Pro-Angiogenic and Osteogenic Effects of Adipose Tissue-Derived Pericytes Synergistically Enhanced by Nel-like Protein-1
Abstract
1. Introduction
2. Methods
2.1. NELL-1 and Human Stromal Vascular Fraction (HSVF) Isolation from Human Adipose Tissue and Purification of Human Pericytes from HSVF
2.2. Osteogenic and Adipogenic Differentiation of Human Pericytes
2.3. Endothelial Cell Culture
2.4. Viability Test
2.5. Tube Formation Assay
2.6. Wound Migration Assay
2.7. Chick Chorioallantoic Membrane Assay
2.8. Animals
2.9. Implant Preparation and Grouping
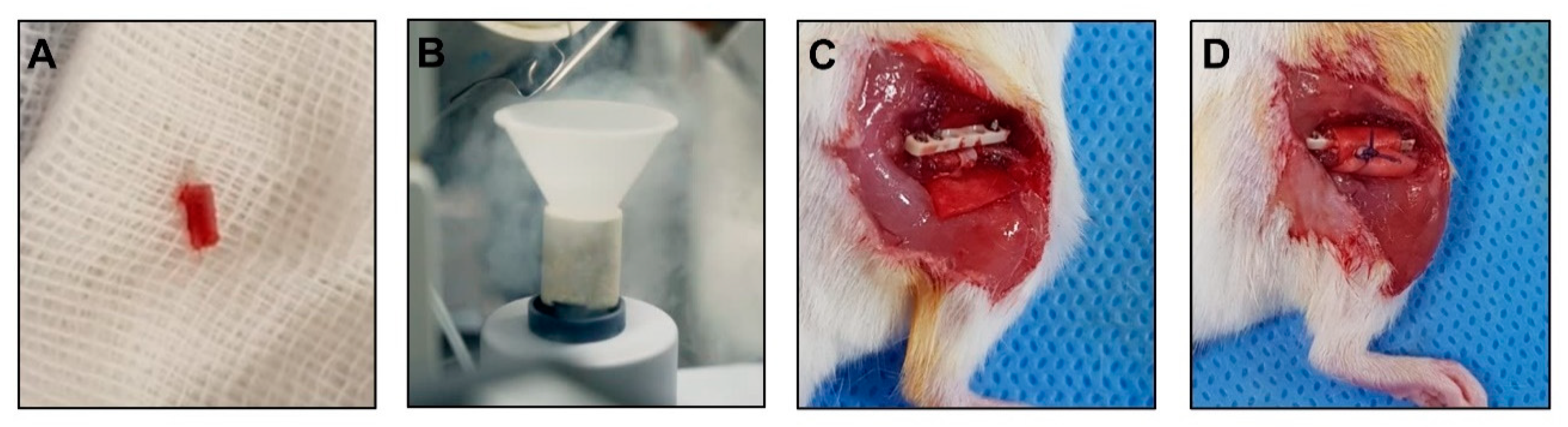
2.10. Surgical Procedure for Mouse Models of Osteonecrosis
2.11. In Vivo Plain Radiograph
2.12. Ex Vivo Micro Computed Tomography
2.13. Histology and Histomorphometric Analysis
2.14. Microarray
2.15. RNA Isolation and QRT-PCR Analysis
2.16. Western Blot
2.17. Statistical Analysis
3. Results
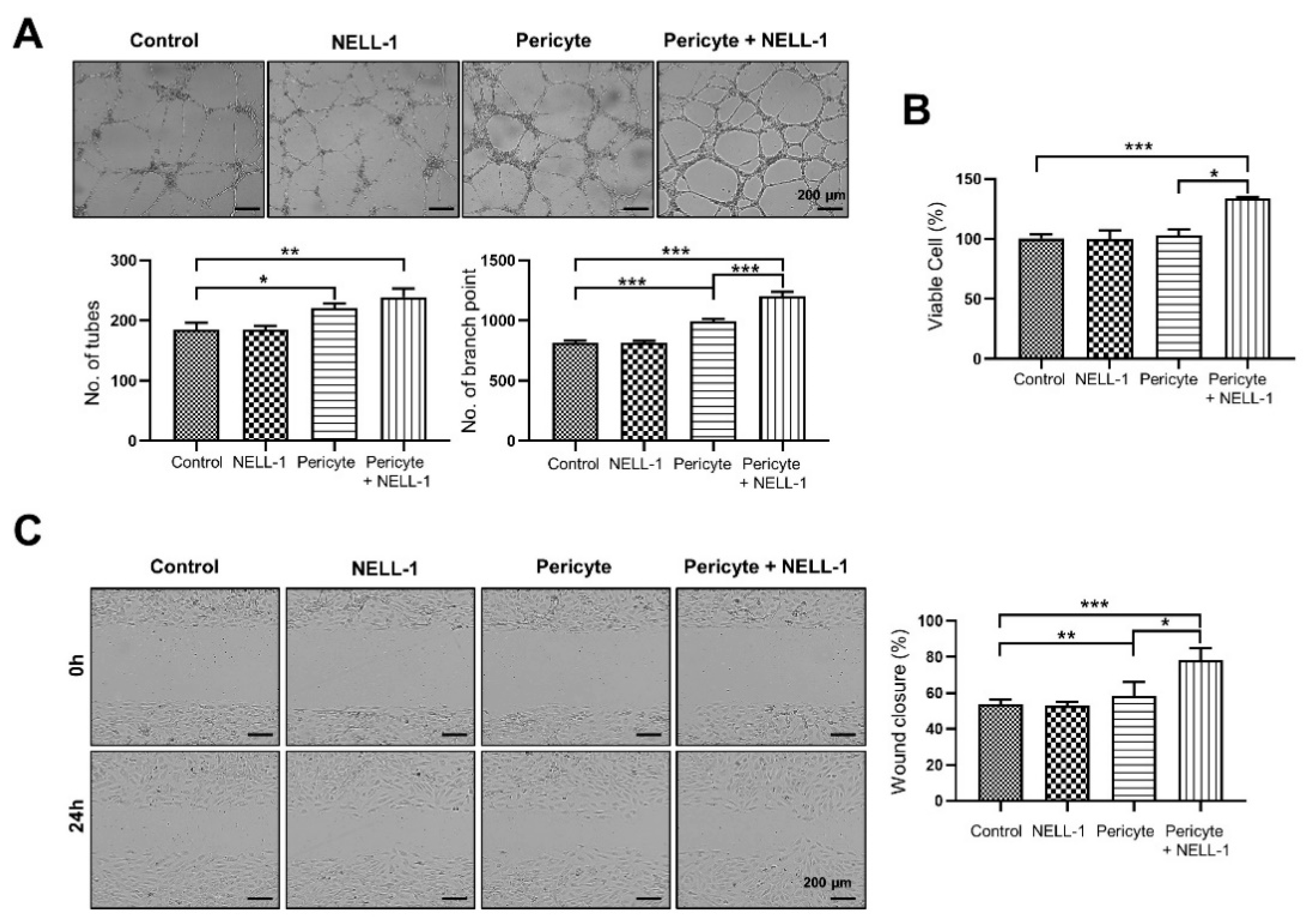
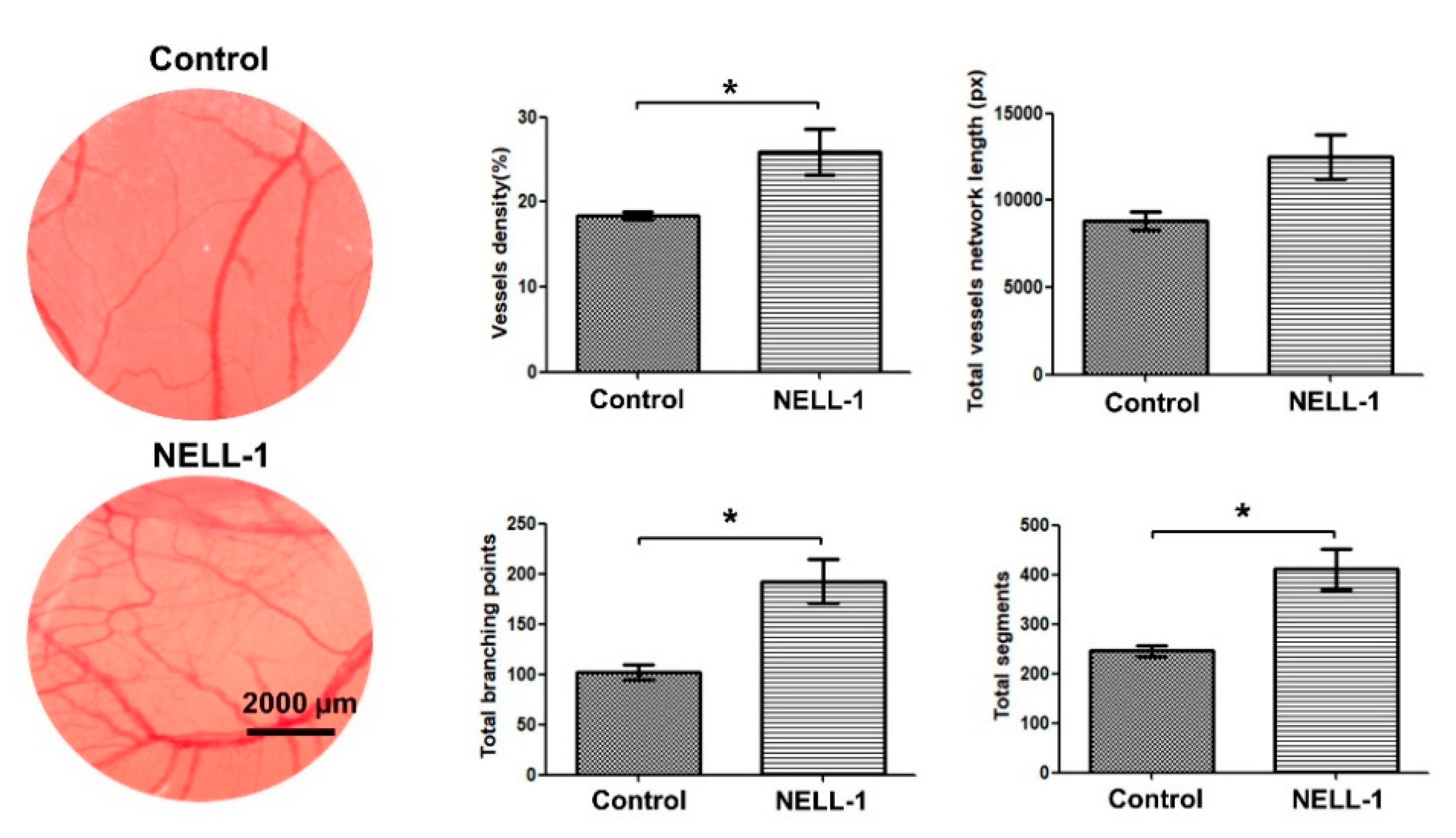
3.1. Pericytes and NELL-1 Promote Angiogenesis and Migration of HUVECs In Vitro and during Embryonic Vascularization In Vivo
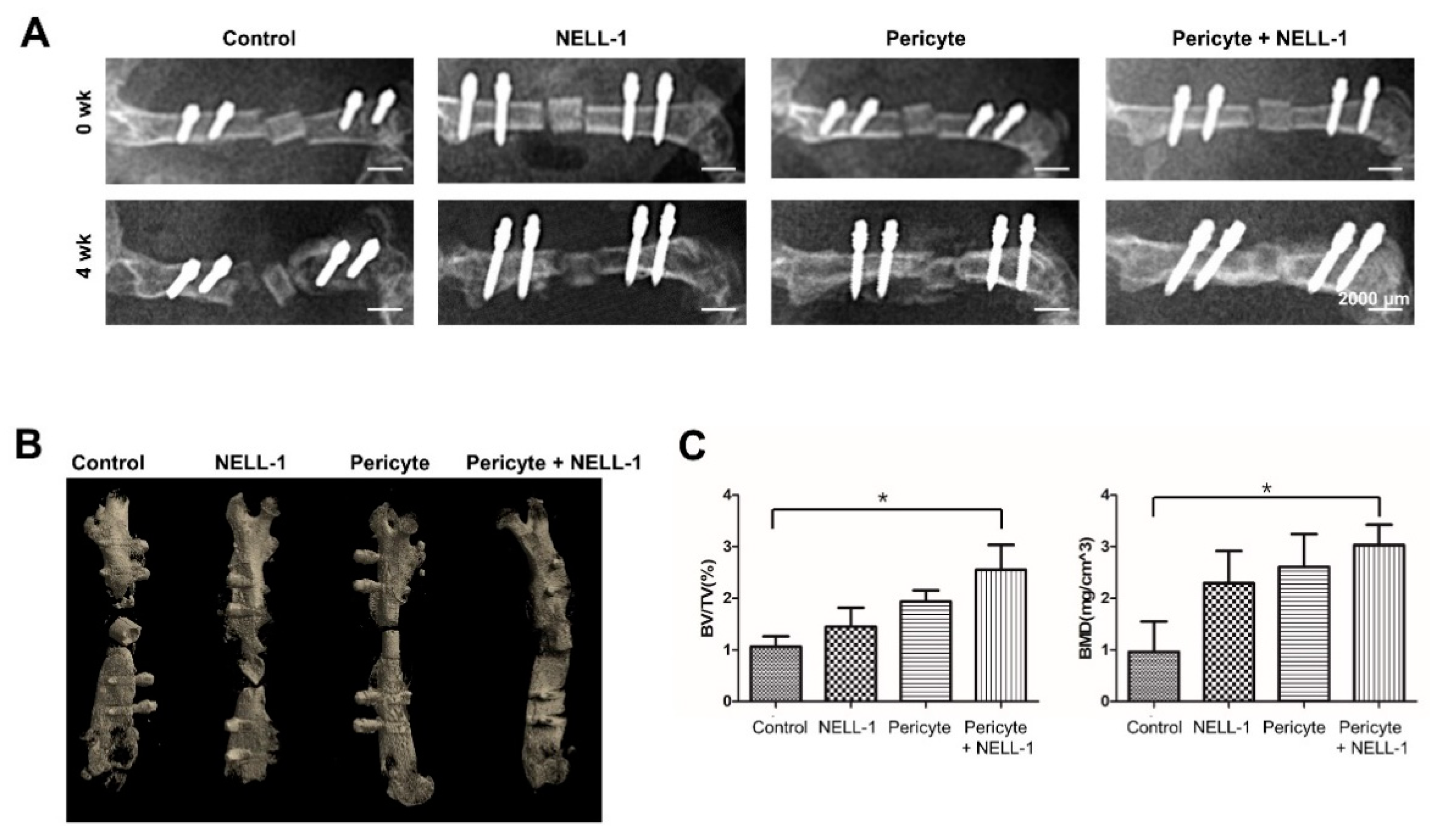
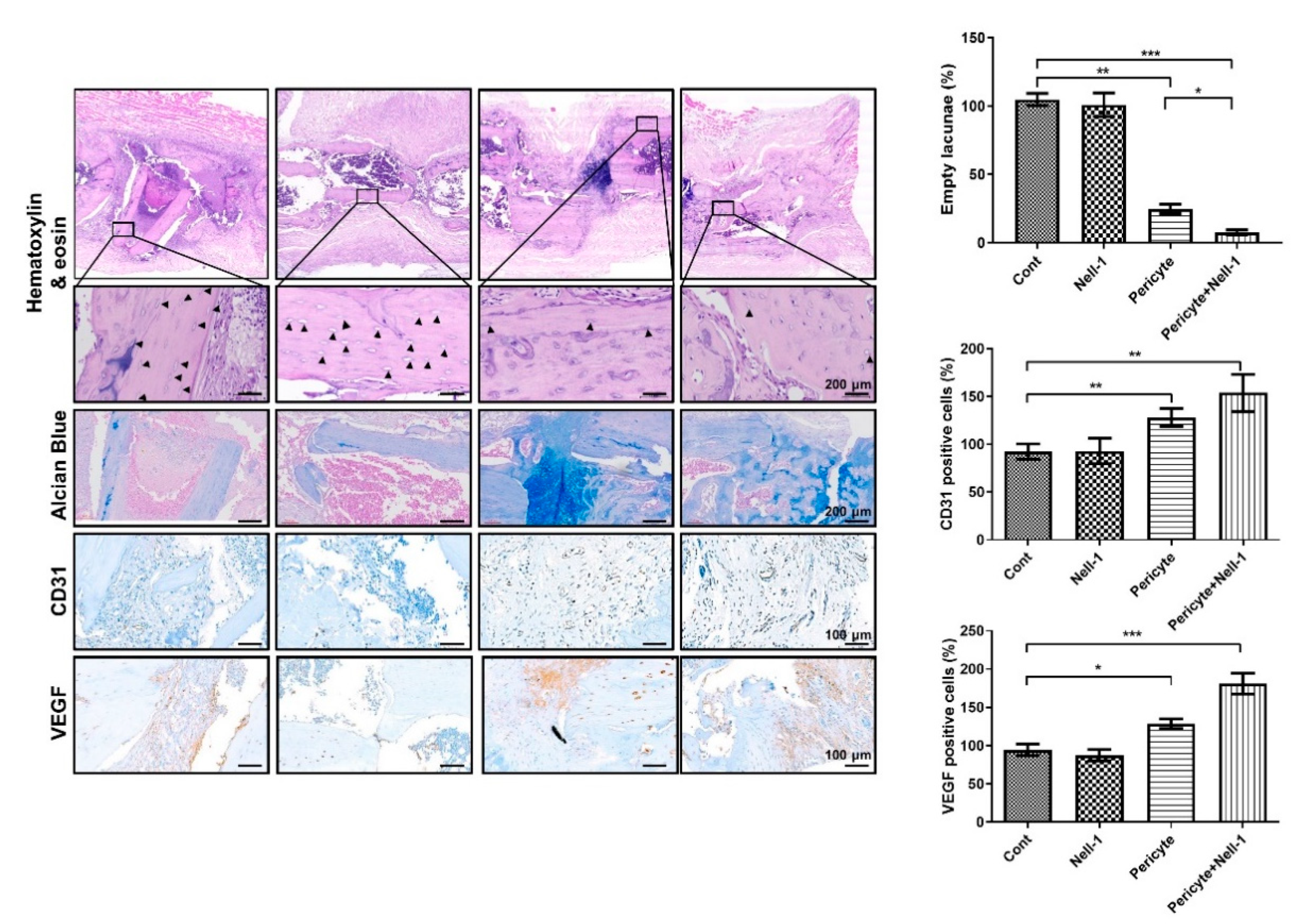
3.2. Pericytes and NELL-1 Promote Bone Formation In Vivo
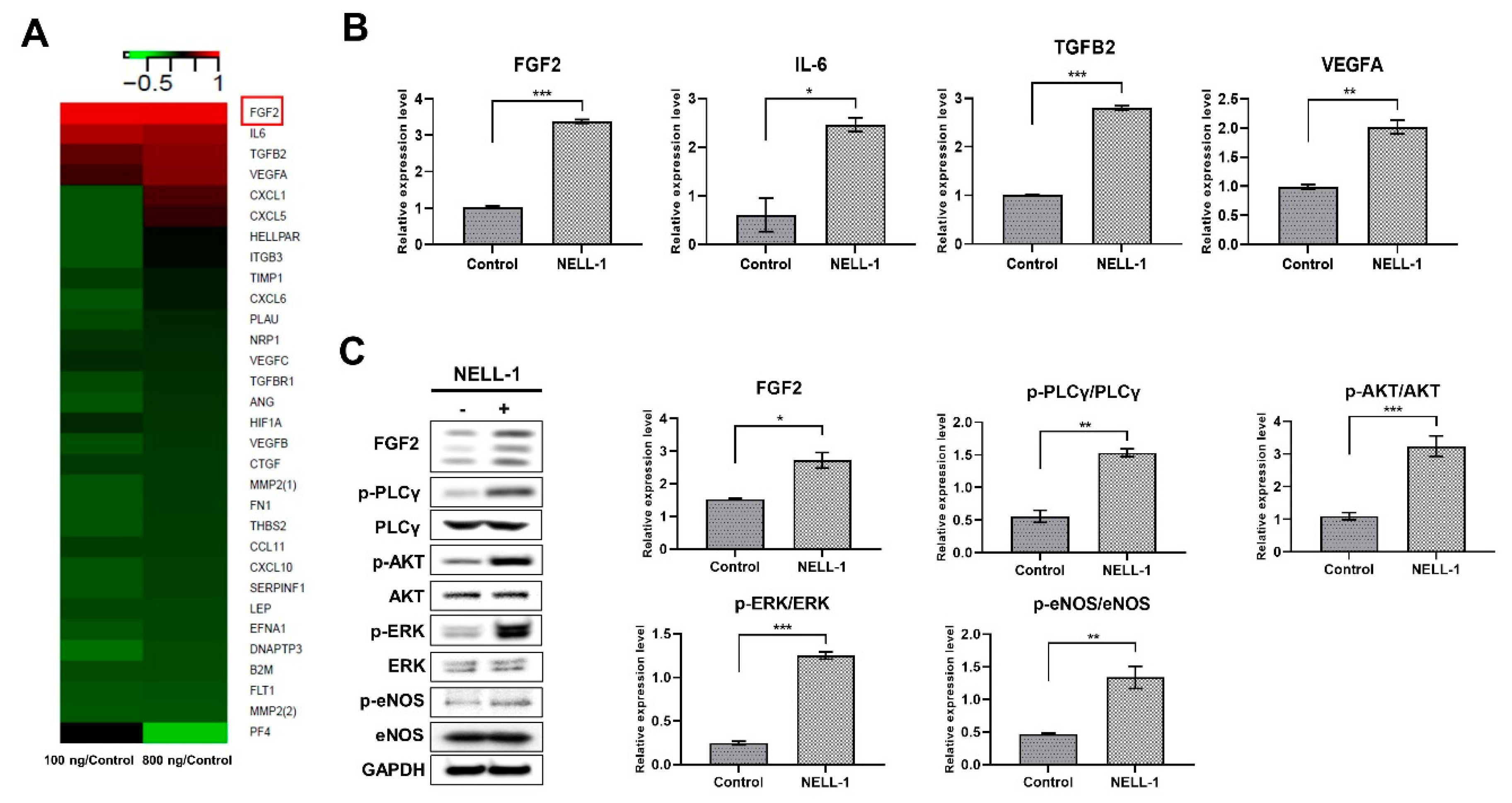
3.3. NELL-1 Promotes the FGF2 Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | adipose tissue-derived |
| NELL-1 | Nel-like protein-1 |
| ONFH | osteonecrosis of the femoral head |
| hSVF | human stromal vascular fraction |
| PBS | phosphate buffered saline |
| DMEM | dulbecco’s modified eagle’s medium |
| FACS | fluorescence-activated cell sorter |
| FBS | fetal bovine serum |
| HUVECs | human umbilical vein endothelial cells |
| WST | water-soluble tetrazolium salt |
| CAM | chick chorioallantoic membrane |
| ACS | absorbable collagen sponge |
| CT | computed tomography |
| BMD | bone mineral density |
| PG | pericytes group |
| PNG | pericytes + NELL-1 group |
References
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. JoVE 2014, 91, e51312. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Benoit, D.S. Depot-Based Delivery Systems for Pro-Angiogenic Peptides: A Review. Front. Bioeng. Biotechnol. 2015, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.; Young, S.W.; Frampton, C.M.; Hooper, G.J. The lifetime risk of revision following total hip arthroplasty. Bone Jt. J. 2021, 103-B, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R. Core decompression for osteonecrosis of the hip. Clin. Orthop. Relat. Res. 2004, 418, 29–33. [Google Scholar] [CrossRef]
- Andriolo, L.; Merli, G.; Tobar, C.; Altamura, S.A.; Kon, E.; Filardo, G. Regenerative therapies increase survivorship of avascular necrosis of the femoral head: A systematic review and meta-analysis. Int. Orthop. 2018, 42, 1689–1704. [Google Scholar] [CrossRef]
- Askarinam, A.; James, A.W.; Zara, J.N.; Goyal, R.; Corselli, M.; Pan, A.; Liang, P.; Chang, L.; Rackohn, T.; Stoker, D.; et al. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng. Part. A 2013, 19, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhang, X.; Shen, J.; James, A.W.; Chung, C.G.; Hardy, R.; Li, C.; Girgius, C.; Zhang, Y.; Stoker, D.; et al. Brief Report: Human Perivascular Stem Cells and Nel-Like Protein-1 Synergistically Enhance Spinal Fusion in Osteoporotic Rats. Stem Cells 2015, 33, 3158–3163. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Chen, C.W.; Montelatici, E.; Crisan, M.; Corselli, M.; Huard, J.; Lazzari, L.; Peault, B. Perivascular multi-lineage progenitor cells in human organs: Regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009, 20, 429–434. [Google Scholar] [CrossRef]
- Corselli, M.; Chen, C.W.; Sun, B.; Yap, S.; Rubin, J.P.; Peault, B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012, 21, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Corselli, M.; Chen, W.C.; Peault, B. Perivascular cells for regenerative medicine. J. Cell Mol. Med. 2012, 16, 2851–2860. [Google Scholar] [CrossRef]
- Canfield, A.E.; Doherty, M.J.; Wood, A.C.; Farrington, C.; Ashton, B.; Begum, N.; Harvey, B.; Poole, A.; Grant, M.E.; Boot-Handford, R.P. Role of pericytes in vascular calcification: A review. Z. Fur Kardiol. 2000, 89 (Suppl. 2), 20–27. [Google Scholar] [CrossRef]
- Schor, A.M.; Allen, T.D.; Canfield, A.E.; Sloan, P.; Schor, S.L. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J. Cell Sci. 1990, 97 Pt 3, 449–461. [Google Scholar] [CrossRef]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B.; You, W.K.; Kucharova, K.; Cejudo-Martin, P.; Yotsumoto, F. NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression. Microcirculation 2016, 23, 122–133. [Google Scholar] [CrossRef]
- Stapor, P.C.; Sweat, R.S.; Dashti, D.C.; Betancourt, A.M.; Murfee, W.L. Pericyte dynamics during angiogenesis: New insights from new identities. J. Vasc. Res. 2014, 51, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.; Vastardis, H.; Mulliken, J.B.; Soo, C.; Tieu, A.; Do, H.; Kwong, E.; Bertolami, C.N.; Kawamoto, H.; Kuroda, S.; et al. Human NELL-1 expressed in unilateral coronal synostosis. J. Bone Miner. Res. 1999, 14, 80–89. [Google Scholar] [CrossRef]
- Aghaloo, T.; Cowan, C.M.; Chou, Y.F.; Zhang, X.; Lee, H.; Miao, S.; Hong, N.; Kuroda, S.; Wu, B.; Ting, K.; et al. Nell-1-induced bone regeneration in calvarial defects. Am. J. Pathol. 2006, 169, 903–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cowan, C.M.; Cheng, S.; Ting, K.; Soo, C.; Walder, B.; Wu, B.; Kuroda, S.; Zhang, X. Nell-1 induced bone formation within the distracted intermaxillary suture. Bone 2006, 38, 48–58. [Google Scholar] [CrossRef]
- Cowan, C.M.; Jiang, X.; Hsu, T.; Soo, C.; Zhang, B.; Wang, J.Z.; Kuroda, S.; Wu, B.; Zhang, Z.; Zhang, X.; et al. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J. Bone Miner. Res. 2007, 22, 918–930. [Google Scholar] [CrossRef]
- Lu, S.S.; Zhang, X.; Soo, C.; Hsu, T.; Napoli, A.; Aghaloo, T.; Wu, B.M.; Tsou, P.; Ting, K.; Wang, J.C. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007, 7, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Siu, R.K.; Lu, S.S.; Li, W.; Whang, J.; McNeill, G.; Zhang, X.; Wu, B.M.; Turner, A.S.; Seim, H.B., 3rd; Hoang, P.; et al. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng. Part. A 2011, 17, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Shen, J.; Liu, Y.; Chen, F.; Zheng, Z.; James, A.W.; Hsu, C.Y.; Zhang, H.; Lee, K.S.; Wang, C.; et al. Proliferation and osteogenic differentiation of mesenchymal stem cells induced by a short isoform of NELL-1. Stem Cells 2015, 33, 904–915. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Péault, B.; Chen, W.; Li, W.; Corselli, M.; James, A.W.; Lee, M.; Siu, R.K.; Shen, P.; Zheng, Z.; et al. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng. Part. A 2011, 17, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Shen, J.; Zhang, X.; Asatrian, G.; Goyal, R.; Kwak, J.H.; Jiang, L.; Bengs, B.; Culiat, C.T.; Turner, A.S.; et al. NELL-1 in the treatment of osteoporotic bone loss. Nat. Commun. 2015, 6, 7362. [Google Scholar] [CrossRef]
- James, A.W.; Zara, J.N.; Corselli, M.; Askarinam, A.; Zhou, A.M.; Hourfar, A.; Nguyen, A.; Megerdichian, S.; Asatrian, G.; Pang, S.; et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl. Med. 2012, 1, 673–684. [Google Scholar] [CrossRef]
- Blazquez, C.; Casanova, M.L.; Planas, A.; Gomez Del Pulgar, T.; Villanueva, C.; Fernandez-Acenero, M.J.; Aragones, J.; Huffman, J.W.; Jorcano, J.L.; Guzman, M. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003, 17, 529–531. [Google Scholar] [CrossRef]
- Lai, S.L.; Cheah, S.C.; Wong, P.F.; Noor, S.M.; Mustafa, M.R. In vitro and in vivo anti-angiogenic activities of Panduratin, A. PLoS ONE 2012, 7, e38103. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Xu, M.; et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chou, M.Y.; Jonas, M.; Tien, Y.T.; Chi, E.Y. A composite graft material containing bone particles and collagen in osteoinduction in mouse. J. Biomed. Mater. Res. 2002, 63, 65–70. [Google Scholar] [CrossRef]
- Wang, C.K.; Ho, M.L.; Wang, G.J.; Chang, J.K.; Chen, C.H.; Fu, Y.C.; Fu, H.H. Controlled-release of rhBMP-2 carriers in the regeneration of osteonecrotic bone. Biomaterials 2009, 30, 4178–4186. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Lee, S.; Ting, K.; Shen, J.; Wang, C.; Nguyen, A.; Berthiaume, E.A.; Zara, J.N.; Turner, A.S.; Seim, H.B., III; et al. Cyst-like osteolytic formations in recombinant human bone morphogenetic protein-2 (rhBMP-2) augmented sheep spinal fusion. Am. J. Path. 2017, 187, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.P.; Das, A.; Cui, Q. Osteonecrosis of the femoral head: An update in year 2012. World J. Orthop. 2012, 3, 49–57. [Google Scholar] [CrossRef]
- Malizos, K.N.; Quarles, L.D.; Seaber, A.V.; Rizk, W.S.; Urbaniak, J.R. An experimental canine model of osteonecrosis: Characterization of the repair process. J. Orthop. Res. 1993, 11, 350–357. [Google Scholar] [CrossRef]
- Kim, H.K.; Su, P.H. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. JBJS 2002, 84, 1329–1334. [Google Scholar] [CrossRef]
- Vélez, R.; Soldado, F.; Hernández, A.; Barber, I.; Aguirre, M. A new preclinical femoral head osteonecrosis model in sheep. Arch. Orthop. Trauma Surg. 2011, 131, 5–9. [Google Scholar] [CrossRef]
- Herrmann, M.; Bara, J.J.; Sprecher, C.M.; Menzel, U.; Jalowiec, J.M.; Osinga, R.; Scherberich, A.; Alini, M.; Verrier, S. Pericyte plasticity—Comparative investigation of the angiogenic and multilineage potential of pericytes from different human tissues. Eur. Cell Mater. 2016, 31, 236–249. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Rothe, L.; Bekker, S.; Anderson, F.; Huang, Y.; Osdoby, P. Basic fibroblast growth factor stimulates osteoclast recruitment, development, and bone pit resorption in association with angiogenesis in vivo on the chick chorioallantoic membrane and activates isolated avian osteoclast resorption in vitro. J. Bone Miner. Res. 2002, 17, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Patterson-Buckendahl, P.; Gospodarowicz, D. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor beta. Endocrinology 1988, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Andre, G.; Edwards, D.; Kim, H.K.W. A rat model of ischemic osteonecrosis for investigating local therapeutics using biomaterials. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
| Samples a | Sex/Age | Past Medical History | SVF Yield | SVF Viability | Ratio of Pericytes in SVF | Pericytes Yield |
|---|---|---|---|---|---|---|
| 1 | M/75 | Hypertension | 22.4 × 106 | 90.3% | 0.27 | 5.46 × 106 |
| 2 | M/68 | Diabetes mellitus | 25.1 × 106 | 85.2% | 0.22 | 4.70 × 106 |
| 3 | F/73 | None | 19.0 × 106 | 88.2% | 0.24 | 4.02 × 106 |
| 4 | F/82 | Hypercholesterolemia, Diabetes mellitus | 31.4 × 106 | 78.3% | 0.31 | 7.62 × 106 |
| 5 | F/66 | None | 29.2 × 106 | 79.2% | 0.22 | 5.09 × 106 |
| 6 | F/72 | None | 22.3 × 106 | 92.4% | 0.19 | 3.91 × 106 |
| 7 | F/65 | Hypertension, HBV carrier | 15.1 × 106 | 83.7% | 0.29 | 3.67 × 106 |
| 8 | F/70 | Hypertension | 17.6 × 106 | 87.9% | 0.23 | 3.56 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.-J.; Ko, K.R.; Baek, M.; Jeong, Y.; Lee, H.H.; Kim, H.; Kim, D.K.; Lee, S.-Y.; Lee, S. Pro-Angiogenic and Osteogenic Effects of Adipose Tissue-Derived Pericytes Synergistically Enhanced by Nel-like Protein-1. Cells 2021, 10, 2244. https://doi.org/10.3390/cells10092244
An H-J, Ko KR, Baek M, Jeong Y, Lee HH, Kim H, Kim DK, Lee S-Y, Lee S. Pro-Angiogenic and Osteogenic Effects of Adipose Tissue-Derived Pericytes Synergistically Enhanced by Nel-like Protein-1. Cells. 2021; 10(9):2244. https://doi.org/10.3390/cells10092244
Chicago/Turabian StyleAn, Hyun-Ju, Kyung Rae Ko, Minjung Baek, Yoonhui Jeong, Hyeon Hae Lee, Hyungkyung Kim, Do Kyung Kim, So-Young Lee, and Soonchul Lee. 2021. "Pro-Angiogenic and Osteogenic Effects of Adipose Tissue-Derived Pericytes Synergistically Enhanced by Nel-like Protein-1" Cells 10, no. 9: 2244. https://doi.org/10.3390/cells10092244
APA StyleAn, H.-J., Ko, K. R., Baek, M., Jeong, Y., Lee, H. H., Kim, H., Kim, D. K., Lee, S.-Y., & Lee, S. (2021). Pro-Angiogenic and Osteogenic Effects of Adipose Tissue-Derived Pericytes Synergistically Enhanced by Nel-like Protein-1. Cells, 10(9), 2244. https://doi.org/10.3390/cells10092244







