Platelets, Not an Insignificant Player in Development of Allergic Asthma
Abstract
1. Introduction
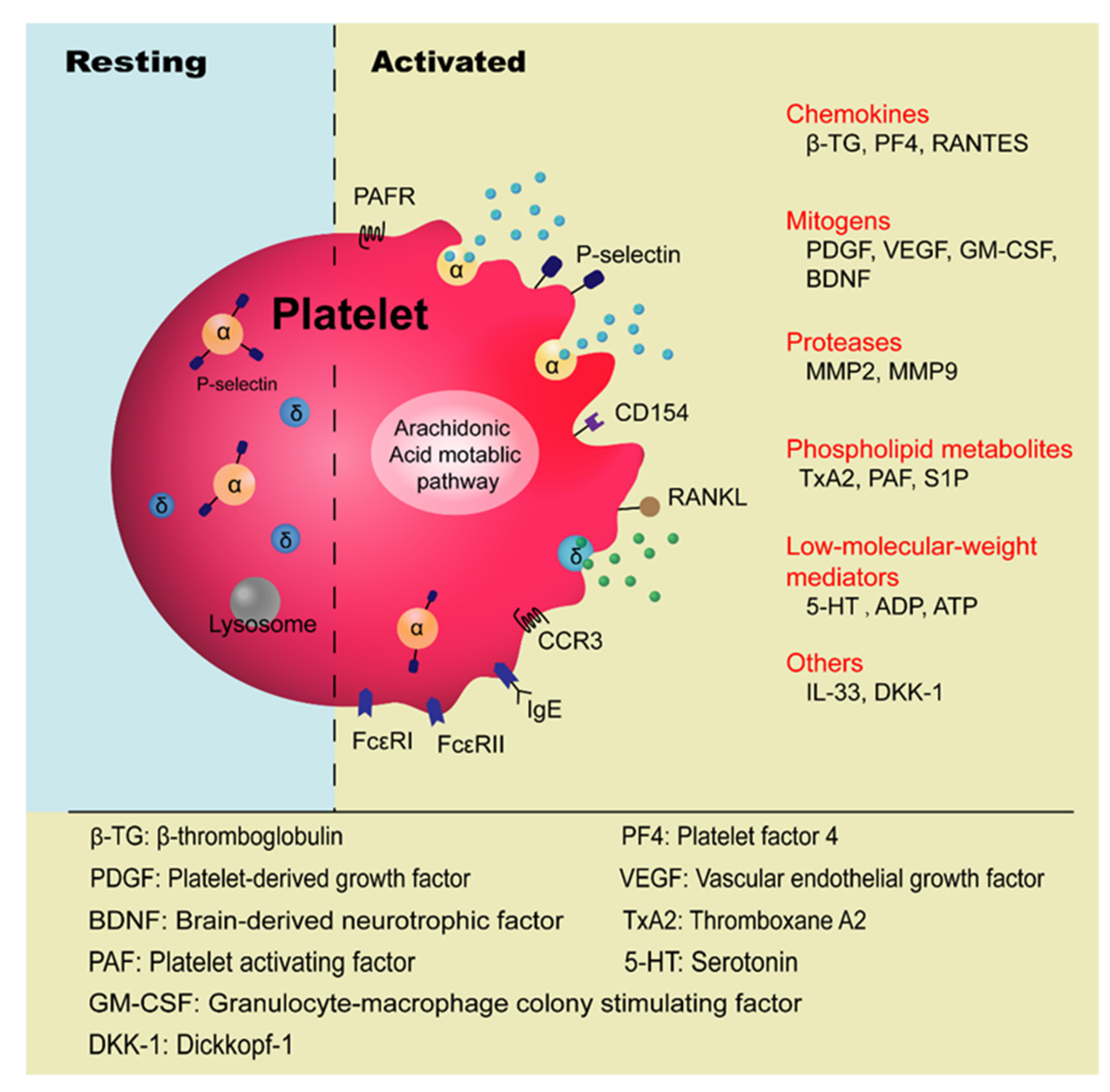
2. Current Understanding of Platelets’ Involvement in Allergic Asthma
3. Mechanisms of Platelets’ Role in the Pathogenesis of Allergic Asthma
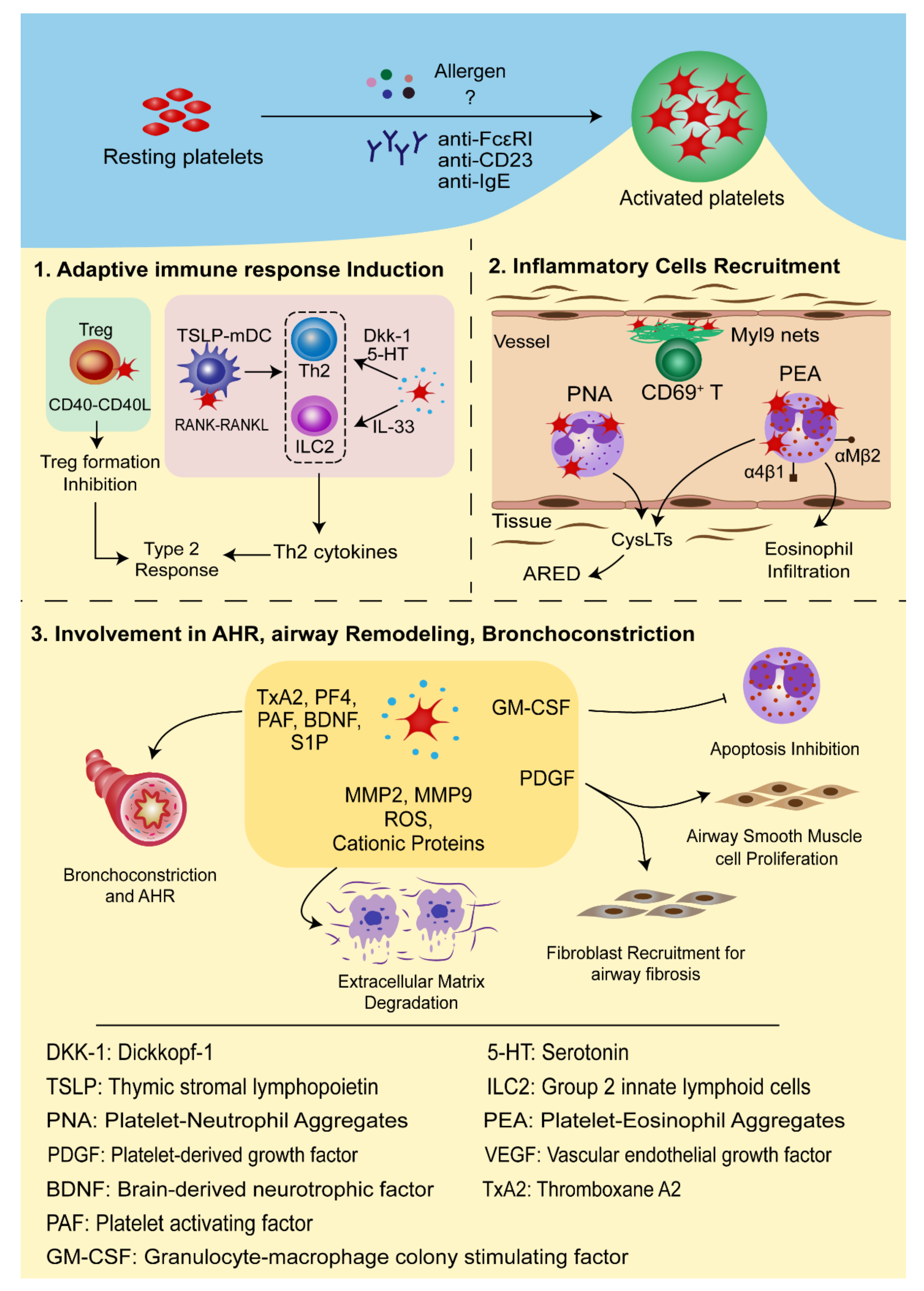
3.1. Platelets in Adaptive Immune Response Induction
3.2. Platelets in the Recruitment of Inflammatory Cells
3.3. The Roles of Platelets in Airway Hyperresponsiveness (AHR), Airway Remodeling, and Bronchoconstriction
4. Antiplatelet Therapies for Asthma Control
5. New Insights of Platelets in Asthma
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-LO | 5-Lipoxygenase |
| 5-HT | Serotonin |
| AHR | Airway hyperresponsiveness |
| AERD | Aspirin-exacerbated respiratory disease |
| AMs | Alveolar macrophages |
| APCs | Antigen-presenting cells |
| BALF | Bronchoalveolar lavage fluid |
| BDNF | Brain-derived neurotrophic factor |
| COX | Cyclooxygenase |
| cysLTs | Cysteinyl leukotriene |
| DCs | Dendritic cells |
| DKK-1 | Dickkopf-1 |
| DTS | Dense tubular system |
| Dp | Dermatophagoides pteronysisnus |
| ECP | Eosinophil cationic protein |
| ECs | Enterochromaffin cells |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GPCRs | G-protein-couple receptors |
| HDM | House dust mite |
| ILC2 | Group 2 innate lymphoid cells |
| MIP-1α | Macrophage inflammatory protein-1α |
| MKs | Megakaryocytes |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OCS | Open canalicular system |
| OVA | Ovalbumin |
| PAF | Platelet-activating factor |
| PDGF | Platelet-derived growth factor |
| PEA | Platelet-eosinophil aggregate |
| PF4 | Platelet factor 4 |
| PGs | Prostaglandins |
| PGE2 | Prostaglandin E2 |
| PMPs | Platelet-derived microparticles |
| PNA | Platelet-neutrophil aggregate |
| RANKL | RANK ligand |
| S1P | Sphingosine-1-phosphate |
| TDI | Toluene diisocyanate |
| TP receptor | Thromboxane-prostanoid receptor |
| TSLP | Thymic stromal lymphopoietin |
| TSLP-mDC | TSLP-stimulated myeloid DCs |
| TxA2 | Thromboxane A2 |
| VEGF | Vascular endothelial growth factor |
| β-TG | β-thromboglobulin |
References
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef]
- Radermecker, C.; Louis, R.; Bureau, F.; Marichal, T. Role of neutrophils in allergic asthma. Curr. Opin. Immunol. 2018, 54, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.S.; Pavlidis, S.; Loza, M.; Baribaud, F.; Rowe, A.; Pandis, I.; Hoda, U.; Rossios, C.; Sousa, A.; Wilson, S.J.; et al. A Transcriptome-driven Analysis of Epithelial Brushings and Bronchial Biopsies to Define Asthma Phenotypes in U-BIOPRED. Am. J. Respir. Crit. Care Med. 2017, 195, 443–455. [Google Scholar] [CrossRef]
- Agache, I.; Rogozea, L. Endotypes in allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 177–183. [Google Scholar] [CrossRef]
- Thon, J.N.; Italiano, J.E. Platelets: Production, morphology and ultrastructure. In Antiplatelet Agents. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. [Google Scholar] [CrossRef]
- Pitchford, S.C. Defining a role for platelets in allergic inflammation. Biochem. Soc. Trans. 2007, 35, 1104–1108. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Katoh, N.; Ueda, E.; Masuda, K.; Kishimoto, S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: Increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol. Int. 2008, 57, 391–396. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Boyce, J.A. Platelets in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2015, 135, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Pitchford, S.; Page, C. Role of platelets in allergic airway inflammation. J. Allergy Clin. Immunol. 2015, 135, 1416–1423. [Google Scholar] [CrossRef]
- Morianos, I.; Semitekolou, M. Dendritic Cells: Critical Regulators of Allergic Asthma. Int. J. Mol. Sci. 2020, 21, 7930. [Google Scholar] [CrossRef]
- Khan, D.A. Allergic rhinitis and asthma: Epidemiology and common pathophysiology. Allergy Asthma Proc. 2014, 35, 357–361. [Google Scholar] [CrossRef]
- Schatz, M.; Rosenwasser, L. The allergic asthma phenotype. J. Allergy Clin. Immunology. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, Y.; He, Q.; Chen, Y.; Wang, D.; Sun, W.; He, Y.; Zou, Z.; Liang, Z.; Chen, R.; et al. Toll-like Receptor 4 Deficiency Aggravates Airway Hyperresponsiveness and Inflammation by Impairing Neutrophil Apoptosis in a Toluene Diisocyanate-Induced Murine Asthma Model. Allergy Asthma Immunol. Res. 2020, 12, 608–625. [Google Scholar] [CrossRef]
- Bazan-Socha, S.; Kuczia, P.; Potaczek, D.P.; Mastalerz, L.; Cybulska, A.; Zareba, L.; Kremers, R.; Hemker, C.; Undas, A. Increased blood levels of cellular fibronectin in asthma: Relation to the asthma severity, inflammation, and prothrombotic blood alterations. Respir. Med. 2018, 141, 64–71. [Google Scholar] [CrossRef]
- De Boer, J.D.; Majoor, C.J.; Van ’t Veer, C.; Bel, E.H.; Van der Poll, T. Asthma and coagulation. Blood 2012, 119, 3236–3244. [Google Scholar] [CrossRef]
- Nastałek, M.; Potaczek, D.P.; Wojas-Pelc, A.; Undas, A. Plasma platelet activation markers in patients with atopic dermatitis and concomitant allergic diseases. J. Dermatol. Sci. 2011, 64, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Pampuch, A.; Kowal-Bielecka, O.; DuBuske, L.M.; Bodzenta-Lukaszyk, A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2006, 36, 426–432. [Google Scholar] [CrossRef]
- Metzger, W.J.; Sjoerdsma, K.; Richerson, H.B.; Moseley, P.; Zavala, D.; Monick, M.; Hunninghake, G.W. Platelets in bronchoalveolar lavage from asthmatic patients and allergic rabbits with allergen-induced late phase responses. Agents Actions Suppl. 1987, 21, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Averill, F.J.; Hubbard, W.C.; Proud, D.; Gleich, G.J.; Liu, M.C. Platelet activation in the lung after antigen challenge in a model of allergic asthma. Am. Rev. Respir. Dis 1992, 145, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, S.C.; Riffo-Vasquez, Y.; Sousa, A.; Momi, S.; Gresele, P.; Spina, D.; Page, C.P. Platelets are necessary for airway wall remodeling in a murine model of chronic allergic inflammation. Blood 2004, 103, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Tutluoglu, B.; Gurel, C.B.; Ozdas, S.B.; Musellim, B.; Erturan, S.; Anakkaya, A.N.; Kilinc, G.; Ulutin, T. Platelet function and fibrinolytic activity in patients with bronchial asthma. Clin. Appl. Thromb. Hemost. 2005, 11, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, S.C.; Yano, H.; Lever, R.; Riffo-Vasquez, Y.; Ciferri, S.; Rose, M.J.; Giannini, S.; Momi, S.; Spina, D.; O’Connor, B.; et al. Platelets are essential for leukocyte recruitment in allergic inflammation. J. Allergy Clin. Immunol. 2003, 112, 109–118. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Rogala, B. Seasonal changes in platelet activity in pollen-induced seasonal allergic rhinitis and asthma. J. Asthma Off. J. Assoc. Care Asthma 2008, 45, 485–487. [Google Scholar] [CrossRef]
- Benton, A.S.; Kumar, N.; Lerner, J.; Wiles, A.A.; Foerster, M.; Teach, S.J.; Freishtat, R.J. Airway platelet activation is associated with airway eosinophilic inflammation in asthma. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2010, 58, 987–990. [Google Scholar] [CrossRef]
- Johansson, M.W.; Han, S.T.; Gunderson, K.A.; Busse, W.W.; Jarjour, N.N.; Mosher, D.F. Platelet activation, P-selectin, and eosinophil beta1-integrin activation in asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.W.; Mosher, D.F. Activation of beta1 integrins on blood eosinophils by P-selectin. Am. J. Respir. Cell Mol. Biol. 2011, 45, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Taveira-Gomes, T.; Sokhatska, O.; Palmares, C.; Costa, R.; Negrao, R.; Guimaraes, J.T.; Delgado, L.; Soares, R.; Moreira, A. Increased circulating platelet microparticles as a potential biomarker in asthma. Allergy 2013, 68, 1073–1075. [Google Scholar] [CrossRef]
- Capron, M.; Jouault, T.; Prin, L.; Joseph, M.; Ameisen, J.C.; Butterworth, A.E.; Papin, J.P.; Kusnierz, J.P.; Capron, A. Functional study of a monoclonal antibody to IgE Fc receptor (Fc epsilon R2) of eosinophils, platelets, and macrophages. J. Exp. Med. 1986, 164, 72–89. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Schloetcke, K.; Klotz, J.; Schuhbaeck, K.; Zingler, D.; Zingler, C.; Schulte-Herbruggen, O.; Gill, H.; Schuff-Werner, P.; Virchow, J.C. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Zebrowska, E.; Chabowski, A. Altered Sphingolipid Metabolism Is Associated With Asthma Phenotype in House Dust Mite-Allergic Patients. Allergy Asthma Immunol. Res. 2019, 11, 330–342. [Google Scholar] [CrossRef]
- Hasegawa, S.; Pawankar, R.; Suzuki, K.; Nakahata, T.; Furukawa, S.; Okumura, K.; Ra, C. Functional expression of the high affinity receptor for IgE (FcepsilonRI) in human platelets and its’ intracellular expression in human megakaryocytes. Blood 1999, 93, 2543–2551. [Google Scholar] [CrossRef]
- Hasegawa, S.; Tashiro, N.; Matsubara, T.; Furukawa, S.; Ra, C. A comparison of FcepsilonRI-mediated RANTES release from human platelets between allergic patients and healthy individuals. Int. Arch. Allergy Immunol. 2001, 125 (Suppl. 1), 42–47. [Google Scholar] [CrossRef]
- Shah, S.A.; Kanabar, V.; Riffo-Vasquez, Y.; Mohamed, Z.; Cleary, S.J.; Corrigan, C.; James, A.L.; Elliot, J.G.; Shute, J.K.; Page, C.P.; et al. Platelets Independently Recruit into Asthmatic Lungs and Models of Allergic Inflammation via CCR3. Am. J. Respir. Cell Mol. Biol. 2021, 64, 557–568. [Google Scholar] [CrossRef]
- Joseph, M.; Gounni, A.S.; Kusnierz, J.P.; Vorng, H.; Sarfati, M.; Kinet, J.P.; Tonnel, A.B.; Capron, A.; Capron, M. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur. J. Immunol. 1997, 27, 2212–2218. [Google Scholar] [CrossRef]
- Pitchford, S.C.; Momi, S.; Baglioni, S.; Casali, L.; Giannini, S.; Rossi, R.; Page, C.P.; Gresele, P. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am. J. Respir. Crit. Care Med. 2008, 177, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cardot, E.; Pestel, J.; Callebaut, I.; Lassalle, P.; Tsicopoulos, A.; Gras-Masse, H.; Capron, A.; Joseph, M. Specific activation of platelets from patients allergic to Dermatophagoides pteronyssinus by synthetic peptides derived from the allergen Der p I. Int. Arch. Allergy Immunol. 1992, 98, 127–134. [Google Scholar] [CrossRef]
- Raiden, S.; Schettini, J.; Salamone, G.; Trevani, A.; Vermeulen, M.; Gamberale, R.; Giordano, M.; Geffner, J. Human platelets produce granulocyte-macrophage colony-stimulating factor and delay eosinophil apoptosis. Lab. Investig. J. Tech. Methods Pathol. 2003, 83, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Nagata, K.; Koike, J.; Todoroki, N.; Irimura, T. Induction of superoxide anion production from monocytes an neutrophils by activated platelets through the P-selectin-sialyl Lewis X interaction. J. Leukoc. Biol. 1994, 56, 583–587. [Google Scholar] [CrossRef]
- Nakanishi, T.; Inaba, M.; Inagaki-Katashiba, N.; Tanaka, A.; Vien, P.T.; Kibata, K.; Ito, T.; Nomura, S. Platelet-derived RANK ligand enhances CCL17 secretion from dendritic cells mediated by thymic stromal lymphopoietin. Platelets 2015, 26, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, S.C.; Momi, S.; Giannini, S.; Casali, L.; Spina, D.; Page, C.P.; Gresele, P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood 2005, 105, 2074–2081. [Google Scholar] [CrossRef]
- Lukacs, N.W.; John, A.; Berlin, A.; Bullard, D.C.; Knibbs, R.; Stoolman, L.M. E- and P-selectins are essential for the development of cockroach allergen-induced airway responses. J. Immunol. (Baltimore, Md.: 1950) 2002, 169, 2120–2125. [Google Scholar] [CrossRef]
- Cardenas, E.I.; Breaux, K.; Da, Q.; Flores, J.R.; Ramos, M.A.; Tuvim, M.J.; Burns, A.R.; Rumbaut, R.E.; Adachi, R. Platelet Munc13-4 regulates hemostasis, thrombosis and airway inflammation. Haematologica 2018, 103, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Ohba, M.; Wu, X.; Sasano, T.; Nakamura, M.; Endo, Y. Accumulation of platelets in the lung and liver and their degranulation following antigen-challenge in sensitized mice. Br. J. Pharmacol. 2002, 137, 146–152. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Rogala, B. Markers of platelet activation in plasma of patients suffering from persistent allergic rhinitis with or without asthma symptoms. Clin. Exp. Allergy 2005, 35, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zajac, A.; Rogala, B. Platelet activity measured by plasma levels of beta-thromboglobulin and platelet factor 4 in seasonal allergic rhinitis during natural pollen exposure. Inflamm. Res. 2003, 52, 477–479. [Google Scholar] [CrossRef]
- Koczy-Baron, E.; Grzanka, A.; Jochem, J.; Gawlik, R.; Kasperska-Zajac, A. Evaluation of circulating vascular endothelial growth factor and its soluble receptors in patients suffering from persistent allergic rhinitis. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2016, 12, 17. [Google Scholar] [CrossRef]
- Potaczek, D.P. Links between allergy and cardiovascular or hemostatic system. Int. J. Cardiol. 2014, 170, 278–285. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Snelgrove, R.J. Type 2 immunity: Expanding our view. Sci. Immunol. 2018, 3, eaat1604. [Google Scholar] [CrossRef]
- Kumar, S.; Jeong, Y.; Ashraf, M.U.; Bae, Y.S. Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int. J. Mol. Sci. 2019, 20, 2159. [Google Scholar] [CrossRef]
- Amison, R.T.; Cleary, S.J.; Riffo-Vasquez, Y.; Bajwa, M.; Page, C.P.; Pitchford, S.C. Platelets Play a Central Role in Sensitization to Allergen. Am. J. Respir. Cell Mol. Biol. 2018, 59, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Page, C.; Pitchford, S. Platelets coming of age: Implications for our understanding of allergic inflammation. Am. J. Respir. Crit. Care Med. 2013, 187, 459–460. [Google Scholar] [CrossRef]
- Durk, T.; Duerschmied, D.; Muller, T.; Grimm, M.; Reuter, S.; Vieira, R.P.; Ayata, K.; Cicko, S.; Sorichter, S.; Walther, D.J.; et al. Production of serotonin by tryptophan hydroxylase 1 and release via platelets contribute to allergic airway inflammation. Am. J. Respir. Crit. Care Med. 2013, 187, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhu, T.; Liu, J.; Guo, Z.; Cao, X. Platelets promote allergic asthma through the expression of CD154. Cell. Mol. Immunol. 2015, 12, 700–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akdis, C.A.; Arkwright, P.D.; Bruggen, M.C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 immunity in the skin and lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef]
- Takeda, T.; Unno, H.; Morita, H.; Futamura, K.; Emi-Sugie, M.; Arae, K.; Shoda, T.; Okada, N.; Igarashi, A.; Inoue, E.; et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2016, 138, 1395–1403. [Google Scholar] [CrossRef]
- Chae, W.J.; Ehrlich, A.K.; Chan, P.Y.; Teixeira, A.M.; Henegariu, O.; Hao, L.; Shin, J.H.; Park, J.H.; Tang, W.H.; Kim, S.T.; et al. The Wnt Antagonist Dickkopf-1 Promotes Pathological Type 2 Cell-Mediated Inflammation. Immunity 2016, 44, 246–258. [Google Scholar] [CrossRef]
- Gomez-Casado, C.; Villasenor, A.; Rodriguez-Nogales, A.; Bueno, J.L.; Barber, D.; Escribese, M.M. Understanding Platelets in Infectious and Allergic Lung Diseases. Int. J. Mol. Sci. 2019, 20, 1730. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Chihara, J.; Kobayashi, Y.; Kakazu, T.; Kurachi, D.; Yamamoto, T.; Nakajima, S. Effect of platelet-activating factor and platelet factor 4 on eosinophil adhesion. Int. Arch. Allergy Immunol. 1994, 104 (Suppl. 1), 57–59. [Google Scholar] [CrossRef]
- Chihara, J.; Yasuba, H.; Tsuda, A.; Urayama, O.; Saito, N.; Honda, K.; Kayaba, H.; Yamashita, T.; Kurimoto, F.; Yamada, H. Elevation of the plasma level of RANTES during asthma attacks. J. Allergy Clin. Immunol. 1997, 100, S52–S55. [Google Scholar] [CrossRef]
- Bisset, L.R.; Schmid-Grendelmeier, P. Chemokines and their receptors in the pathogenesis of allergic asthma: Progress and perspective. Curr. Opin. Pulm. Med. 2005, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hayashizaki, K.; Kimura, M.Y.; Tokoyoda, K.; Hosokawa, H.; Shinoda, K.; Hirahara, K.; Ichikawa, T.; Onodera, A.; Hanazawa, A.; Iwamura, C.; et al. Myosin light chains 9 and 12 are functional ligands for CD69 that regulate airway inflammation. Sci. Immunol. 2016, 1, eaaf9154. [Google Scholar] [CrossRef]
- Amison, R.T.; Momi, S.; Morris, A.; Manni, G.; Keir, S.; Gresele, P.; Page, C.P.; Pitchford, S.C. RhoA signaling through platelet P2Y(1) receptor controls leukocyte recruitment in allergic mice. J. Allergy Clin. Immunol. 2015, 135, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.G.; Chen, M.; Chou, K.C. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr. Med. Chem. 2004, 11, 2153–2160. [Google Scholar] [CrossRef]
- Johansson, M.W.; Mosher, D.F. Integrin activation States and eosinophil recruitment in asthma. Front. Pharmacol. 2013, 4, 33. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Kidder, M.S.; Bhattacharyya, N.; Xing, W.; Shen, S.; Milne, G.L.; Castells, M.C.; Chhay, H.; Boyce, J.A. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 2012, 119, 3790–3798. [Google Scholar] [CrossRef]
- Mitsui, C.; Kajiwara, K.; Hayashi, H.; Ito, J.; Mita, H.; Ono, E.; Higashi, N.; Fukutomi, Y.; Sekiya, K.; Tsuburai, T.; et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2016, 137, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Mitsui, C.; Hayashi, H.; Ono, E.; Kajiwara, K.; Mita, H.; Watai, K.; Kamide, Y.; Fukutomi, Y.; Sekiya, K.; et al. Aspirin-exacerbated respiratory disease (AERD): Current understanding of AERD. Allergol. Int. 2019, 68, 289–295. [Google Scholar] [CrossRef]
- Dominas, C.; Gadkaree, S.; Maxfield, A.Z.; Gray, S.T.; Bergmark, R.W. Aspirin-exacerbated respiratory disease: A review. Laryngoscope Investig. Otolaryngol. 2020, 5, 360–367. [Google Scholar] [CrossRef]
- Rusznak, M.; Peebles, R.S., Jr. Prostaglandin E2 in NSAID-exacerbated respiratory disease: Protection against cysteinyl leukotrienes and group 2 innate lymphoid cells. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 38–45. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Julius, P.; Kuepper, M.; Garn, H.; Bratke, K.; Irmscher, S.; Luttmann, W.; Renz, H.; Braun, A.; Virchow, J.C. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J. Allergy Clin. Immunol. 2006, 118, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.; Oddera, S.; Sacco, O.; Balbo, A.; Crimi, E.; Rossi, G.A. Bronchial and bronchoalveolar inflammation in single early and dual responders after allergen inhalation challenge. Lung 1997, 175, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Herd, C.M.; Page, C.P. Pulmonary immune cells in health and disease: Platelets. Eur. Respir. J. 1994, 7, 1145–1160. [Google Scholar] [PubMed]
- Yoshimi, Y.; Fujimura, M.; Myou, S.; Tachibana, H.; Hirose, T. Effect of thromboxane A2 (TXA2) synthase inhibitor and TXA2 receptor antagonist alone and in combination on antigen-induced bronchoconstriction in guinea pigs. Prostaglandins Other Lipid Mediat. 2001, 65, 1–9. [Google Scholar] [CrossRef]
- Coyle, A.J.; Page, C.P.; Atkinson, L.; Flanagan, R.; Metzger, W.J. The requirement for platelets in allergen-induced late asthmatic airway obstruction. Eosinophil infiltration and heightened airway responsiveness in allergic rabbits. Am. Rev. Respir. Dis. 1990, 142, 587–593. [Google Scholar] [CrossRef]
- Keir, S.D.; Spina, D.; Page, C.P. Bradykinin and capsaicin induced airways obstruction in the guinea pig are platelet dependent. Pulm. Pharmacol. Ther. 2015, 33, 25–31. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Rogala, B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation 2008, 31, 112–120. [Google Scholar] [CrossRef]
- Braun, A.; Lommatzsch, M.; Neuhaus-Steinmetz, U.; Quarcoo, D.; Glaab, T.; McGregor, G.P.; Fischer, A.; Renz, H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br. J. Pharmacol. 2004, 141, 431–440. [Google Scholar] [CrossRef]
- Barnes, P.J. New aspects of asthma. J. Intern. Med. 1992, 231, 453–461. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, X.Y.; Li, N.; Zhao, L.M.; Guo, Y.L.; Li, X.S.; Tian, C.J.; Cheng, D.J.; Chen, Z.C.; Zhang, L.X. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018, 212, 93–101. [Google Scholar] [CrossRef]
- Johnson, J.R.; Folestad, E.; Rowley, J.E.; Noll, E.M.; Walker, S.A.; Lloyd, C.M.; Rankin, S.M.; Pietras, K.; Eriksson, U.; Fuxe, J. Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L658–L671. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, X.; Hu, B.; Xia, Q.; Ni, X.; Song, Y. P2X4R promotes airway remodeling by acting on the phenotype switching of bronchial smooth muscle cells in rats. Purinergic Signal. 2018, 14, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Sekine, K.; Miyasaka, T.; Kawashima, R.; Nakajima, Y.; Nakano, J.; Yamamoto, T.; Horiuchi, T.; Hirai, K.; Ohta, K. Platelet-derived growth factor is involved in the augmentation of airway responsiveness through remodeling of airways in diesel exhaust particulate-treated mice. J. Allergy Clin. Immunol. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wynendaele, W.; Derua, R.; Hoylaerts, M.F.; Pawinski, A.; Waelkens, E.; De Bruijn, E.A.; Paridaens, R.; Merlevede, W.; Van Oosterom, A.T. Vascular endothelial growth factor measured in platelet poor plasma allows optimal separation between cancer patients and volunteers: A key to study an angiogenic marker in vivo? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1999, 10, 965–971. [Google Scholar] [CrossRef]
- Hur, G.Y.; Broide, D.H. Genes and Pathways Regulating Decline in Lung Function and Airway Remodeling in Asthma. Allergy Asthma Immunol. Res. 2019, 11, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Finotto, S. Resolution of allergic asthma. Semin. Immunopathol. 2019, 41, 665–674. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb. Haemost. 2015, 114, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Judge, H.M.; Wilcox, R.G.; Heptinstall, S. Inhibition of ADP-induced P-selectin expression and platelet-leukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR-C69931MX but not aspirin. Thromb. Haemost. 2002, 88, 488–494. [Google Scholar]
- Suh, D.H.; Trinh, H.K.; Liu, J.N.; Pham le, D.; Park, S.M.; Park, H.S.; Shin, Y.S. P2Y12 antagonist attenuates eosinophilic inflammation and airway hyperresponsiveness in a mouse model of asthma. J. Cell. Mol. Med. 2016, 20, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Di Marco, F.; Terraneo, S.; Parati, M.; Razzari, C.; Scavone, M.; Femia, E.A.; Moro, A.; Centanni, S.; Cattaneo, M. Effect of prasugrel in patients with asthma: Results of PRINA, a randomized, double-blind, placebo-controlled, cross-over study. J. Thromb. Haemost. JTH 2015, 13, 136–141. [Google Scholar] [CrossRef]
- Shi, H.; Yokoyama, A.; Kohno, N.; Hirasawa, Y.; Kondo, K.; Sakai, K.; Hiwada, K. Effect of thromboxane A2 inhibitors on allergic pulmonary inflammation in mice. Eur. Respir. J. 1998, 11, 624–629. [Google Scholar] [PubMed]
- Hayashi, M.; Koya, T.; Kawakami, H.; Sakagami, T.; Hasegawa, T.; Kagamu, H.; Takada, T.; Sakai, Y.; Suzuki, E.; Gelfand, E.W.; et al. A prostacyclin agonist with thromboxane inhibitory activity for airway allergic inflammation in mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 317–326. [Google Scholar] [CrossRef]
- Fukuoka, T.; Miyake, S.; Umino, T.; Inase, N.; Tojo, N.; Yoshizawa, Y. The effect of seratrodast on eosinophil cationic protein and symptoms in asthmatics. J. Asthma Off. J. Assoc. Care Asthma 2003, 40, 257–264. [Google Scholar] [CrossRef]
- Chamba, G.; Lemoine, P.; Flachaire, E.; Ferry, N.; Quincy, C.; Sassard, J.; Ferber, C.; Mocaer, E.; Kamoun, A.; Renaud, B. Increased serotonin platelet uptake after tianeptine administration in depressed patients. Biol. Psychiatry 1991, 30, 609–617. [Google Scholar] [CrossRef]
- Lechin, F.; Van der Dijs, B.; Lechin, A.E. Treatment of bronchial asthma with tianeptine. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Pitchford, S.; Cleary, S.; Arkless, K.; Amison, R. Pharmacological strategies for targeting platelet activation in asthma. Curr. Opin. Pharm. 2019, 46, 55–64. [Google Scholar] [CrossRef]
- Cattaneo, M. The platelet P2 receptors in inflammation. Hamostaseologie 2015, 35, 262–266. [Google Scholar] [CrossRef]
- Kunapuli, S.P.; Dorsam, R.T.; Kim, S.; Quinton, T.M. Platelet purinergic receptors. Curr. Opin. Pharmacol. 2003, 3, 175–180. [Google Scholar] [CrossRef]
- Mansour, A.; Bachelot-Loza, C.; Nesseler, N.; Gaussem, P.; Gouin-Thibault, I. P2Y(12) Inhibition beyond Thrombosis: Effects on Inflammation. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, X.H.; Zhang, J.D.; Li, R.B.; Jia, M.; Cui, X.R. Compared efficacy of clopidogrel and ticagrelor in treating acute coronary syndrome: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 217. [Google Scholar] [CrossRef]
- Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Damaskos, C.; Athanasiou, A.; Moris, D.; Politou, M. The role of prasugrel in the management of acute coronary syndromes: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4733–4743. [Google Scholar]
- Trinh, H.K.T.; Nguyen, T.V.T.; Choi, Y.; Park, H.S.; Shin, Y.S. The synergistic effects of clopidogrel with montelukast may be beneficial for asthma treatment. J. Cell. Mol. Med. 2019, 23, 3441–3450. [Google Scholar] [CrossRef]
- Amison, R.T.; Arnold, S.; O’Shaughnessy, B.G.; Cleary, S.J.; Ofoedu, J.; Idzko, M.; Page, C.P.; Pitchford, S.C. Lipopolysaccharide (LPS) induced pulmonary neutrophil recruitment and platelet activation is mediated via the P2Y1 and P2Y14 receptors in mice. Pulm. Pharmacol. Ther. 2017, 45, 62–68. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Z.G.; Zhang, K.; Kiselev, E.; Crane, S.; Wang, J.; Paoletta, S.; Yi, C.; Ma, L.; Zhang, W.; et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 2015, 520, 317–321. [Google Scholar] [CrossRef]
- Dogne, J.M.; De Leval, X.; Benoit, P.; Rolin, S.; Pirotte, B.; Masereel, B. Therapeutic potential of thromboxane inhibitors in asthma. Expert Opin. Investig. Drugs 2002, 11, 275–281. [Google Scholar] [CrossRef]
- Hernandez, J.M.; Janssen, L.J. Revisiting the usefulness of thromboxane-A2 modulation in the treatment of bronchoconstriction in asthma. Can. J. Physiol. Pharm. 2015, 93, 111–117. [Google Scholar] [CrossRef]
- Fujimura, M.; Sasaki, F.; Nakatsumi, Y.; Takahashi, Y.; Hifumi, S.; Taga, K.; Mifune, J.; Tanaka, T.; Matsuda, T. Effects of a thromboxane synthetase inhibitor (OKY-046) and a lipoxygenase inhibitor (AA-861) on bronchial responsiveness to acetylcholine in asthmatic subjects. Thorax 1986, 41, 955–959. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Mauler, M.; Braun, A.; Duerschmied, D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets 2018, 29, 541–548. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lechin, F.; Van der Dijs, B.; Orozco, B.; Lechin, M.; Lechin, A.E. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 1996, 77, 245–253. [Google Scholar] [CrossRef]
- McNicol, A.; Israels, S.J. Platelet dense granules: Structure, function and implications for haemostasis. Thromb. Res. 1999, 95, 1–18. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The Key Contribution of Platelet and Vascular Arachidonic Acid Metabolism To The Pathophysiology Of Atherothrombosis. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Resendiz-Hernandez, J.M.; Falfan-Valencia, R.; Teran, L.M. Biochemical pathogenesis of aspirin exacerbated respiratory disease (AERD). Clin. Biochem. 2013, 46, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Le Pham, D.; Lee, J.H.; Park, H.S. Aspirin-exacerbated respiratory disease: An update. Curr. Opin. Pulm. Med. 2017, 23, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P. The involvement of platelets in non-thrombotic processes. Trends Pharmacol. Sci. 1988, 9, 66–71. [Google Scholar] [CrossRef]
- Laumonnier, Y.; Wiese, A.V.; Figge, J.; Karsten, C. Regulation and function of anaphylatoxins and their receptors in allergic asthma. Mol. Immunol. 2017, 84, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Polley, M.J.; Nachman, R.L. Human platelet activation by C3a and C3a des-arg. J. Exp. Med. 1983, 158, 603–615. [Google Scholar] [CrossRef]
- Sauter, R.J.; Sauter, M.; Obrich, M.; Emschermann, F.N.; Nording, H.; Patzelt, J.; Wendel, H.P.; Reil, J.C.; Edlich, F.; Langer, H.F. Anaphylatoxin Receptor C3aR Contributes to Platelet Function, Thrombus Formation and In Vivo Haemostasis. Thromb. Haemost. 2019, 119, 179–182. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jurk, K.; Hobohm, L.; Jäckel, S.; Saffarzadeplatelet activation and fibrin formatioh, M.; Schwierczek, K.; Wenzel, P.; Langer, F.; Reinhardt, C.; Ruf, W. Distinct contributions of complement factors to n in venous thrombus development. Blood 2017, 129, 2291–2302. [Google Scholar] [CrossRef]
- Martel, C.; Cointe, S.; Maurice, P.; Matar, S.; Ghitescu, M.; Théroux, P.; Bonnefoy, A. Requirements for membrane attack complex formation and anaphylatoxins binding to collagen-activated platelets. PLoS ONE 2011, 6, e18812. [Google Scholar] [CrossRef]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. J. Lab. Clin. Med. 2018, 191, 1–14. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, L. Update on the role of alternatively activated macrophages in asthma. J. Asthma Allergy 2016, 9, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Thomas, K.; Mersmann, S.; Skupski, J.; Margraf, A.; Tekath, T.; Jouvene, C.C.; Dalli, J.; Hidalgo, A.; Meuth, S.G.; et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Winnica, D.; Corey, C.; Mullett, S.; Reynolds, M.; Hill, G.; Wendell, S.; Que, L.; Holguin, F.; Shiva, S. Bioenergetic Differences in the Airway Epithelium of Lean Versus Obese Asthmatics Are Driven by Nitric Oxide and Reflected in Circulating Platelets. Antioxid. Redox Signal. 2019, 31, 673–686. [Google Scholar] [CrossRef]
- Xu, W.; Cardenes, N.; Corey, C.; Erzurum, S.C.; Shiva, S. Platelets from Asthmatic Individuals Show Less Reliance on Glycolysis. PLoS ONE 2015, 10, e0132007. [Google Scholar] [CrossRef]
- Newcomb, D.C.; Peebles, R.S., Jr. Th17-mediated inflammation in asthma. Curr. Opin. Immunol. 2013, 25, 755–760. [Google Scholar] [CrossRef]
- Affandi, A.J.; Silva-Cardoso, S.C.; Garcia, S.; Leijten, E.F.A.; Van Kempen, T.S.; Marut, W.; Van Roon, J.A.G.; Radstake, T. CXCL4 is a novel inducer of human Th17 cells and correlates with IL-17 and IL-22 in psoriatic arthritis. Eur. J. Immunol. 2018, 48, 522–531. [Google Scholar] [CrossRef]
- Ponomarev, E.D. Fresh Evidence for Platelets as Neuronal and Innate Immune Cells: Their Role in the Activation, Differentiation, and Deactivation of Th1, Th17, and Tregs during Tissue Inflammation. Front. Immunol 2018, 9, 406. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, Z.; Stalesen, R.; Hansson, G.K.; Li, N. Platelets provoke distinct dynamics of immune responses by differentially regulating CD4+ T-cell proliferation. J. Thromb. Haemost. JTH 2014, 12, 1156–1165. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Zimmerman, G.A. Platelets in lung biology. Annu. Rev. Physiol. 2013, 75, 569–591. [Google Scholar] [CrossRef]
- Lefrançais, E.; Ortiz-Muñoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef]
- Yeung, A.K.; Villacorta-Martin, C.; Hon, S.; Rock, J.R.; Murphy, G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020, 4, 6204–6217. [Google Scholar] [CrossRef]
- Pariser, D.N.; Hilt, Z.T.; Ture, S.K.; Blick-Nitko, S.K.; Looney, M.R.; Cleary, S.J.; Roman-Pagan, E.; Saunders, J., 2nd; Georas, S.N.; Veazey, J.; et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Investig. 2021, 131, e137377. [Google Scholar] [CrossRef]
- Hogan, K.A.; Weiler, H.; Lord, S.T. Mouse models in coagulation. Thromb. Haemost. 2002, 87, 563–574. [Google Scholar] [CrossRef]
- Alessandrini, F.; Musiol, S.; Schneider, E.; Blanco-Pérez, F.; Albrecht, M. Mimicking Antigen-Driven Asthma in Rodent Models-How Close Can We Get? Front. Immunol 2020, 11, 575936. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef]
- Looney, M.R.; Headley, M.B. Live imaging of the pulmonary immune environment. Cell. Immunol. 2020, 350, 103862. [Google Scholar] [CrossRef]
| Models | Samples | Indicators | Subjects | References |
|---|---|---|---|---|
| In vivo | Peripheral blood | Platelet-leukocyte conjugates↑ | Asthmatic patients after allergen exposure | [25] |
| Platelet-derived microparticles (PMPs) ↑ | Asthmatic patients | [30] | ||
| Percentage of IgE+ platelets↑ | Asthmatic patients | [31] | ||
| Eosinophil β1-integrin activation↑ | Asthmatic patients | [28,29] | ||
| Platelet BDNF↑ | Patients with allergic asthma | [32] | ||
| Plasma | β-TG and PF4↑ | Atopic dermatitis patients with concomitant asthma and allergic rhinitis | [19] | |
| β-TG, PF4↑ Platelet count↓ | House-dust-mite-sensitive asthmatic patients intrabronchially challenged with Dp extract | [20] | ||
| PF4↑ (during the grass pollen season) PF4↓(off season) | Patients with pollen-induced seasonal allergic rhinitis and asthma | [26] | ||
| BDNF↑ | Patients with allergic asthma | [32] | ||
| Phingosine-1-phosphate (SIP)↑ | House-dust-mite-allergic patients | [33] | ||
| BALF | Isolation of platelets | Asthmatic patients | [21] | |
| 5-HT↑ | Asthmatic patients | [31,34,35] | ||
| β-TG, PF4↑ | Ragweed-allergic asthmatic subjects after challenge with ragweed antigen. | [22] | ||
| Bronchial biopsies | Platelet deposition on interalveolar septum walls Platelet number↑ | Asthmatic patients | [23,24,36] | |
| Nasal lavage fluid | P-selectin positively correlated with ECP level | Asthmatic patients | [27] | |
| In vitro | Platelet | FcεRI and FcεRII/CD23 expression | Human platelets and megakaryocyte | [31,34,35,37,38] |
| RANTES release↑ Cytotoxicity against schistosomula | Platelets treated by anti-FcεRI, anti-CD23, anti-IgE | [34,35,37] | ||
| RANTES release↑ | Platelet from allergic patients stimulated with IgE and anti-IgE | [35] | ||
| Allergen-specific cytotoxicity against schistosomula↑ | Patients allergic to Dermatophagoides pteronyssinus | [39] | ||
| Allergen-specific platelet chemotaxis | Allergic asthmatic patients | [38] | ||
| GM-CSF↑ Eosinophils apoptosis↓ | Human platelets and Eosinophils coculture | [40] | ||
| P-selectin↑ Neutrophil superoxide anions↑ | Human platelets and neutrophils coculture | [41] | ||
| RANKL in platelets↑ CCL17 (Th2-attracting chemokine)↑ | TRAP6-activated platelets with TSLP-stimulated DCs coculture | [42] |
| Mechanism Studied | Method/Animal Model | Findings | References |
|---|---|---|---|
| Platelet degranulation | Munc13-4−/−mice | Platelets Munc13-4 deficiency abolishes dense granule release to reduce AHR and eosinophilic inflammation. | [45] |
| Platelet migration | FcRγ−/−mice Platelet depletion Platelet transfusion Allergen or anti-IgE antibody in vitro stimulation | Platelet migration is allergen-IgE-FcεRI dependent. | [38] |
| CCR3 antagonist | Platelet rolling, adhesion, and extravascular migration are CCR3 dependent. | [36] | |
| Sensitization of allergic asthma | Platelet depletion FcRγ−/−mice | Platelets rely on an IgE-FcεRI-dependent pathway to induce Type 2 immune response formation during sensitization stage. | [53] |
| Leukocyte recruitment | Platelet depletion Platelet transfusion | Depletion of platelets reduces, while transfusion of platelets restores the allergen-induced pulmonary leukocyte recruitment. | [25] |
| Platelet depletion Platelet transfusion | Platelets P-selectin is required for pulmonary eosinophils and lymphocytes recruitment. | [43] | |
| Selectin−/−mice | P-selectin is critical in the development of allergen-induced airway response. | [43,44] | |
| Anti-Myl9/12 antibody CD69-deficient mice | Platelet secreted myl9 upon activation and formed intravascular net-like structures to bind to CD69+ CD4+ T cells, regulating the pathological process of allergic asthma. | [64] | |
| P2Y1 antagonists Platelet depletion Platelet transfusion | Purine receptor P2Y1 on platelets regulates leukocyte recruitment in allergic mice through the RhoA signaling pathway. | [65] | |
| Type 2 immunity induction | TPH1−/−mice BM chimera Mast cell–deficient mice Platelet transfusion | Platelets secrete 5-HT to enhance the ability of mature DCs to polarize Type 2 immunity formation. | [55] |
| Platelet depletion Platelet transfusion Cd154−/−mice | Platelets inhibit Treg generation via CD154 to promote asthma development. | [56] | |
| Platelet depletion Platelet transfusion IL-33-deficient mice | IL-33 is essential for eosinophilic inflammation in the airway. | [58] | |
| Dkk1d/d mice | Dickkopf-1 (DKK-1) facilitated leukocyte migration and promoted Th2 cell differentiation and Type 2 cytokine production. | [59] | |
| Airway remodeling | Platelet depletion | Platelets are necessary for epithelial and smooth-muscle thickening and the deposition of reticular fibers in the extracellular matrix (ECM). | [23] |
| Category | Target | Drug | Mechanism | References |
|---|---|---|---|---|
| ADP receptor antagonists | P2Y12 receptor | Clopidogrel Prasugrel Ticagrelor | Reduce the release of platelets and the formation of platelets-leukocyte aggregates, and inhibit eosinophilic inflammation and airway hyperreactivity. | [89,90,91,92] |
| P2Y1 receptor | MRS2179 MRS2500 | Inhibit the recruitment of eosinophils and lymphocytes to the lung. | [65] | |
| TxA2 synthase inhibitor | TxA2 synthase | Ozagrel (OKY-46) ONO-1301 | Inhibit the production of proinflammatory cytokines and alleviate the eosinophil infiltration in the airways; suppress AHR and airway inflammation. | [93,94] |
| TP receptor antagonist | TP receptor | Seratrodast (AA-2414) S-1452 | Reduce bronchial hyperresponsiveness by reducing airway inflammation. | [93,95] |
| 5-HT modifier | 5-HT-specific transporter | Tianeptine | Enhance the uptake of free 5-HT in peripheral blood. | [96,97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Zhang, J.; Lee, J.; Tao, A. Platelets, Not an Insignificant Player in Development of Allergic Asthma. Cells 2021, 10, 2038. https://doi.org/10.3390/cells10082038
Luo L, Zhang J, Lee J, Tao A. Platelets, Not an Insignificant Player in Development of Allergic Asthma. Cells. 2021; 10(8):2038. https://doi.org/10.3390/cells10082038
Chicago/Turabian StyleLuo, Liping, Junyan Zhang, Jongdae Lee, and Ailin Tao. 2021. "Platelets, Not an Insignificant Player in Development of Allergic Asthma" Cells 10, no. 8: 2038. https://doi.org/10.3390/cells10082038
APA StyleLuo, L., Zhang, J., Lee, J., & Tao, A. (2021). Platelets, Not an Insignificant Player in Development of Allergic Asthma. Cells, 10(8), 2038. https://doi.org/10.3390/cells10082038






