Bacillus subtilis Spore-Trained Dendritic Cells Enhance the Generation of Memory T Cells via ICAM1
Abstract
:1. Introduction
2. Methods
2.1. Animals and Ethics Statement
2.2. Vaccine Preparation
2.3. Immunogenicity Study
2.4. Intestinal Mucosa-Associated Lymphocyte (IMAL) Isolation
2.5. Flow Cytometry and Cell Sorting
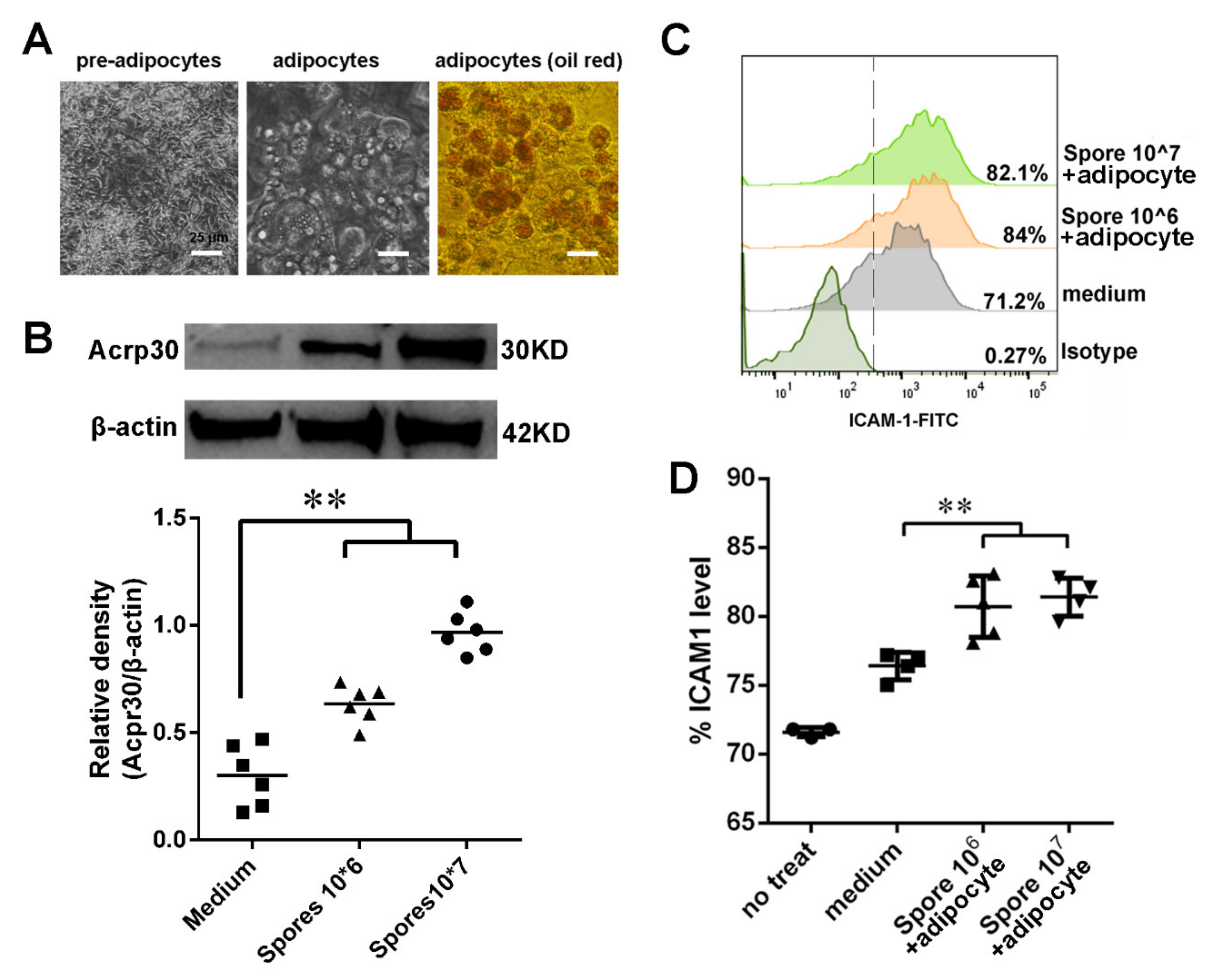
2.6. Adipocyte Differentiation in Cell Culture
2.7. FTY720 Treatments and Tetramer Staining
2.8. DC/EC Coculture System
2.9. Ligated Loop Experiments
2.10. Mouse Cytokine Array by a Proteome Profiler
2.11. Histology and Immunohistochemistry
2.12. Immunofluorescence (IF)
2.13. Quantitative RT-PCR (qRT-PCR)
2.14. Western Blot Assay
2.15. Statistical Analysis
3. Results
3.1. Spore-Adjuvant H9N2 WIV Induces the Production of Specific Antibodies and Prompts Lymphocyte Proliferation
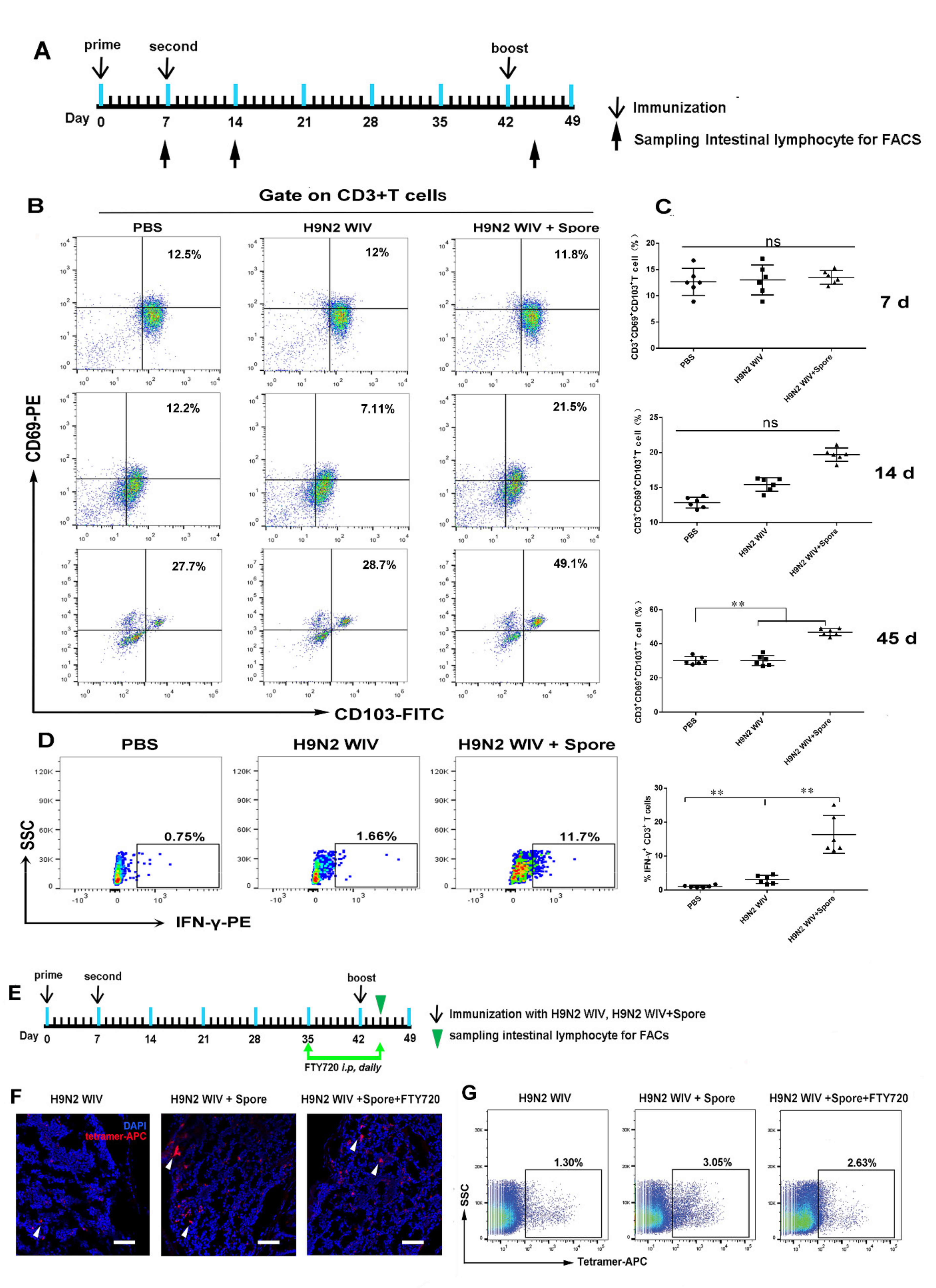
3.2. Spores Plus H9N2 WIV Induce CD69+ CD103+ Trms in Intestinal Tissue
3.3. Spore-Adjuvant Immunization Induces HA-Specific Trms in Intestinal Tissue
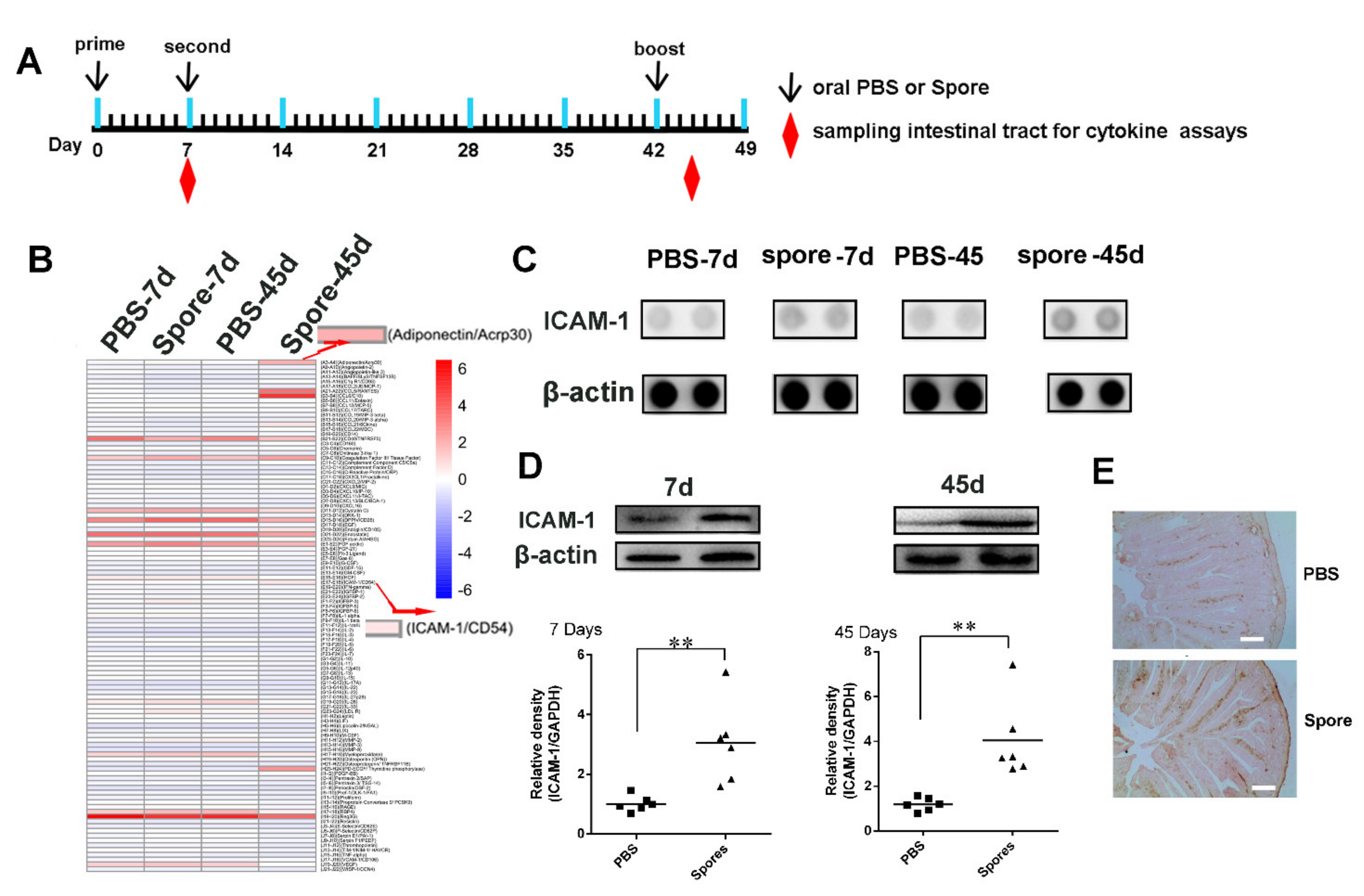
3.4. Spores Upregulate ICAM1 to Recruit and Activate DCs
3.4.1. Spores Effectively Recruit and Activate Submucosal DCs
3.4.2. Spores Upregulate ICAM1 to Recruit and Activate DCs
3.5. Spore Stimulate Acrp30 Secretion Leading to High Expression of ICAM1
3.6. DCs Induce ICAM1-Dependent Generation of HA-Specific T Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMDCs | bone marrow-derived dendritic cells | |
| AIV | avian influenza virus | |
| Tcms | central memory T cells | |
| Trms | tissue-resident memory T cells | |
| Tems | effector memory T cells | |
| S1P | sphingosine-1-phosphate | |
| TEDs | transepithelial dendrites | |
| EC | epithelial cell | |
| GM-CSF | granulocyte colony-stimulating factor | |
| MHC-II | major histocompatibility complex class II | |
| FACS | Fluorescence-Activated Cell Sorter | |
| Key Resources Table | ||
| Reagent or Resource | Source | Clone |
| anti-mouse CD11c–APC | eBioscience | N418 |
| anti-mouse CD40–PE | eBioscience | 1C10 |
| anti-mouse CD80–FITC | eBioscience | 16-10A1 |
| anti-mouse CD86–PE | eBioscience | GL1 |
| anti-mouse MHC class II I-A–FITC | eBioscience | NIMR-4 |
| anti-mouse CD3e–APC | eBioscience | 145-2C11 |
| anti-mouse CD62L–APC | Miltenyi | REA828 |
| anti-mouse CCR7–PE | Miltenyi | REA685 |
| recombinant murine GM-CSF | Peprotech | Cat#214-14-204G |
| recombinant murine IL-4 | Peprotech | Cat#315-03-204G 2E7 |
| anti-mouse CD69–PE | eBioscience | M290 |
| anti-mouse CD103–FITC | eBioscience | XMG1.2 |
| anti-mouse INF-γ–PE | eBioscience | IM7 |
| anti-mouse CD44–FITC | eBioscience | EPR22161-284 |
| rabbit anti-mouse ICAM1 | Abcam | PA1-054 |
| rabbit anti-mouse Acrp30 | Thermo Fisher | 4D3 |
| rabbit anti-mouse β-actin | Bioworld | N/A |
| influenza HA518–526 (IYSTVASSL) peptide | Genscript | N/A |
| HA-specific (HA518–526) | MBL | |
| H-2Kd tetramer-IYSTVASSL | ||
References
- Iqbal, M.; Yaqub, T.; Mukhtar, N.; Shabbir, M.Z.; McCauley, J.W. Infectivity and Transmissibility of H9N2 Avian Influenza Virus in Chickens and Wild Terrestrial Birds. Vet. Res. 2013, 44, 100. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.P.; Liu, J.H. H9N2 Influenza Virus in China: A Cause of Concern. Protein Cell 2015, 6, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Qu, B.Q.; Li, X.; Gao, W.; Sun, W.K.; Jin, Y.; Cardona, C.J.; Xing, Z. Human Intestinal Epithelial Cells Are Susceptible to Influenza Virus Subtype H9N2. Virus Res. 2012, 163, 151–159. [Google Scholar] [CrossRef]
- Wille, M.; Brojer, C.; Lundkvist, A.; Jarhult, J.D. Alternate Routes of Influenza a Virus Infection in Mallard (Anas Platyrhynchos). Vet. Res. 2018, 49, 110. [Google Scholar] [CrossRef] [Green Version]
- Van Splunter, M.; van Hoffen, E.; Floris-Vollenbroek, E.G.; Timmerman, H.; Lucas-van de Bos, E.; Meijer, B.; Ulfman, L.H.; Witteman, B.; Wells, J.M.; Brugman, S.; et al. Oral Cholera Vaccination Promotes Homing of IgA(+) Memory B Cells to the Large Intestine and the Respiratory Tract. Mucosal Immunol. 2018, 11, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, X.P.; Xiong, F.F.; Gao, F.X.; Tan, W.S. Enhancing Immune Response and Heterosubtypic Protection Ability of Inactivated H7N9 Vaccine by Using STING Agonist as a Mucosal Adjuvan. Front. Immunol. 2019, 10, 2274. [Google Scholar] [CrossRef] [Green Version]
- Ji, E.L.; Kye, Y.C.; Park, S.M.; Shim, B.S.; Yun, C.H. Bacillus Subtilis Spores as Adjuvants Against Avian Influenza h9n2 Induce Antigen-Specific Antibody and T Cell Responses in White Leghorn Chickens. Vet. Res. 2020, 51, 1–12. [Google Scholar]
- Zhao, G.Y.; Miao, Y.; Guo, Y.; Qiu, H.J.; Sun, S.H.; Kou, Z.H.; Yu, H.; Li, J.F.; Chen, Y.; Jiang, S.B.; et al. Development of a Heat-Stable and Orally Delivered Recombinant M2e-Expressing B. Subtilis Spore-Based Influenza Vaccine. Hum. Vacc. Immunother. 2014, 10, 3649–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Hong, H.A.; Huang, J.M.; Colenutt, C.; Khang, D.D.; Thi, V.A.N.; Park, S.M.; Shim, B.S.; Song, H.H.; Cheon, I.S.; et al. Killed Bacillus Subtilis Spores as a Mucosal Adjuvant for an H5N1 Vaccine. Vaccine 2012, 30, 3266–3277. [Google Scholar] [CrossRef]
- Esparza-Gonzalez, S.C.; Troy, A.R.; Izzo, A.A. Comparative Analysis of Bacillus Subtilis Spores and Monophosphoryl Lipid a As Adjuvants of Protein-Based Mycobacterium Tuberculosis-Based Vaccines: Partial Requirement for Interleukin-17a for Induction of Protective Immunity. Clin. Vaccine Immunol. 2014, 21, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copland, A.; Hart, P.; Diogo, G.R.; Harris, S.; Paul, M.; Singh, M.; Cutting, S.M.; Reljic, R. Mucosal Delivery of Fusion Proteins with Bacillus subtilis Spores Enhances Protection against Tuberculosis by BCG. Front. Immunol. 2018, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Yang, I.-Y.; Kim, J.; Lee, K.-Y.; Jang, Y.-S. Antimicrobial Peptide Ll-37 Promotes Antigen-Specific Immune Responses in Mice by Enhancing th17-Skewed Mucosal and Systemic Immunities. Eur. J. Immunol. 2015, 45, 1402–1413. [Google Scholar] [CrossRef]
- Wu, T.; Hu, Y.H.; Lee, Y.T.; Bouchard, K.R.; Benechet, A.; Khanna, K.; Cauley, L.S. Lung-Resident Memory CD8 T Cells (TRM) Are Indispensable for Optimal Crossprotection Against Pulmonary Virus Infection. J. Leukocyte Biol. 2014, 95, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-Generated Lung Tissue-Resident Memory T Cells Provide Heterosubtypic Protection to Influenza Infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Wang, J.L.; Wang, Y.H.; Zhang, E.; Li, Y.C.; Yu, Q.H.; Yang, Q. Upregulation of CD4(+) CD8 (+) Memory Cells in the Piglet Intestine Following Oral Administration of Bacillus subtilis Spores Combined with PEDV Whole Inactivated Virus. Vet. Microbiol. 2019, 235, 1–9. [Google Scholar] [CrossRef]
- Qin, T.; Yin, Y.Y.; Yu, Q.H.; Huang, L.L.; Wang, X.Q.; Lin, J.; Yang, Q. CpG Oligodeoxynucleotides Facilitate Delivery of Whole Inactivated H9N2 Influenza Virus via Transepithelial Dendrites of Dendritic Cells in Nasal Mucosa. J. Virol. 2015, 89, 5904–5918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.T.; Zhang, B.H.; Chen, C.; Ma, J.Y.; Meng, D.Y.; Huang, J.; Hu, R.; Liu, X.G.; Otsu, K.; Liu, A.C.; et al. Protein Kinase p38 Alpha Signaling in Dendritic Cells Regulates Colon Inflammation and Tumorigenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E12313–E12322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, M.A.; Barnum, S.R.; Bullard, D.C.; Zajac, A.J. ICAM-1-Dependent Tuning of Memory CD8 T-Cell Responses Following Acute Infection. Proc. Natl. Acad. Sci. USA 2013, 110, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- McNamara, H.A.; Cai, Y.; Wagle, M.V.; Sontani, Y.; Roots, C.M.; Miosge, L.A.; O’Connor, J.H.; Sutton, H.J.; Ganusov, V.V.; Heath, W.R.; et al. Up-Regulation of LFA-1 Allows Liver-Resident Memory T Cells to Patrol and Remain in the Hepatic Sinusoids. Sci. Immunol. 2017, 2, eaaj1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Fu, S.; Xu, X.; Yang, Y.; Du, L.; Li, W.; Kan, S.; Li, Z.; Zhang, X.; Wang, L.; et al. Leptin-Mediated Regulation of ICAM-1 Is Rho/ROCK Dependent and Enhances Gastric Cancer Cell Migration. Brit. J. Cancer 2014, 110, 1801–1810. [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhang, R.F.; Wang, D.D.; Qiu, M.H.; Feng, H.C.; Zhang, N.; Shen, Q.R. Enhanced Control of Cucumber Wilt Disease by Bacillus amyloliquefaciens SQR9 by Altering the Regulation of Its DegU Phosphorylation. Appl. Environ. Microb. 2014, 80, 2941–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeraedts, F.; Goutagny, N.; Hornung, V.; Severa, M.; de Haan, A.; Pool, J.; Wilschut, J.; Fitzgerald, K.A.; Huckriede, A. Superior Immunogenicity of Inactivated Whole Virus H5N1 Influenza Vaccine Is Primarily Controlled by Toll-Like Receptor Signalling. PLoS Pathog. 2008, 4, e1000138. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor ROR Gamma T Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, S.; Reynolds, J.M. Mouse Naive CD4(+) T Cell Isolation and In vitro Differentiation into T Cell Subsets. J. Vis. Exp. JoVE 2015, 98, 52739. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Do, H.J.; Shin, M.J.; Seong, S.I.; Hwang, K.Y.; Lee, J.Y.; Kwon, O.; Jin, T.; Chung, J.H. 1-Deoxynojirimycin Isolated from a Bacillus Subtilis Stimulates Adiponectin and GLUT4 Expressions in 3T3-L1 Adipocytes. J. Microbiol. Biotechn. 2013, 23, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Huang, L.L.; Zhu, L.Q.; Mou, C.X.; Hou, Q.H.; Yu, Q.H. Inhibition of H9N2 Virus Invasion into Dendritic Cells by the S-Layer Protein from L. Acidophilus ATCC 4356. Front. Cell. Infect. Microbiol. 2016, 6, 137. [Google Scholar] [CrossRef]
- Wein, A.N.; Mcmaster, S.R.; Takamura, S.; Dunbar, P.R.; Cartwright, E.K.; Hayward, S.L. Cxcr6 Regulates Localization of Tissue-Resident Memory cd8 T Cells to the Airways. J. Exp. Med. 2019, 216, 2748–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzavecchia, A.; Sallusto, F. Progressive Differentiation and Selection of the Fittest in the Immune Response. Nat. Rev. Immunol. 2002, 2, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Wakim, L.M.; Smith, J.; Caminschi, I.; Lahoud, M.H.; Villadangos, J.A. Antibody-Targeted Vaccination to Lung Dendritic Cells Generates Tissue-Resident Memory CD8 Tcells That Are Highly Protective Against Influenza Virus Infection. Mucosal Immunol. 2015, 8, 1060–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapuente, D.; Bonsmann, M.S.G.; Maaske, A.; Stab, V.; Heinecke, V.; Watzstedt, K.; Hess, R.; Westendorf, A.M.; Bayer, W.; Ehrhardt, C.; et al. IL-1 Beta as Mucosal Vaccine Adjuvant: The Specific Induction of Tissue-Resident Memory T Cells Improves the Heterosubtypic Im-Munity Against Influenza a Viruses. Mucosal Immunol. 2018, 11, 1265–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholer, A.; Hugues, S.; Boissonnas, A.; Fetler, L.; Amigorena, S. Intercellular Adhesion Molecule-1-Dependent Stable Interactions Between T Cells and Dendritic Cells Determine CD8(+) T Cell Memory. Immunity 2008, 28, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The Developmental Pathway for CD103(+)CD8(+) Tissue-Resident Memory T Cells of Skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. 2016, 11, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; De Rosa, A.; Nigro, E.; Scudiero, O.; Capasso, M.; Masullo, M.; de Laurentiis, G.; Oriani, G.; Sofia, M.; Bianco, A. Adiponectin Oligomerization State and Adiponectin Receptors Airway Expression in Chronic Obstructive Pulmonary Disease. Int. J. Biochem. Cell B 2012, 44, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Daniele, A.; Scudiero, O.; Monaco, M.L.; Roviezzo, F.; D’Agostino, B.; Mazzarella, G.; Bianco, A. Adiponectin in Asthma: Implications for Phenotyping. Curr. Protein Pept. Sci. 2015, 16, 182–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacedonia, D.; Nigro, E.; Matera, M.G.; Scudiero, O.; Monaco, M.L.; Polito, R.; Carpagnano, G.E.; Barbaro, M.P.F.; Mazzarella, G.; Bianco, A.; et al. Evaluation of Adiponectin Profile in Italian Patients Affected by Obstructive Sleep Apnea Syndrome. Pulm. Pharmacol. Ther. 2016, 40, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Oritani, K.; Takahashi, I.; Ishikawa, J.; Matsuyama, A.; Ouchi, N.; Kihara, S.; Funahashi, T.; Tenner, A.J.; Tomiyama, Y.; et al. Adiponectin, a New Member of the Family of Soluble Defense Collagens, Negatively Regulates the Growth of Myelomonocytic Progenitors and the Functions of Macrophages. Blood 2000, 96, 1723–1732. [Google Scholar] [CrossRef]
- Pang, T.T.L.; Narendran, P. The Distribution of Adiponectin Receptors on Human Peripheral Blood Mononuclear Cells. Ann. N. Y. Acad. Sci. 2008, 1150, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Lihn, A.S.; Verdich, C.; Pedersen, S.B.; Toubro, S.; Astrup, A.; Richelsen, B. Regulation of Adiponectin by Adipose Tissue-Derived Cytokines: In Vivo and In Vitro Investigations in Humans. Am. J. Physiol. Endoc. M 2003, 285, E527–E533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.; Prins, J.; Venkatesh, B. Clinical Review: Adiponectin Biology and Its Role in Inflammation and Critical Illness. Crit. Care 2011, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsanis, C.; Zacharioudaki, V.; Androulidaki, A.; Dermitzaki, E.; Charalampopoulos, I.; Minas, V.; Gravanis, A.; Margioris, A.N. Adiponectin Induces TNF-Alpha and IL-6 in Macrophages and Promotes Tolerance to Itself and other Pro-Inflammatory Stimuli. Biochem. Biophys. Res. Commun. 2005, 335, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Iwasaki, A. Tissue Instruction for Migration and Retention of T-RM Cells. Trends Immunol. 2015, 36, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Salzman, N.H.; de Jong, H.; Paterson, Y.; Harmsen, H.J.M.; Welling, G.W.; Bos, N.A. Analysis of 16S Libraries of Mouse Gastrointestinal Microflora Reveals a Large New Group of Mouse Intestinal Bacteria. Microbiology 2002, 148, 3651–3660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.J.; et al. A Mucosal Vaccine Against Chlamydia Trachomatis Generates Two Waves of Protective Memory T Cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Huang, L.; Li, Y.; Zhang, P.; Yu, Q.; Yang, Q. Bacillus subtilis Spore-Trained Dendritic Cells Enhance the Generation of Memory T Cells via ICAM1. Cells 2021, 10, 2267. https://doi.org/10.3390/cells10092267
Lin J, Huang L, Li Y, Zhang P, Yu Q, Yang Q. Bacillus subtilis Spore-Trained Dendritic Cells Enhance the Generation of Memory T Cells via ICAM1. Cells. 2021; 10(9):2267. https://doi.org/10.3390/cells10092267
Chicago/Turabian StyleLin, Jian, Lulu Huang, Yuchen Li, Penghao Zhang, Qinghua Yu, and Qian Yang. 2021. "Bacillus subtilis Spore-Trained Dendritic Cells Enhance the Generation of Memory T Cells via ICAM1" Cells 10, no. 9: 2267. https://doi.org/10.3390/cells10092267
APA StyleLin, J., Huang, L., Li, Y., Zhang, P., Yu, Q., & Yang, Q. (2021). Bacillus subtilis Spore-Trained Dendritic Cells Enhance the Generation of Memory T Cells via ICAM1. Cells, 10(9), 2267. https://doi.org/10.3390/cells10092267






