The Cytoskeleton and Its Roles in Self-Organization Phenomena: Insights from Xenopus Egg Extracts
Abstract
1. Introduction
2. Recapitulating Interphase Aster Growth and Expansion in Xenopus Egg Extracts
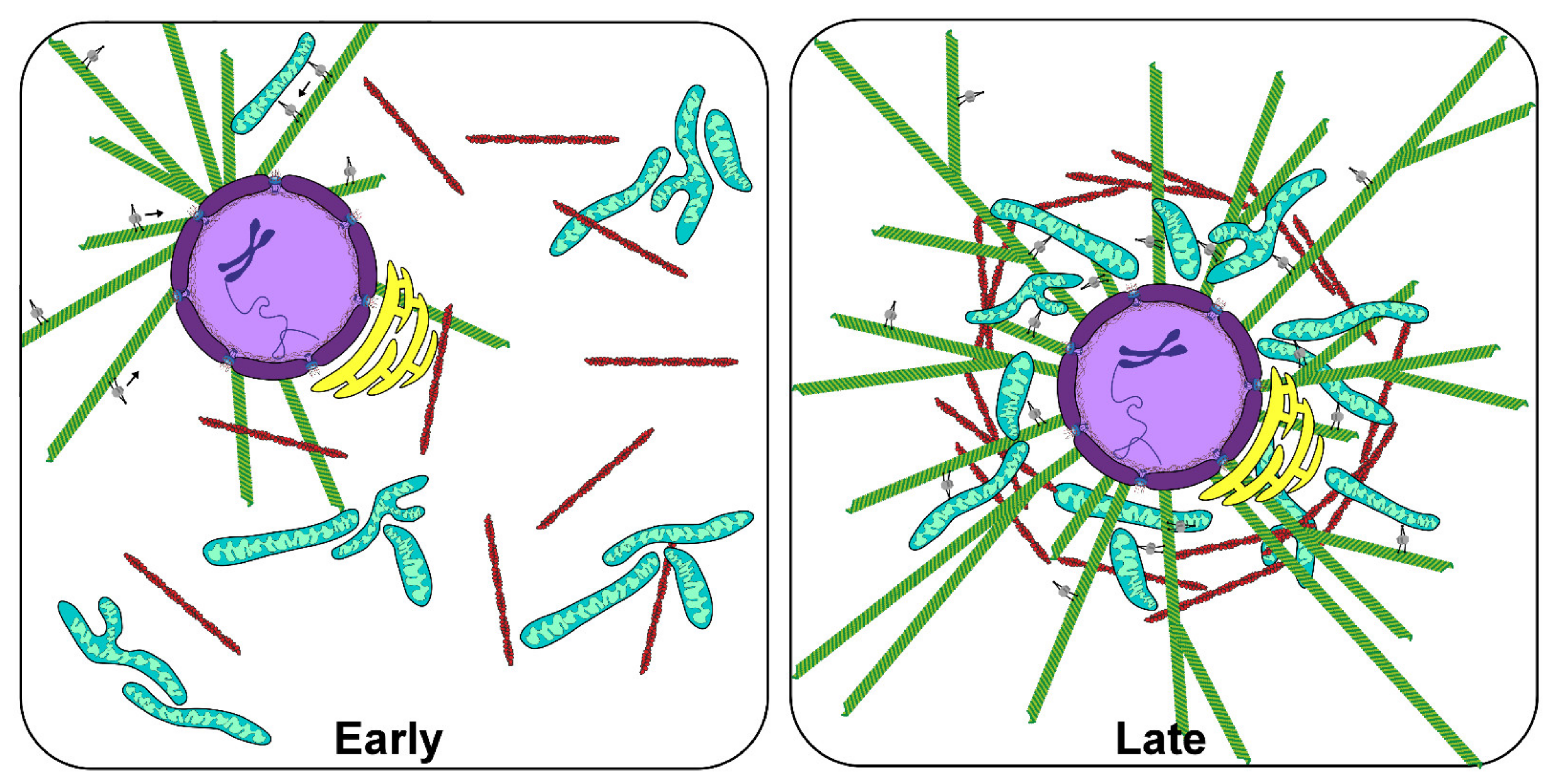
3. Aster Centration and Cell-Like Compartmentalization in Xenopus Egg Extracts
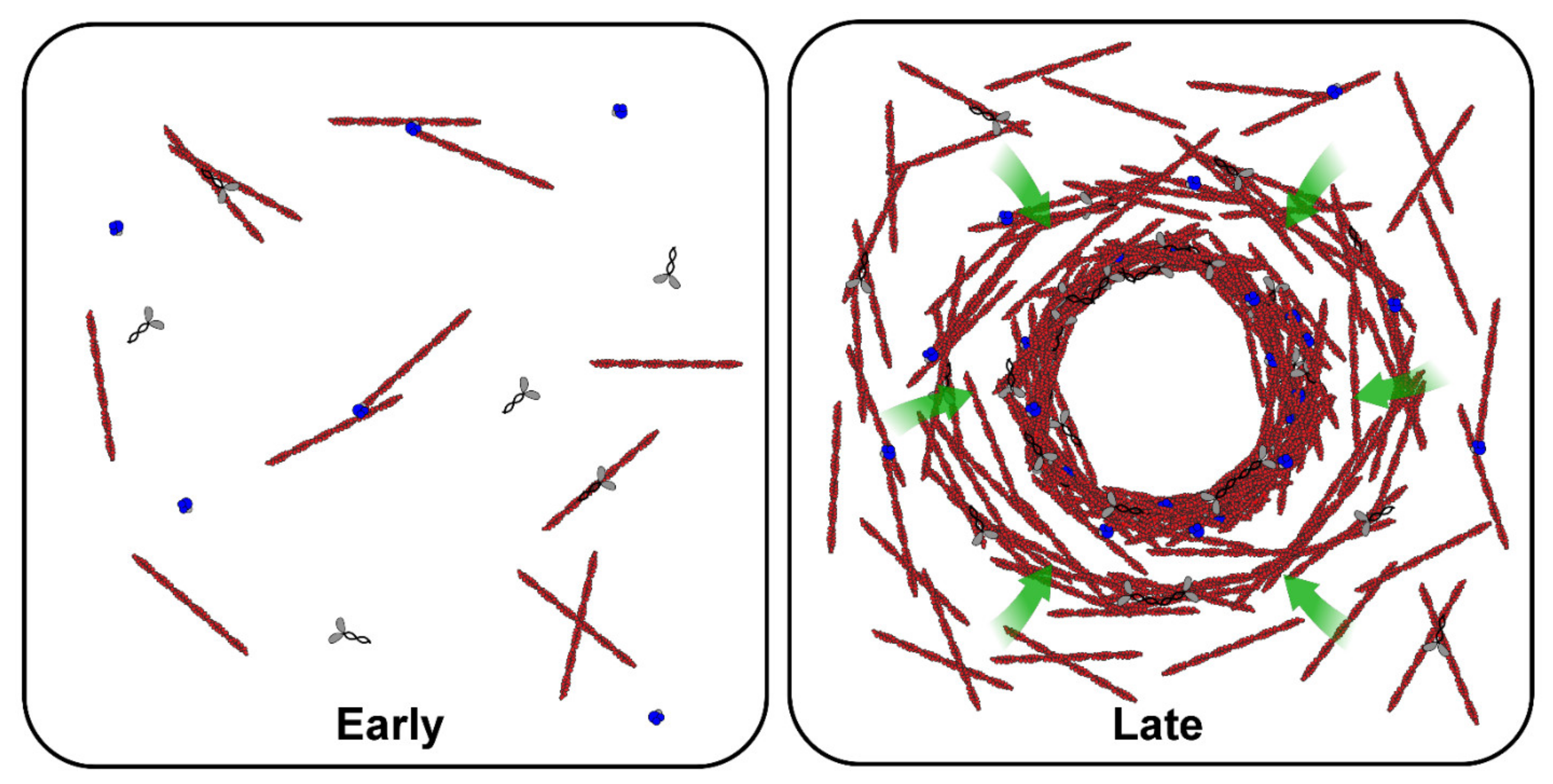
4. Studies of the Actomyosin Cytoskeleton in Bulk Xenopus Egg Extracts
5. Studies of the Actomyosin Cytoskeleton in Discrete Volumes of Xenopus Egg Extracts
6. Importance of Nuclear Lamina Assembly for Nuclear Processes and Envelope Integrity
7. The Roles of Nuclear Actin from Structuring the Nucleus to Regulating Chromatin States
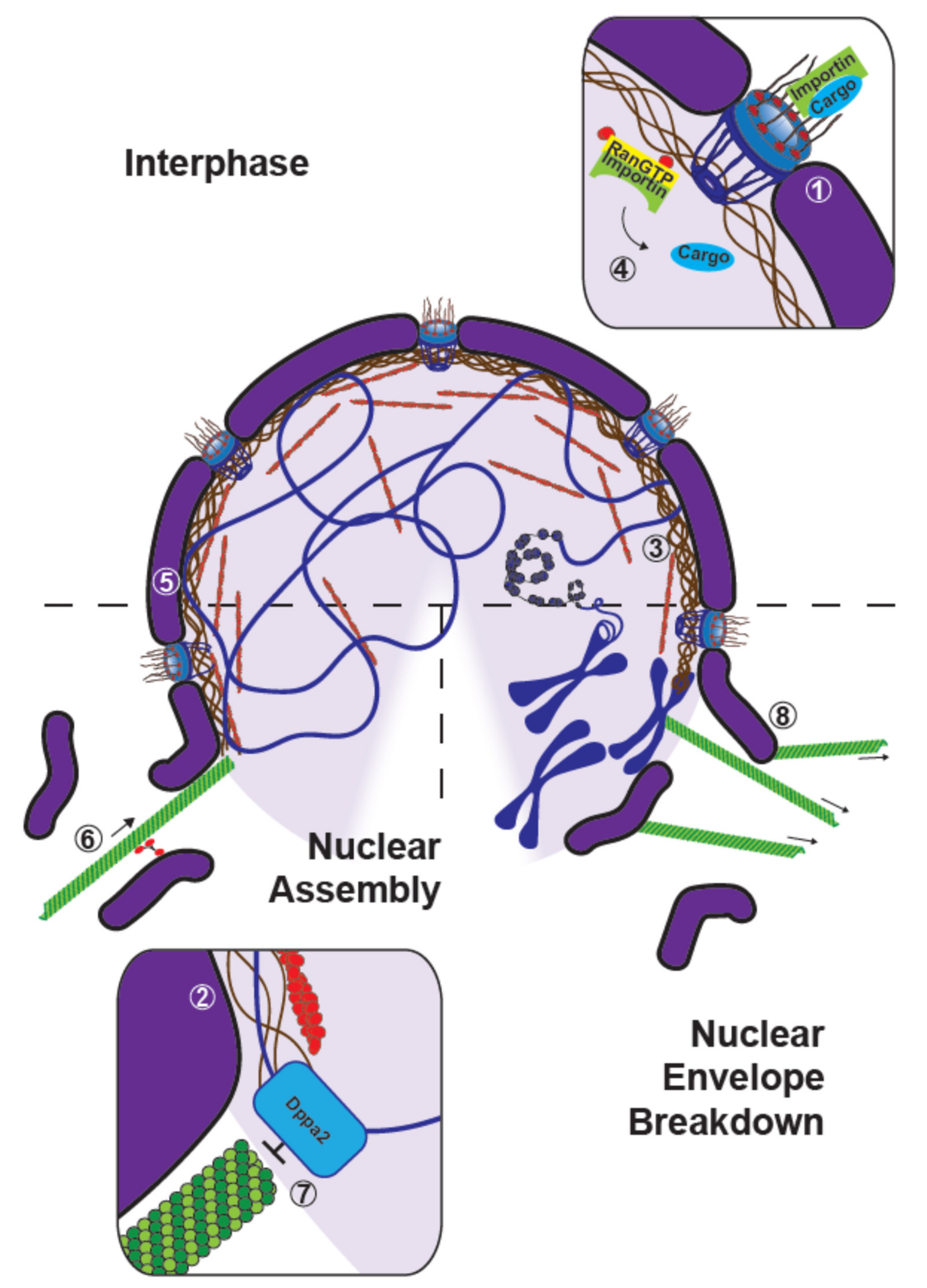
8. The Control of Nuclear Assembly and Disassembly by the Microtubule Cytoskeleton
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lohka, M.J.; Masui, Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science 1983, 220, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Lohka, M.J.; Maller, J.L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J. Cell Biol. 1985, 101, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Miake-Lye, R.; Kirschner, M.W. Induction of early mitotic events in a cell-free system. Cell 1985, 41, 165–175. [Google Scholar] [CrossRef]
- Murray, A.W.; Solomon, M.J.; Kirschner, M.W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 1989, 339, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W.; Kirschner, M.W. Cyclin synthesis drives the early embryonic cell cycle. Nature 1989, 339, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W. Cell cycle extracts. Methods Cell Biol. 1991, 36, 581–605. [Google Scholar] [PubMed]
- Blow, J.J.; Laskey, R.A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 1986, 47, 577–587. [Google Scholar] [CrossRef]
- Tuomikoski, T.; Felix, M.-A.; Dorée, M.; Gruenberg, J. Inhibition of endocytic vesicle fusion in vitro by the cell-cycle control protein kinase cdc2. Nature 1989, 342, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Glotzer, M.; Murray, A.W.; Kirschner, M.W. Cyclin is degraded by the ubiquitin pathway. Nature 1991, 349, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Belmont, L.D.; Hyman, A.A.; Sawin, K.E.; Mitchison, T.J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 1990, 62, 579–589. [Google Scholar] [CrossRef]
- Verde, F.; Berrez, J.M.; Antony, C.; Karsenti, E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs: Requirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 1991, 112, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Sawin, K.E.; Mitchison, T.J. Poleward microtubule flux in mitotic spindles assembled in vitro. J. Cell Biol. 1991, 112, 941–954. [Google Scholar] [CrossRef]
- Wuhr, M.; Freeman, R.M., Jr.; Presler, M.; Horb, M.E.; Peshkin, L.; Gygi, S.P.; Kirschner, M.W. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr. Biol. 2014, 24, 1467–1475. [Google Scholar] [CrossRef]
- Humphrey, D.; Duggan, C.; Saha, D.; Smith, D.; Käs, J. Active fluidization of polymer networks through molecular motors. Nature 2002, 416, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D.; Milligan, R.A. The Way Things Move: Looking Under the Hood of Molecular Motor Proteins. Science 2000, 288, 88–95. [Google Scholar] [CrossRef]
- Holy, T.E.; Dogterom, M.; Yurke, B.; Leibler, S. Assembly and positioning of microtubule asters in microfabricated chambers. Proc. Natl. Acad. Sci. USA 1997, 94, 6228–6231. [Google Scholar] [CrossRef]
- Papaseit, C.; Pochon, N.; Tabony, J. Microtubule self-organization is gravity-dependent. Proc. Natl. Acad. Sci. USA 2000, 97, 8364–8368. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, G.; Gibeaux, R. Mechanisms of spindle assembly and size control. Biol. Cell 2020, 112, 369–382. [Google Scholar] [CrossRef]
- Ishihara, K.; Nguyen, P.A.; Groen, A.C.; Field, C.M.; Mitchison, T.J. Microtubule nucleation remote from centrosomes may explain how asters span large cells. Proc. Natl. Acad. Sci. USA 2014, 111, 17715–17722. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Groen, A.C.; Loose, M.; Ishihara, K.; Wuhr, M.; Field, C.M.; Mitchison, T.J. Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Science 2014, 346, 244–247. [Google Scholar] [CrossRef]
- Geisterfer, Z.M.; Zhu, D.Y.; Mitchison, T.J.; Oakey, J.; Gatlin, J.C. Microtubule Growth Rates Are Sensitive to Global and Local Changes in Microtubule Plus-End Density. Curr. Biol. 2020, 30, 3016–3023.e3. [Google Scholar] [CrossRef]
- Cheng, X.; Ferrell, J.E., Jr. Spontaneous emergence of cell-like organization in Xenopus egg extracts. Science 2019, 366, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.F.; Field, C.M.; Furthauer, S.; Sonnett, M.; Mitchison, T.J. Co-movement of astral microtubules, organelles and F-actin by dynein and actomyosin forces in frog egg cytoplasm. eLife 2020, 9, e60047. [Google Scholar] [CrossRef] [PubMed]
- Mühlhäusser, P.; Kutay, U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J. Cell Biol. 2007, 178, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Z.; Woo, E.M.; Postow, L.; Chait, B.T.; Funabiki, H. Chromatin-bound Xenopus dppa2 shapes the nucleus by locally inhibiting microtubule assembly. Dev. Cell 2013, 27, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Merten, C.A. Dynein-Based Accumulation of Membranes Regulates Nuclear Expansion in Xenopus laevis Egg Extracts. Dev. Cell 2015, 33, 562–575. [Google Scholar] [CrossRef]
- Krauss, S.W.; Heald, R.; Lee, G.; Nunomura, W.; Gimm, J.A.; Mohandas, N.; Chasis, J.A. Two distinct domains of protein 4.1 critical for assembly of functional nuclei in vitro. J. Biol. Chem. 2002, 277, 44339–44346. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.W.; Chen, C.; Penman, S.; Heald, R. Nuclear actin and protein 4.1: Essential interactions during nuclear assembly in vitro. Proc. Natl. Acad. Sci. USA 2003, 100, 10752–10757. [Google Scholar] [CrossRef]
- Waterman-Storer, C.; Duey, D.Y.; Weber, K.L.; Keech, J.; Cheney, R.E.; Salmon, E.D.; Bement, W.M. Microtubules remodel actomyosin networks in Xenopus egg extracts via two mechanisms of F-actin transport. J. Cell Biol. 2000, 150, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Field, C.M.; Wuhr, M.; Anderson, G.A.; Kueh, H.Y.; Strickland, D.; Mitchison, T.J. Actin behavior in bulk cytoplasm is cell cycle regulated in early vertebrate embryos. J. Cell Sci. 2011, 124, 2086–2095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinot, M.; Steiner, V.; Dehapiot, B.; Yoo, B.K.; Chesnel, F.; Blanchoin, L.; Kervrann, C.; Gueroui, Z. Confinement induces actin flow in a meiotic cytoplasm. Proc. Natl. Acad. Sci. USA 2012, 109, 11705–11710. [Google Scholar] [CrossRef] [PubMed]
- Malik-Garbi, M.; Ierushalmi, N.; Jansen, S.; Abu-Shah, E.; Goode, B.L.; Mogilner, A.; Keren, K. Scaling behaviour in steady-state contracting actomyosin networks. Nat. Phys. 2019, 15, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ierushalmi, N.; Malik-Garbi, M.; Manhart, A.; Shah, E.A.; Goode, B.L.; Mogilner, A.; Keren, K. Centering and symmetry breaking in confined contracting actomyosin networks. eLife 2020, 9, e55368. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Tanabe, M.; Hiraiwa, T.; Suzuki, K.; Ishiwata, S.; Maeda, Y.T.; Miyazaki, M. Tug-of-war between actomyosin-driven antagonistic forces determines the positioning symmetry in cell-sized confinement. Nat. Commun. 2020, 11, 3063. [Google Scholar] [CrossRef]
- Newport, J. Nuclear reconstitution in vitro: Stages of assembly around protein-free DNA. Cell 1987, 48, 205–217. [Google Scholar] [CrossRef]
- Lourim, D.; Krohne, G. Membrane-associated lamins in Xenopus egg extracts: Identification of two vesicle populations. J. Cell Biol. 1993, 123, 501–512. [Google Scholar] [CrossRef]
- Lourim, D.; Kempf, A.; Krohne, G. Characterization and quantitation of three B-type lamins in Xenopus oocytes and eggs: Increase of lamin LI protein synthesis during meiotic maturation. J. Cell Sci. 1996, 109, 1775–1785. [Google Scholar] [CrossRef]
- Lourim, D.; Krohne, G. Lamin-dependent nuclear envelope reassembly following mitosis: An argument. Trends Cell Biol. 1994, 4, 314–318. [Google Scholar] [CrossRef]
- Goldberg, M.W.; Allen, T.D. Structural and functional organization of the nuclear envelope. Curr. Opin. Cell Biol. 1995, 7, 301–309. [Google Scholar] [CrossRef]
- Smythe, C.; Jenkins, H.E.; Hutchison, C.J. Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: Evidence from cell-free extracts of Xenopus eggs. EMBO J. 2000, 19, 3918–3931. [Google Scholar] [CrossRef] [PubMed]
- Spann, T.P.; Moir, R.D.; Goldman, A.E.; Stick, R.; Goldman, R.D. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 1997, 136, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Moir, R.D.; Spann, T.P.; Herrmann, H.; Goldman, R.D. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J. Cell Biol. 2000, 149, 1179–1191. [Google Scholar] [CrossRef]
- Lopez-Soler, R.I.; Moir, R.D.; Spann, T.P.; Stick, R.; Goldman, R.D. A role for nuclear lamins in nuclear envelope assembly. J. Cell Biol. 2001, 154, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.L.; Heald, R. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell 2010, 143, 288–298. [Google Scholar] [CrossRef]
- Edens, L.J.; Dilsaver, M.R.; Levy, D.L. PKC-mediated phosphorylation of nuclear lamins at a single serine residue regulates interphase nuclear size in Xenopus and mammalian cells. Mol. Biol. Cell 2017, 28, 1389–1399. [Google Scholar] [CrossRef]
- Field, C.M.; Pelletier, J.F.; Mitchison, T.J. Disassembly of Actin and Keratin Networks by Aurora B Kinase at the Midplane of Cleaving Xenopus laevis Eggs. Curr. Biol. 2019, 29, 1999–2008.e4. [Google Scholar] [CrossRef]
- Weber, K.L.; Bement, W.M. F-actin serves as a template for cytokeratin organization in cell free extracts. J. Cell Sci. 2002, 115, 1373–1382. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Zheng, Y. Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr. Biol. 2005, 15, 2156–2163. [Google Scholar] [CrossRef]
- Wuhr, M.; Chen, Y.; Dumont, S.; Groen, A.C.; Needleman, D.J.; Salic, A.; Mitchison, T.J. Evidence for an upper limit to mitotic spindle length. Curr. Biol. 2008, 18, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, B.R. Microtubule Organizing Centers. Annu. Rev. Cell Biol. 1985, 1, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Nguyen, P.A.; Wuhr, M.; Groen, A.C.; Field, C.M.; Mitchison, T.J. Organization of early frog embryos by chemical waves emanating from centrosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130454. [Google Scholar] [CrossRef]
- Ishihara, K.; Korolev, K.S.; Mitchison, T.J. Physical basis of large microtubule aster growth. eLife 2016, 5, e19145. [Google Scholar] [CrossRef] [PubMed]
- Lüders, J.; Stearns, T. Microtubule-organizing centres: A re-evaluation. Nat. Rev. Mol. Cell Biol. 2007, 8, 161–167. [Google Scholar] [CrossRef]
- Petry, S.; Groen, A.C.; Ishihara, K.; Mitchison, T.J.; Vale, R.D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 2013, 152, 768–777. [Google Scholar] [CrossRef]
- Alfaro-Aco, R.; Thawani, A.; Petry, S. Structural analysis of the role of TPX2 in branching microtubule nucleation. J. Cell Biol. 2017, 216, 983–997. [Google Scholar] [CrossRef]
- Alfaro-Aco, R.; Thawani, A.; Petry, S. Biochemical reconstitution of branching microtubule nucleation. eLife 2020, 9, e49797. [Google Scholar] [CrossRef]
- Thawani, A.; Stone, H.A.; Shaevitz, J.W.; Petry, S. Spatiotemporal organization of branched microtubule networks. eLife 2019, 8, e43890. [Google Scholar] [CrossRef]
- Wittmann, T.; Wilm, M.; Karsenti, E.; Vernos, I. TPX2, A novel xenopus MAP involved in spindle pole organization. J. Cell Biol. 2000, 149, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Wuhr, M.; Guttler, T.; Peshkin, L.; McAlister, G.C.; Sonnett, M.; Ishihara, K.; Groen, A.C.; Presler, M.; Erickson, B.K.; Mitchison, T.J.; et al. The Nuclear Proteome of a Vertebrate. Curr. Biol. 2015, 25, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- McNally, F.J. Mechanisms of spindle positioning. J. Cell Biol. 2013, 200, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Rieckhoff, E.M.; Berndt, F.; Elsner, M.; Golfier, S.; Decker, F.; Ishihara, K.; Brugues, J. Spindle Scaling Is Governed by Cell Boundary Regulation of Microtubule Nucleation. Curr. Biol. 2020, 30, 4973–4983.e10. [Google Scholar] [CrossRef] [PubMed]
- Dinarina, A.; Pugieux, C.; Corral, M.M.; Loose, M.; Spatz, J.; Karsenti, E.; Nedelec, F. Chromatin shapes the mitotic spindle. Cell 2009, 138, 502–513. [Google Scholar] [CrossRef]
- Reinsch, S.; Gonczy, P. Mechanisms of nuclear positioning. J. Cell Sci. 1998, 111, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Wuhr, M.; Dumont, S.; Groen, A.C.; Needleman, D.J.; Mitchison, T.J. How does a millimeter-sized cell find its center? Cell Cycle 2009, 8, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Sulerud, T.; Sami, A.B.; Li, G.; Kloxin, A.; Oakey, J.; Gatlin, J. Microtubule-dependent pushing forces contribute to long-distance aster movement and centration in Xenopus laevis egg extracts. Mol. Biol. Cell 2020, 31, 2791–2802. [Google Scholar] [CrossRef]
- Meaders, J.L.; de Matos, S.N.; Burgess, D.R. A Pushing Mechanism for Microtubule Aster Positioning in a Large Cell Type. Cell Rep. 2020, 33, 108213. [Google Scholar] [CrossRef]
- Mitchison, T.; Wuhr, M.; Nguyen, P.; Ishihara, K.; Groen, A.; Field, C.M. Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells. Cytoskeleton 2012, 69, 738–750. [Google Scholar] [CrossRef]
- Rappaport, R.; Rappaport, B.N. Cleavage in conical sand dollar eggs. Dev. Biol. 1994, 164, 258–266. [Google Scholar] [CrossRef]
- Condeelis, J.S.; Taylor, D.L. The contractile basis of amoeboid movement: V. The control of gelation, solation, and contraction in extracts from Dictyostelium discoideum. J. Cell Biol. 1977, 74, 901–927. [Google Scholar] [CrossRef]
- Kane, R.E. Actin polymerization and interaction with other proteins in temperature-induced gelation of sea urchin egg extracts. J. Cell Biol. 1976, 71, 704–714. [Google Scholar] [CrossRef]
- Pollard, T.D. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J. Cell Biol. 1976, 68, 579–601. [Google Scholar] [CrossRef]
- Elinson, R.P. Cytoplasmic phases in the first cell cycle of the activated frog egg. Dev. Biol. 1983, 100, 440–451. [Google Scholar] [CrossRef]
- Clark, T.G.; Merriam, R.W. Actin in Xenopus oocytes. J. Cell Biol. 1978, 77, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Thery, M.; Racine, V.; Piel, M.; Pepin, A.; Dimitrov, A.; Chen, Y.; Sibarita, J.B.; Bornens, M. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl. Acad. Sci. USA 2006, 103, 19771–19776. [Google Scholar] [CrossRef] [PubMed]
- Minc, N.; Burgess, D.; Chang, F. Influence of cell geometry on division-plane positioning. Cell 2011, 144, 414–426. [Google Scholar] [CrossRef]
- Good, M.C.; Vahey, M.D.; Skandarajah, A.; Fletcher, D.A.; Heald, R. Cytoplasmic volume modulates spindle size during embryogenesis. Science 2013, 342, 856–860. [Google Scholar] [CrossRef]
- Hazel, J.; Krutkramelis, K.; Mooney, P.; Tomschik, M.; Gerow, K.; Oakey, J.; Gatlin, J.C. Changes in cytoplasmic volume are sufficient to drive spindle scaling. Science 2013, 342, 853–856. [Google Scholar] [CrossRef]
- Pinot, M.; Chesnel, F.; Kubiak, J.Z.; Arnal, I.; Nedelec, F.J.; Gueroui, Z. Effects of confinement on the self-organization of microtubules and motors. Curr. Biol. 2009, 19, 954–960. [Google Scholar] [CrossRef]
- Colin, A.; Singaravelu, P.; Thery, M.; Blanchoin, L.; Gueroui, Z. Actin-Network Architecture Regulates Microtubule Dynamics. Curr. Biol. 2018, 28, 2647–2656.e4. [Google Scholar] [CrossRef]
- Brownlee, C.; Heald, R. Importin alpha Partitioning to the Plasma Membrane Regulates Intracellular Scaling. Cell 2019, 176, 805–815.e8. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.D. The fine structure of the nuclear envelope of Amoeba proteus. J. Biophys Biochem. Cytol. 1956, 2, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Beams, H.W.; Tahmisian, T.N.; Devine, R.; Anderson, E. Ultrastructure of the nuclear membrane of a gregarine parasitic in grasshoppers. Exp. Cell Res. 1957, 13, 200–204. [Google Scholar] [CrossRef]
- McKeon, F.D.; Kirschner, M.W.; Caput, D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 1986, 319, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Newport, J.W.; Wilson, K.L.; Dunphy, W.G. A lamin-independent pathway for nuclear envelope assembly. J. Cell Biol. 1990, 111, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Krohne, G.; Dabauvalle, M.-C.; Franke, W.W. Cell type-specific differences in protein composition of nuclear pore complex-lamina structures in oocytes and erythrocytes of Xenopus laevis. J. Mol. Biol. 1981, 151, 121–141. [Google Scholar] [CrossRef]
- Heald, R.; Tournebize, R.; Blank, T.; Sandaltzopoulos, R.; Becker, P.; Hyman, A.; Karsenti, E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 1996, 382, 420–425. [Google Scholar] [CrossRef]
- Ellenberg, J.; Siggia, E.D.; Moreira, J.E.; Smith, C.L.; Presley, J.F.; Worman, H.J.; Lippincott-Schwartz, J. Nuclear membrane dynamics and reassembly in living cells: Targeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 1997, 138, 1193–1206. [Google Scholar] [CrossRef]
- Anderson, D.J.; Hetzer, M.W. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 2007, 9, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Hetzer, M.W. Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J. Cell Biol. 2008, 182, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Puhka, M.; Vihinen, H.; Joensuu, M.; Jokitalo, E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J. Cell Biol. 2007, 179, 895–909. [Google Scholar] [CrossRef]
- Goldberg, M.; Jenkins, H.; Allen, T.; Whitfield, W.G.F.; Hutchison, C.J. Xenopus lamin B3 has a direct role in the assembly of a replication competent nucleus: Evidence from cell-free egg extracts. J. Cell Sci. 1995, 108, 3451–3461. [Google Scholar] [CrossRef]
- Meier, J.; Campbell, K.H.S.; Ford, C.C.; Stick, R.; Hutchison, C.J. The role of lamin LIII in nuclear assembly and DNA replication, in cell-free extracts of Xenopus eggs. J. Cell Sci. 1991, 98, 271–279. [Google Scholar] [CrossRef]
- Gant, T.M.; Harris, C.A.; Wilson, K.L. Roles of LAP2 Proteins in Nuclear Assembly and DNA Replication: Truncated LAP2β Proteins Alter Lamina Assembly, Envelope Formation, Nuclear Size, and DNA Replication Efficiency in Xenopus laevis Extracts. J. Cell Biol. 1999, 144, 1083–1096. [Google Scholar] [CrossRef]
- O’Brien, L.L.; Wiese, C. TPX2 is required for postmitotic nuclear assembly in cell-free Xenopus laevis egg extracts. J. Cell Biol. 2006, 173, 685–694. [Google Scholar] [CrossRef]
- Adam, S.A.; Sengupta, K.; Goldman, R.D. Regulation of Nuclear Lamin Polymerization by Importin α. J. Biol. Chem. 2008, 283, 8462–8468. [Google Scholar] [CrossRef]
- Jevtic, P.; Edens, L.J.; Li, X.; Nguyen, T.; Chen, P.; Levy, D.L. Concentration-dependent Effects of Nuclear Lamins on Nuclear Size in Xenopus and Mammalian Cells. J. Biol. Chem. 2015, 290, 27557–27571. [Google Scholar] [CrossRef] [PubMed]
- Serebryannyy, L.; de Lanerolle, P. Nuclear actin: The new normal. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2020, 821, 111714. [Google Scholar] [CrossRef] [PubMed]
- Kelpsch, D.J.; Tootle, T.L. Nuclear Actin: From Discovery to Function. Anat. Rec. 2018, 301, 1999–2013. [Google Scholar] [CrossRef]
- Rungger, D.; Rungger-Brändle, E.; Chaponnier, C.; Gabbiani, G. Intranuclear injection of anti-actin antibodies into Xenopus oocytes blocks chromosome condensation. Nature 1979, 282, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, M.T.; Stüven, T.; Kuhn, C.; Cordes, V.C.; Görlich, D. A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nat. Cell Biol. 2006, 8, 257–263. [Google Scholar] [CrossRef]
- Feric, M.; Brangwynne, C.P. A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat. Cell Biol. 2013, 15, 1253–1259. [Google Scholar] [CrossRef]
- Oda, H.; Shirai, N.; Ura, N.; Ohsumi, K.; Iwabuchi, M. Chromatin tethering to the nuclear envelope by nuclear actin filaments: A novel role of the actin cytoskeleton in the Xenopus blastula. Genes Cells Devoted Mol. Cell. Mech. 2017, 22, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Parisis, N.; Krasinska, L.; Harker, B.; Urbach, S.; Rossignol, M.; Camasses, A.; Dewar, J.; Morin, N.; Fisher, D. Initiation of DNA replication requires actin dynamics and formin activity. EMBO J. 2017, 36, 3212–3231. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Zünkler, C.; Lourim, D.; Dabauvalle, M.C. Microtubule-dependent assembly of the nuclear envelope in Xenopus laevis egg extract. Eur. J. Cell Biol. 2001, 80, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Cordes, V.C.; Reidenbach, S.; Franke, W.W. Cytoplasmic annulate lamellae in cultured cells: Composition, distribution, and mitotic behavior. Cell Tissue Res. 1996, 284, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Kossner, U.; Scheer, U.; Dabauvalle, M.C. A biochemical and immunological comparison of nuclear and cytoplasmic pore complexes. J. Cell Sci. 1996, 109, 1813–1824. [Google Scholar] [CrossRef]
- Schlaitz, A.L.; Thompson, J.; Wong, C.C.; Yates, J.R., 3rd; Heald, R. REEP3/4 Ensure Endoplasmic Reticulum Clearance from Metaphase Chromatin and Proper Nuclear Envelope Architecture. Dev. Cell 2013, 26, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Rosselló, C.A.; Lindström, L.; Glindre, J.; Eklund, G.; Alvarado-Kristensson, M. Gamma-tubulin coordinates nuclear envelope assembly around chromatin. Heliyon 2016, 2, e00166. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisterfer, Z.M.; Guilloux, G.; Gatlin, J.C.; Gibeaux, R. The Cytoskeleton and Its Roles in Self-Organization Phenomena: Insights from Xenopus Egg Extracts. Cells 2021, 10, 2197. https://doi.org/10.3390/cells10092197
Geisterfer ZM, Guilloux G, Gatlin JC, Gibeaux R. The Cytoskeleton and Its Roles in Self-Organization Phenomena: Insights from Xenopus Egg Extracts. Cells. 2021; 10(9):2197. https://doi.org/10.3390/cells10092197
Chicago/Turabian StyleGeisterfer, Zachary M., Gabriel Guilloux, Jesse C. Gatlin, and Romain Gibeaux. 2021. "The Cytoskeleton and Its Roles in Self-Organization Phenomena: Insights from Xenopus Egg Extracts" Cells 10, no. 9: 2197. https://doi.org/10.3390/cells10092197
APA StyleGeisterfer, Z. M., Guilloux, G., Gatlin, J. C., & Gibeaux, R. (2021). The Cytoskeleton and Its Roles in Self-Organization Phenomena: Insights from Xenopus Egg Extracts. Cells, 10(9), 2197. https://doi.org/10.3390/cells10092197





