 0.1% hydrocortisone hemisuccinate, 0.25% HLL supplement, 0.1% rh FGF basic, 0.1% TGF β-1 supplement, 0.1% rh insulin, and 0.1% ascorbic acid. The cell line was cultured at 37 °C in a humidified incubator with 5% CO2.
2.3. RNA Extraction, RNA Library Preparation and RNASeq Assays
Total RNA was isolated from cell line, LS and the matched adjacent normal tissue using the TRIzol reagent (Cat#15596–026, Invitrogen, Carlsbad, CA, USA). Blood RNA was isolated by the RiboPure™ RNA Purification Kit (Cat#AM1928, Life Technologies, Frederick, MD, USA). The total RNA from LS and the matched adjacent normal tissue was subsequently purified with the miRNeasy Mini kit (Cat#217004, Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. RNA quality, quantity, and purity were determined using a Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Cat# 5067-1511, Agilent, Foster City, CA, USA). For RNA sequencing, 1 μg of the total RNA was used to prepare small a RNA library using TruSeq Small RNA Sample Prep Kits (Illumina, San Diego, CA, USA). miRNA deep sequencing (RNASeq) was performed by LC Sciences (Houston, TX, USA) using Illumina Hiseq 4000 Sequencer (Illumina, San Diego, CA, USA). Raw sequencing reads were obtained using the Illumina’s Sequencing Control Studio software (v2.8; Illumina, Inc. San Diego, CA, USA) (https://www.lcsciences.com/documents/sample_data/microrna_sequencing/miRNA_sequencing_report_DEMO.html, accessed on 21 March 2019).
2.4. Data Preprocessing
Preliminary quality control analysis was performed by removing adapter dimers, junk, low complexity, common RNA families such as snoRNA snRNA, rRNA and tRNA, and repeats sequences from raw sequence reads obtained from sequencing using the ACGT101-miR program (LC Sciences, Houston, TX, USA). Only unique sequences of 18–26 nucleotides (nt) in length were retained and compared to known homo sapiens miRNAs in miRBase 21.0 by BLAST search. Length variation at both the 3′ and 5′ ends and one mismatch inside of the sequence were allowed in the alignment. The unique sequences mapping to human mature miRNAs in hairpin arms were identified as known miRNAs. The unique sequences mapping to the other arm of known specific species precursor hairpin, opposite the annotated mature miRNA-containing arm, were considered to be novel 5p- or 3p-derived miRNA candidates. The remaining sequences were mapped to other selected precursors in miRBase 21.0 by BLAST search, and the mapped pre-miRNAs were further BLASTed against the human genomes to determine their genomic locations.
2.5. Identification of Differentially Expressed miRNAs
The quantitative miRNAs data were normalized to reads per million (RPM), which was calculated as RPM = miRNA counts/total counts of each sample × 1,000,000, and then log 2-transformed. The differently expressed miRNAs between LS and the matched normal tissues were identified using the paired Student’s t-test. miRNAs were considered to be differentially expressed at p < 0.05, fold change (FC) >1.5 or <1/1.5 and average expression > (log2) 5 RPM. To determine whether the differentially expressed miRNAs were able to differentiate LS from the normal control, clustering analysis was performed to generate a heat map using the hclust function implemented in the R package (v2.14.1; http://www.R-project.org, accessed on 20 April 2020), which uses the Euclidean distance and Ward’s method.
2.6. Pathway Analysis
The differentially expressed miRNAs were subjected to molecular pathway analysis using the Ingenuity Pathway Analysis (IPA) software. Focus miRNA genes were identified as having direct interactions with other genes in the database. Pathways of highly interconnected miRNAs and their target genes were identified by statistical likelihood (p < 0.05). The networks were generated through the use of IPA (QIAGEN, Germantown, MD, USA) (https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis, accessed on 5 June 2021).
2.7. Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR)
To verify miRNA sequencing results, miRNA expression was assayed by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) using the Taqman MiRNA Reverse Transcript Kit (Cat# 4366596, Thermo Fisher, Waltham, MA, USA). SYBR Green-based qRT-PCR was used to measure the relative expression of selected target genes. Five hundred nanograms of the total RNA were reverse transcribed to generate complementary DNA (cDNA) employing the High Capacity cDNA Reverse Transcript Kit (Thermo Scientific, Rockford, IL, USA). The QuantStudio™ 3 Real-Time PCR System (ABI) was used for these assays. Relative expression was calculated using the 2−ΔΔCT method [23].
2.8. Target Gene Prediction
To determine the function of a set of selected miRNAs in LS pathogenesis, we performed target prediction using miRDB, DIANA and TargetScan databases [24,25,26,27,28]. Target genes of interest were further evaluated by literature search focusing on their involvement in LS linked inflammatory pathways.
2.9. miRNA Precursors and Plasmid Transfection
miRNA transient-transfection was performed as described [23,29]. Briefly, the miRNA precursors (miR-142-5p mimic, inhibitor and mock control) were transiently transfected into the normal human dermal fibroblast, PCS-201-010 cell line by the Lipofectamine RNAiMAX (Life Technologies) using the Opti-MEM I Reduced Serum Medium (Life Technologies). Cells were subjected to further analysis after 24 h, 48 h and 72 h post-transfection.
2.10. Matrigel Invasion Assays
Matrigel invasion assays were performed using the BD BioCoat™ Matrigel™ Invasion Chamber (Cat# 354480, BD Biosciences, San Jose, CA, USA) as previously described [23,30]. Briefly, prior to the start of each experiment, 500 μL of warm (37 °C) serum-free DMEM medium was added to the upper and lower chambers and allowed to rehydrate for 2 h in a 37 °C cell culture incubator, while 8 × 104 cells were transfected by either miR-142-5p mimic or an inhibitor with the mock controls for 24 h and seeded onto the top chamber of pre-wetted inserts. Cells were incubated in a Matrigel chamber in a 37 °C humidified incubator with 5% CO2 for 24 h. The invasive cells present were fixed, stained with the Diff-Quick staining solution and counted (five microscope fields under the 10× lens). Experiments were done in duplicates for each cell line twice. Cell counts were performed on five non-overlapping random fields for each chamber and four chambers were counted for each experimental point, with the percentage of invasive cells being normalized to corresponding controls.
2.11. Statistical Analysis
Statistical analysis for the differentially expressed miRNAs by RNASeq was described above. All other data are distributed approximately normally by histogram analysis. Data are expressed as the mean ± standard deviation. Differences between groups were determined by Student’s t-test or pair t-test as appropriate. p-value < 0.05 was considered statistically significant.
3. Results
3.1. miRNA Profiles in LS and Matched Adjacent Tissue
Twelve pairs of LS and their matched adjacent normal tissue derived from active LS patients were used to conduct the RNASeq assay (Table 1). A total of 781 differentially expressed miRNAs were identified (Supplementary Table S1). Using the Benjamini–Hochberg False Discovery Rate (FDR) multiple testing correction (p (corr) < 0.05) method and 1.5-fold change cut-off, we identified 170 miRNAs differentially expressed between LS and normal tissue, including 119 upregulated and 51 downregulated miRNAs (Figure 1).
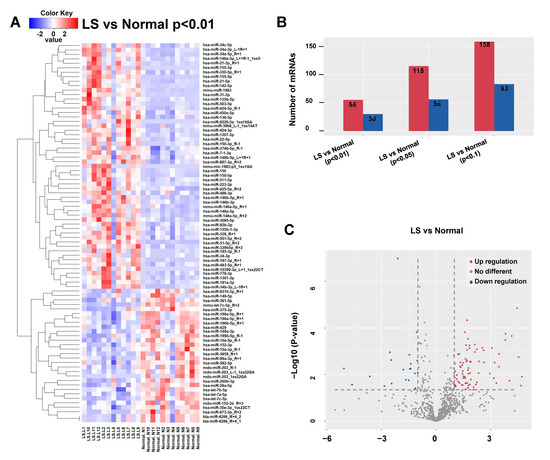
Figure 1.
Differentially expressed microRNAs in LS tissue. Red signal, high relative expression; blue signal, low relative expression. (A) Clustering analysis of differentially expressed miRNAs between LS and matched adjacent normal tissue. The inclusion criteria were a 2-fold-change in either direction with a p-value < 0.01. (B) The numbers of differentially expressed miRNAs with 2-fold change under different p values (0.01, 0.05 and 0.10). (C) The volcano plot revealed more upregulated miRNAs than downregulated ones in LS. The inclusion criteria were a 2-fold-change in either direction with a p-value < 0.05.
We subsequently verified the expression levels of a set of selected candidate miRNAs that are related to allergic and autoimmune diseases, such as upregulated miRNAs (miR-326, miR-142-5p) and downregulated miRNAs (miR-644a, miR-181a2-3p) [31,32,33], by qRT-PCR in LS tissues from the same patients examined by RNASeq (Figure 2A) and from an additional cohort of LS patients by qRT-PCR. Our data showed that the upregulated miRNAs, miR-326, miR-142-5p and downregulated miRNAs, miR-644a, miR-181a-2-3p were concordant with our RNASeq data (Figure 2B). The full dataset is available upon request.
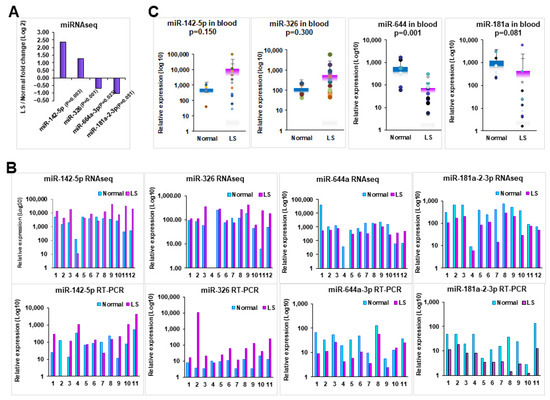
Figure 2.
Expression analysis of selected candidate miRNAs in tissue and blood samples. (A) Expression of represented miRNAs by RNASeq analysis. (B) Expression comparison of dysregulated miRNAs between by RNASeq and qRT-PCR. (C) Expression of representative miRNAs in blood samples from patients with LS compared with healthy female volunteers. p-values were calculated for statistical significance as indicated for each comparison.
3.2. Verifying the Dysregulation of miRNAs in Peripheral Blood
As an attempt to establish whether miRNA profiling could be used as biomarker for LS, we investigated whether there is a similar miRNA expression pattern in peripheral blood samples. To address this, we examined the miRNA expression in 33 peripheral blood samples including 12 patients subjected to RNASeq, and 21 additional LS patients (Table 1). We found that significantly increased miR-326, miR-142-5p and decreased miR-644a, miR-181a-2-3p in blood samples from LS patients compared to the healthy female volunteers (Figure 2C), which is consistent with the RNASeq data we obtained from tissue samples. This suggests that miRNAs may serve as potential biomarkers for LS diagnosis and treatment.
3.3. Gene Network and Pathway Analysis
To gain insights about the broader biological context in which the discovered miRNAs operate, we performed network analysis using the significantly differentially expressed miRNAs. The analysis revealed 14 molecular networks enriched for or targeted by miRNAs highly involved in cancer–injury–reproductive diseases, inflammatory diseases, cell morphology and infectious diseases, cell–cell interaction, as well as cell death and survival. Several miRNAs, such as miR-487b-3p, miR-342-3p, miR-218-5p, and miR-126a-5p, were found to be targeting the AKT gene involved in cancer–injury–reproductive diseases (Figure 3A). Other miRNAs targeted genes involved in cell–cell interaction, cell death, cell survival and cellular assembly and organization network including miR-142-5p, miR-151-3p, miR-218-1-3p, miR-335, and miR-340 related with AGO2, AGO3, CDH1, GABRA3 (Figure 3C). These results are similar to previous reports in renal cell carcinoma [34] and thyroid cancer [35]. The results of network analysis are presented in Figure 3.
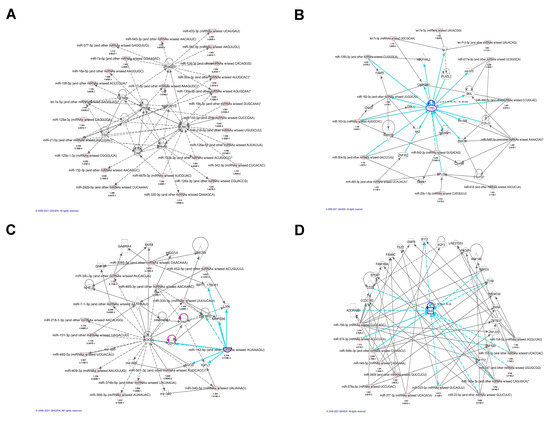
Figure 3.
Top representative molecular networks involving differentially expressed miRNAs in LS identified by IPA analysis. (A) Differentially expressed miRNAs that are involved in AKT gene regulation. (B) Differentially expressed miRNAs that are involved in TP53 regulation. (C) Top representative network involving miR-142-5p in cell–cell interaction, cell death, cell survival and cellular assembly and organization0. (D) A network involving IL-6 regulation related to inflammatory diseases, organismal injury and abnormalities.
3.4. Forced Expression of miR-142-5p in Human Dermal Fibroblast PCS-201-010 Resulted in Decreased Cell Migration
miR-142-5p has been reported as a critical modulator of immune cells as well as inflammatory and immunological responses [20,36]. Pathway analysis showed that miR-142-5p involved in cell–cell interaction and the cell death and survival network, suggesting its essential role in LS development. To demonstrate the function of miR-142-5p in LS, we transfected miR-142-5p into human dermal fibroblast PCS-201-010 cells. Since a migrating capability change is essential in fibrogenic disease [37], we measured the migration ability by Matrigel assay. As presented in Figure 4, the forced expression of miR-142-5p exhibited the remarkable inhibition of cell migration compared to the mock transfection, suggesting that miR-142-5p may be involved in the pathogenesis of LS.
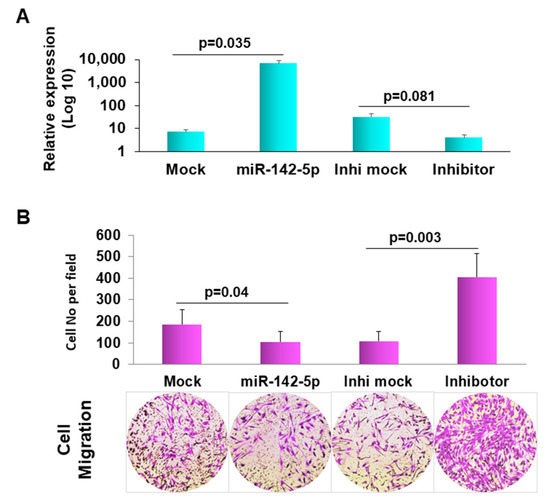
Figure 4.
Forced expression of miR-142-5p reduced cell migration. (A) Confirmation of transfection of miR-142-5p, inhibitor and the mock controls in normal human dermal fibroblast, PCS-201-010 by qRT-PCR. (B) matrigel invasion images (representative ones at the bottom) were pixelized using the Photoshop software version 19.1.3. The migration ability of the cells was displayed as a percentage of the absolute cell numbers. Results are displayed as mean data ± SE (p < 0.05). Five fields of unit area on each membrane or whole membrane were counted for cell numbers, and the experiments were repeated three times with triplicates.
3.5. Target Gene Prediction and Verification
Bioinformatics tools were used to predict the potential target genes of hsa-miR-142-5p. For the primary screening, three databases were used including TargetScan (http://www.targetscan.org, accessed on 6 April 2019), miRDB (http://mirdb.org/, accessed on 6 April 2019) and DIANA Tools (http://diana.imis.athena-innovation.gr, accessed on 6 April 2019) to obtain a dataset of potential downstream target genes of miR-142-5p [38,39,40,41]. Seventeen potential target genes (PPARGC1B, RNF165, PAPPA, SLC22A23, CALM1, HDLBP, GAS7, SREBF1, TMOD1, TCF21, HSD11B1, DACH1, ABHD5, RNF128, CADM3, RBM24, DACH1, and LPP) were predicted. The expression level of these genes was assessed after transfection of miR-142-5p.
To verify the effect of miR-142-5p on the predicted target gene, we transfected miR-142-5p into the PCS-201-010 cell line and measured the expression of the predicted genes. Among the predicted genes, CALM1 and LPP were significantly downregulated in miR-142-5p transfected cells compared to the mock transfected ones, while they were upregulated in miR-142-5p inhibitor transfected cells compared to the inhibitor mock transfected ones (Figure 5A,B). To determine miR-142-5p target gene expression in LS tissue, we performed qRT-PCR in 11 paired samples. As expected, we detected a low expression level of CALM1 and LPP in LS compared to their matched adjacent normal tissues (Figure 5C).
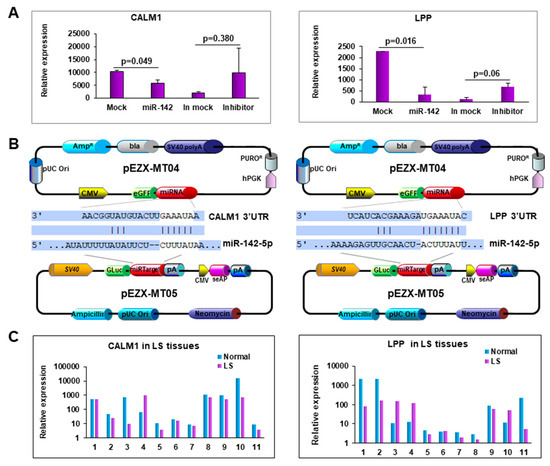
Figure 5.
Forced expression of miR-142-5p reduced CALM1 and LPP expression. (A) Expression of CALM1 and LPP when miR-142-5p was either overexpressed or knocked-down. (B) Map of the plasmids, pEZX-MT04 containing miR-142-5p, and pEZX-MT05 containing 3′-UTR of CALM1 or LPP to illustrate the binding site of miR-1425p to its target genes. (C) Expression of CALM1 and LPP in LS tissue.
4. Discussion
We hypothesized that miRNAs could be promising biomarkers for LS diagnosis and treatment based on the following premises: firstly, that miRNA expression is frequently dysregulated in human diseases [42]; secondly, that miRNAs appear to be tissue-specific [43] and thirdly, that miRNAs are exceptionally stable in tissue and blood [44]. To date, no systematic studies aiming to analyze miRNA expression profiles in vulvar LS have been reported. Here, we assessed these miRNA alterations for the first time in a cohort from LS tissue and blood samples in order to identify miRNA biomarker(s) for LS diagnosis and treatment. We identified 170 dysregulated miRNAs, including 119 upregulated miRNAs and 51 downregulated miRNAs in LS. We selected miRNA candidates to further verify their expression in LS by qRT-PCR. Through transcriptome analysis, functional and pathway analyses, we showed that dysregulated miRNAs may play an essential role in LS development through a number of molecular networks. Our IPA analysis revealed 10 top molecular networks (Supplementary Table S2) enriched for or targeted by miRNAs that are highly likely to be involved in cancer–injury–reproductive diseases, inflammatory diseases, cell morphology infections disease, cell–cell interaction and cell death and survival (Figure 3), which may be involved in LS pathogenesis.
miR-155 has numerous functions, including lymphocyte homeostasis maintenance, inflammation and immune system regulation [45]. Increased levels of miR-155 have been found in various inflammatory diseases [46,47,48], including oral lichen planus (OLP) [49] and LS [50]. An increasing number of studies [51,52] have focused on signaling pathways and cytokine release regulated by miR-155. The expression of miR-155 is usually correlated with increased cytokine release, including IFN-γ [53]. Our RNASeq data confirmed that miR-155 was overexpressed in LS using the adjacent normal tissue from the same patients as controls, while Terlou et al. [54] reported similar findings by microarray analysis when compared with normal volunteers. We further confirmed that miR-155 was upregulated in blood samples from LS patients compared to healthy female volunteers. miR-155 targets signaling pathways that are involved in cellular phenotypes which could play an important role in LS development.
Of the differentially expressed miRNAs, we further focused on one of the most significantly dysregulated miRNAs, miR-142-5p. We found that miR-142-5p expression was increased in both the tissue and blood from LS patients. The miR-142 gene is located on chromosome 17 and is broadly conserved between different species [55]. miR-142-5p was noted to be aberrantly expressed in pathological conditions such as cancer, immunologically related disorders, small bowel inflammation, renal fibrosis where inflammation occurs and in biopsies from renal transplant patients with acute rejection [56]. miR-142-5p has been found to regulate macrophage profibrogenic gene expression in chronic inflammation [57] and is overexpressed in oral lichen planus, a chronic T cell mediated inflammatory disease [58]. Two target genes of miR-142-5p, CALM1 and LPP, were downregulated in LS tissue samples. Gene Ontology (GO) annotations indicate that CALM1 is involved in calcium ion binding and protein domain specific binding [59]. CALM1 stimulates protein kinases and phosphatases, and is involved in a molecular pathway that regulate the centrosome cycle and progression through cytokinesis [60]. LPP, a transcriptional co-activator, localizes to the cell periphery, which involves cell–cell adhesion and cell motility [61]. LPP often appears at the junction of chromosomal translocations, resulting in chimeric proteins that may promote pathogenesis, such as tumor growth and other disorders [62]. LPP also plays a structural and transcriptional role at sites of cell adhesion in maintaining cell shape and motility [63], which may be crucial for LS development and progression. These studies suggest that miR-142-5p may be an essential player in LS, and may serve as a potential target for LS management.
5. Conclusions
Differentially expressed miRNAs and their related molecular networks may shed light on the pathogenesis of LS. Candidate miRNAs, such as miR-155 and miR-142-5p may serve as diagnostic and/or therapeutic biomarkers for LS.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cells10092291/s1, Table S1: A complete list of differentially expressed miRNAs in LS; Table S2: IPA analysis network list (top 10).
Author Contributions
Conceptualization, A.T.G., C.J.M. and S.W.F.; methodology, X.T., S.R. (Shuyang Ren), C.Y., S.R. (Shuchang Ren), M.Z.F., A.R.G., X.L. and L.M.; software, X.T. and S.W.F.; validation, S.R. (Shuyang Ren), C.Y. and S.R. (Shuchang Ren).; formal analysis, X.T., A.T.G. and S.W.F.; investigation, X.T., J.M.K., C.J.M., A.T.G. and S.W.F.; resources, A.T.G.; data curation, X.T.; writing—original draft preparation, X.T. and S.W.F.; writing—review and editing, X.T., M.Z.F., J.M.K., C.J.M., A.T.G. and S.W.F.; supervision, A.T.G. and S.W.F.; project administration, S.W.F.; funding acquisition, S.W.F. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Gynecologic Cancer Research Foundation (to S.W.F. and A.T.G.), the Virginia Gray Research Fund (to S.W.F.), and the Elaine H. Snyder Cancer Research Award (to S.W.F.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the George Washington University (protocol# 091739 approved on 27 November 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The RNASeq data will be deposited into GEO database.
Conflicts of Interest
A.T.G. is the President of the Gynecologic Cancers Research Foundation, a Maryland 501c3 non-profit corporation, which provided funding for this study. Additionally, he is a part-time employee of Dare Bioscience and is a consultant of Ipsen. He has received research funding from SST, Ipsen, and the Gynecologic Cancers Research Foundation. S.W.F. has received research funding from the Gynecologic Cancers Research Foundation.
References
- Dongre, H.; Rana, N.; Fromreide, S.; Rajthala, S.; Engelsen, I.B.; Paradis, J.; Gutkind, J.S.; Vintermyr, O.K.; Johannessen, A.C.; Bjørge, L.; et al. Establishment of a novel cancer cell line derived from vulvar carcinoma associated with lichen sclerosus exhibiting a fibroblast-dependent tumorigenic potential. Exp. Cell Res. 2019, 386, 111684. [Google Scholar] [CrossRef] [PubMed]
- Kirtschig, G.; Becker, K.L.; Gunthert, A.R.; Jasaitiene, D.; Cooper, S.; Chi, C.-C.; Kreuter, A.; Rall, K.; Aberer, W.; Riechardt, S.; et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2015, 29, e1–e43. [Google Scholar] [CrossRef]
- Kreuter, A.; Kryvosheyeva, Y.; Terras, S.; Moritz, R.; Möllenhoff, K.; Altmeyer, P.; Scola, N.; Gambichler, T. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm. Venereol. 2013, 93, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheinis, M.; Selk, A. Development of the adult vulvar lichen sclerosus severity scale—A delphi consensus exercise for item generation. J. Low. Genit. Tract Dis. 2018, 22, 66–73. [Google Scholar] [CrossRef]
- Haefner, H.K.; Aldrich, N.Z.; Dalton, V.K.; Gagné, H.M.; Marcus, S.B.; Patel, D.A.; Berger, M. The impact of vulvar lichen sclerosus on sexual dysfunction. J. Women’s Health 2014, 23, 765–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, E.G.; MacLaren, N.K. Genetic aspects of vulvar lichen sclerosus. Am. J. Obstet. Gynecol. 1984, 150, 161–166. [Google Scholar] [CrossRef]
- Lambert, J. Pruritus in female patients. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Niamh, L.; Naveen, S.; Hazel, B. Diagnosis of vulval inflammatory dermatoses: A pathological study with clinical correlation. Int. J. Gynecol. Pathol. 2009, 28, 554–558. [Google Scholar] [CrossRef] [PubMed]
- McPherson, T.; Cooper, S. Vulval lichen sclerosus and lichen planus. Dermatol. Ther. 2010, 23, 523–532. [Google Scholar] [CrossRef]
- Fistarol, S.K.; Itin, P.H. Diagnosis and treatment of lichen sclerosus. Am. J. Clin. Dermatol. 2013, 14, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Krapf, J.M.; Mitchell, L.; Holton, M.A.; Goldstein, A.T. Vulvar lichen sclerosus: Current perspectives. Int. J. Women’s Health 2020, 12, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, D.A.; Tan, X.; Macri, C.J.; Goldstein, A.T.; Fu, S.W. Lichen sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int. J. Biol. Sci. 2019, 15, 1429–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marren, P.; Yell, J.; Charnock, F.; Bunce, M.; Welsh, K.; Wojnarowska, F.; Jell, J. The association between lichen sclerosus and antigens of the HLA system. Br. J. Dermatol. 2006, 132, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.J.; Creasey, A.; Goldstein, A. The Treatment of vulvar lichen sclerosus and female sexual dysfunction. J. Sex. Med. 2011, 8, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.; Burrows, L.J. Surgical treatment of clitoral phimosis caused by lichen sclerosus. Am. J. Obstet. Gynecol. 2007, 196, 126.e1–126.e4. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Scott, H.S.; Goodall, G.J. A network-biology perspective of microRNA function and dysfunction in cancer. Nat. Rev. Genet. 2016, 17, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.K.; Klein, K.; Winter, S.; Zanger, U.M. Expression variability of absorption, distribution, metabolism, excretion–Related microRNAs in human liver: Influence of nongenetic factors and association with gene expression. Drug Metab. Dispos. 2013, 41, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Singh, R.P.; Massachi, I.; Manickavel, S.; Singh, S.; Rao, N.P.; Hasan, S.; Mc Curdy, D.K.; Sharma, S.; Wong, D.; Hahn, B.H.; et al. The role of miRNA in inflammation and autoimmunity. Autoimmun. Rev. 2013, 12, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Talebi, F.; Ghorbani, S.; Chan, W.F.; Boghozian, R.; Masoumi, F.; Ghasemi, S.; Vojgani, M.; Power, C.; Noorbakhsh, F. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J. Neuroinflamm. 2017, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Zhang, H.; Li, L.; Wang, K. Clinical significance of miR-142-3p in oral lichen planus and its regulatory role in keratinocyte proliferation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021. [Google Scholar] [CrossRef]
- Nymark, P.; Guled, M.; Borze, I.; Faisal, A.; Lahti, L.; Salmenkivi, K.; Kettunen, E.; Anttila, S.; Knuutila, S. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosom. Cancer 2011, 50, 585–597. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Fu, Y.; Chen, L.; Lee, W.; Lai, Y.; Rezaei, K.; Tabbara, S.; Latham, P.; Teal, C.B.; Man, Y.G.; et al. MiR-671-5p inhibits epithelial-to-mesenchymal transition by downregulating FOXM1 expression in breast cancer. Oncotarget 2016, 7, 293–307. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. MiRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. MiRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [Green Version]
- Kozomara, A.; Griffiths-Jones, S. MiRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Peng, J.; Fu, Y.; An, S.; Rezaei, K.; Tabbara, S.; Teal, C.B.; Man, Y.G.; Brem, R.F.; Fu, S.W. MiR-638 mediated regulation of BRCA1 affects DNA repair and sensitivity to UV and cisplatin in triple-negative breast cancer. Breast Cancer Res. 2014, 16, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Ren, S.; Fu, M.Z.; Ren, S.; Yang, C.; Wu, X.; Chen, T.; Latham, P.S.; Meltzer, S.J.; Fu, S.W. microRNA-196b promotes esophageal squamous cell carcinogenesis and chemoradioresistance by inhibiting EPHA7, thereby restoring EPHA2 activity. Am. J. Cancer Res. 2021, 11, 3594–3610. [Google Scholar]
- Jadideslam, G.; Ansarin, K.; Sakhinia, E.; Babaloo, Z.; Abhari, A.; Alipour, S.; Farhadi, J.; Shirvani, S.S.; Ghojazadeh, M.; Khabbazi, A. Expression levels of miR-21, miR-146b and miR-326 as potential biomarkers in Behcet’s disease. Biomark. Med. 2019, 13, 1339–1348. [Google Scholar] [CrossRef]
- Rożalski, M.; Rudnicka, L.; Samochocki, Z. MiRNA in atopic dermatitis. Adv. Dermatol. Allergol. 2016, 33, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specjalski, K.; Jassem, E. MicroRNAs: Potential biomarkers and targets of therapy in allergic diseases? Arch. Immunol. Ther. Exp. 2019, 67, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Zou, L.; Lu, F.; Ye, L.; Su, B.; Yang, K.; Lin, M.; Fu, J.; Li, Y. MiR-142-5p promotes renal cell tumorigenesis by targeting TFAP2B. Oncol. Lett. 2020, 20, 324. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Fang, X.; Yang, H. MiR-142-5p Suppresses tumorigenesis by targeting PIK3CA in non-small cell lung cancer. Cell. Physiol. Biochem. 2017, 43, 2505–2515. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhang, Y.; Yan, R.; Huang, L.; Mellor, A.L.; Yang, Y.; Chen, X.; Wei, W.; Wu, X.; Yu, L.; et al. Exosome-derived miR-142-5p remodels lymphatic vessels and induces IDO to promote immune privilege in the tumour microenvironment. Cell Death Differ. 2021, 28, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Akhmetshina, A.; Schett, G.; Distler, O. Monocyte chemoattractant proteins in the pathogenesis of systemic sclerosis. Rheumatology 2008, 48, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. MiRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef] [Green Version]
- Reczko, M.; Maragkakis, M.; Alexiou, P.; Grosse, I.; Hatzigeorgiou, A.G. Functional microRNA targets in protein coding sequences. Bioinformatics 2012, 28, 771–776. [Google Scholar] [CrossRef]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddiki, N.; Brezar, V.; Ruffin, N.; Lévy, Y.; Swaminathan, S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 2014, 142, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, G.; Biswas, R. MicroRNA-155: A master regulator of inflammation. J. Interf. Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. MiR-146 and miR-155: Two key modulators of immune response and tumor development. Noncoding RNA 2017, 3, 22. [Google Scholar] [CrossRef]
- Vigorito, E.; Kohlhaas, S.; Lu, D.; Leyland, R. MiR-155: An ancient regulator of the immune system. Immunol. Rev. 2013, 253, 146–157. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Chen, J.; Li, Y.; Xu, M.; Cai, Y. MicroRNA microarray-based identification of involvement of miR-155 and miR-19a in development of oral lichen planus (OLP) by modulating Th1/Th2 balance via targeting eNOS and toll-like receptor 2 (TLR2). Med. Sci. Monit. 2018, 24, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhao, Y.; Huo, X.; Wu, X. MiR-155-5p promotes fibroblast cell proliferation and inhibits FOXO signaling pathway in vulvar lichen sclerosis by targeting FOXO3 and CDKN1B. Gene 2018, 653, 43–50. [Google Scholar] [CrossRef]
- Lind, E.F.; Millar, D.G.; Dissanayake, D.; Savage, J.C.; Grimshaw, N.K.; Kerr, W.G.; Ohashi, P.S. MiR-155 upregulation in dendritic cells is sufficient to break tolerance in vivo by negatively regulating SHIP1. J. Immunol. 2015, 195, 4632–4640. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.G.; Zhang, X.D.; Sun, X.D.; Wang, X.Q.; Chang, B.P.; Zhang, M.Z. MiRNA-155 modulates the malignant biological characteristics of NK/T-cell lymphoma cells by targeting FOXO3a gene. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 882–888. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, J.; Ma, J.Z.; Liang, X.Y.; Chen, G.Y.; Lu, R.; Du, G.F.; Zhou, G. MicroRNA-155-IFN-gamma feedback loop in CD4(+)t cells of erosive type oral lichen planus. Sci. Rep. 2015, 5, 16935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlou, A.; Santegoets, L.A.; van der Meijden, W.I.; Heijmans-Antonissen, C.; Swagemakers, S.M.; van der Spek, P.J.; Ewing, P.C.; van Beurden, M.; Helmerhorst, T.J.; Blok, L.J. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: A Th1 response and high levels of microRNA. J. Investig. Dermatol. 2012, 132, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Carraro, G.; El Agha, E.; Mukhametshina, R.; Chao, C.-M.; Rizvanov, A.; Barreto, G.; Bellusci, S. Generation and validation of miR-142 knock out mice. PLoS ONE 2015, 10, e0136913. [Google Scholar] [CrossRef]
- Islam, F.; Gopalan, V.; Vider, J.; Lu, C.-T.; Lam, A.K.-Y. MiR-142-5p act as an oncogenic microRNA in colorectal cancer: Clinicopathological and functional insights. Exp. Mol. Pathol. 2018, 104, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Zhao, Q.; He, C.; Huang, D.; Liu, J.; Chen, F.; Chen, J.; Liao, J.Y.; Cui, X.; Zeng, Y.; et al. MiR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat. Commun. 2015, 6, 8523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Du, G.; Wang, Y.; Shi, L.; Mi, J.; Tang, G. Integrative analysis of mRNA and miRNA expression profiles in oral lichen planus: Preliminary results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 390–402.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Day, D.H.; Taylor, R.J.; Myre, M.A. Calmodulin and calmodulin binding proteins in dictyostelium: A primer. Int. J. Mol. Sci. 2020, 21, 1210. [Google Scholar] [CrossRef] [Green Version]
- Lingle, W.L.; Lukasiewicz, K.; Salisbury, J. Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer. Chem. Biol. Pteridines Folates 2006, 570, 393–421. [Google Scholar] [CrossRef]
- Paksa, A.; Bandemer, J.; Hoeckendorf, B.; Razin, N.; Tarbashevich, K.; Minina, S.; Meyen, D.; Biundo, A.; Leidel, S.; Peyrieras, N.; et al. Repulsive cues combined with physical barriers and cell–cell adhesion determine progenitor cell positioning during organogenesis. Nat. Commun. 2016, 7, 11288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrington, C.B.; Patel, A.; Bacino, C.A.; Bowles, N.E. Haploinsufficiency of the LIM domain containing preferred translocation partner in lipoma (LPP) gene in patients with tetralogy of Fallot and VACTERL association. Am. J. Med Genet. Part A 2010, 152A, 2919–2923. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.M.R.; Meulemans, S.M.P.; Alen, P.; Ayoubi, T.A.Y.; Jansen, E.; Van De Ven, W.J.M. The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol. 2005, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).