Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Culture Conditions
2.2. Assessment of Cell Viability
2.3. Detection of Apoptosis Using Annexin V and Propidium Iodide (PI) Test
2.4. Assessment of Bcl-2 Protein Phosphorylation
2.5. Analysis of Changes in Mitochondrial Membrane Potential
2.6. Measurement of the Mitochondrial Membrane Potential (Δψm)
2.7. Measurement of the Production of Reactive Oxygen Species (ROS)
2.8. Test for Caspase 3/7 Activity
2.9. Transmission Electron Microscopy
2.10. Detection of LC3 Antibodies
2.11. DAPI Staining
2.12. Cell Cycle Analysis
2.13. Evaluation of Morphological Changes and Determination of the Mitotic Index
2.14. Statistical Analysis
3. Results
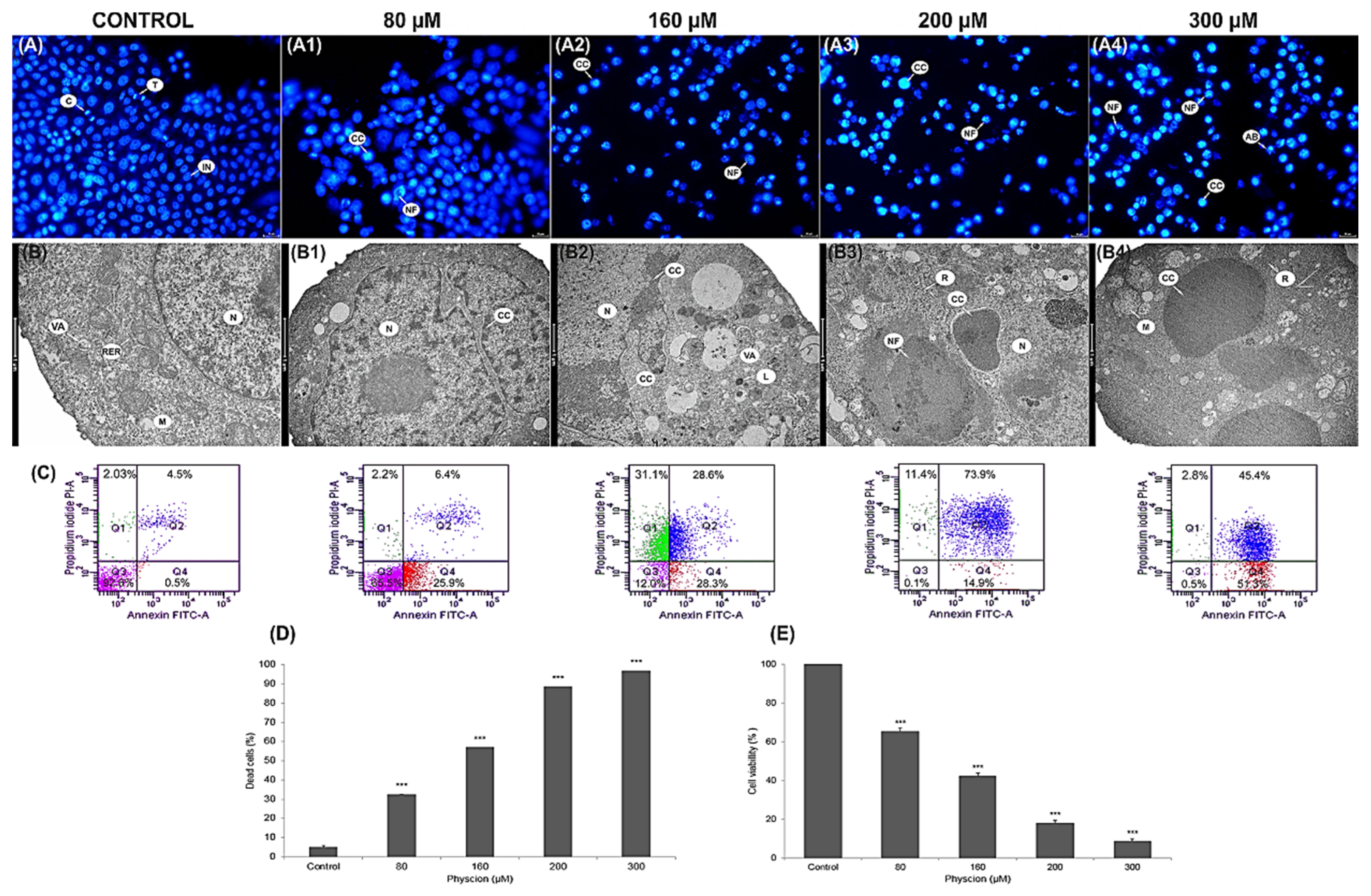
3.1. Induction of Apoptosis in HeLa Cells
3.2. Inhibition of the Viability of HeLa Cells through Mitochondrial Dysfunction
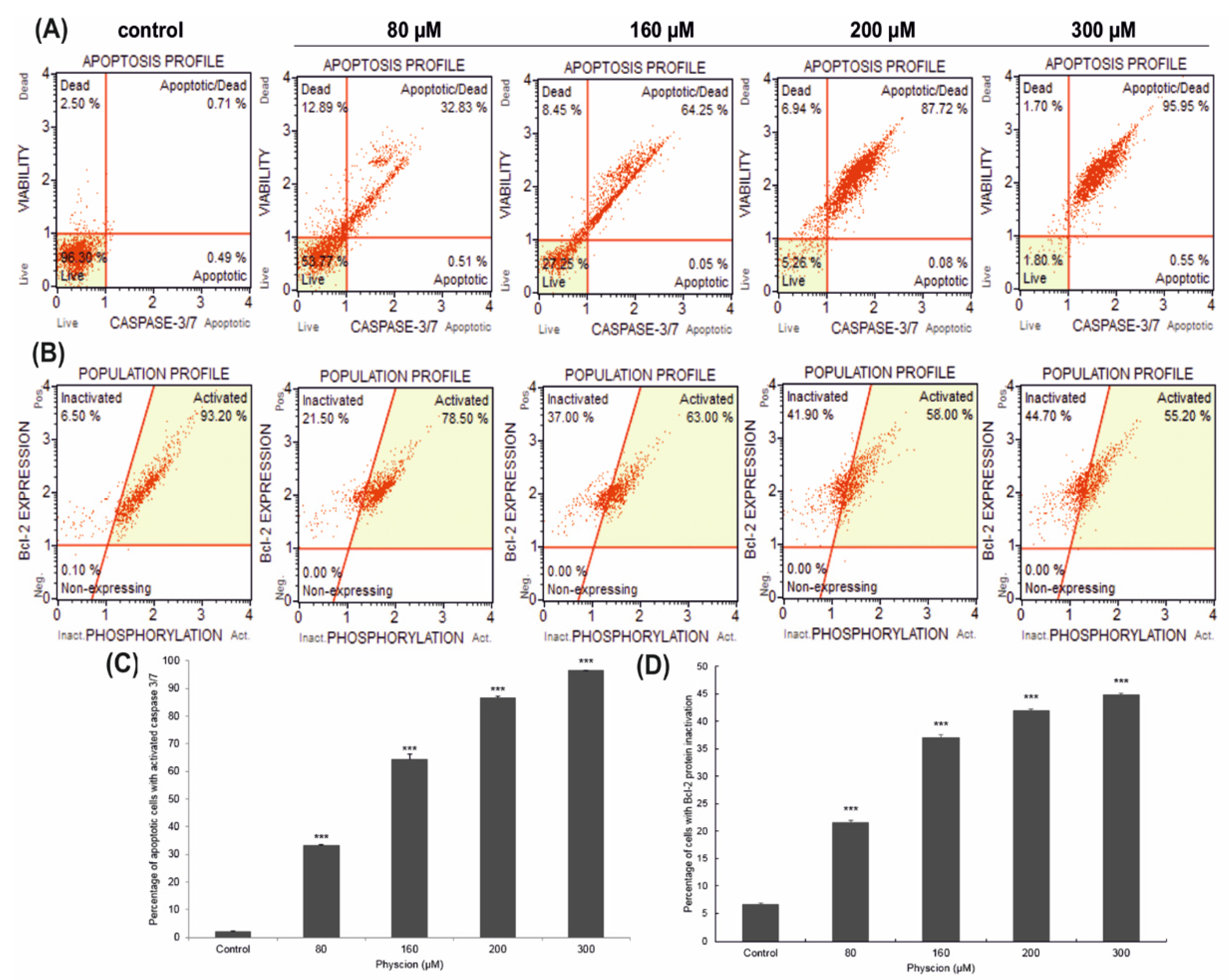
3.3. Changes in Caspase 3/7 Activity
3.4. Effect of Physcion on the Inactivation of Bcl-2
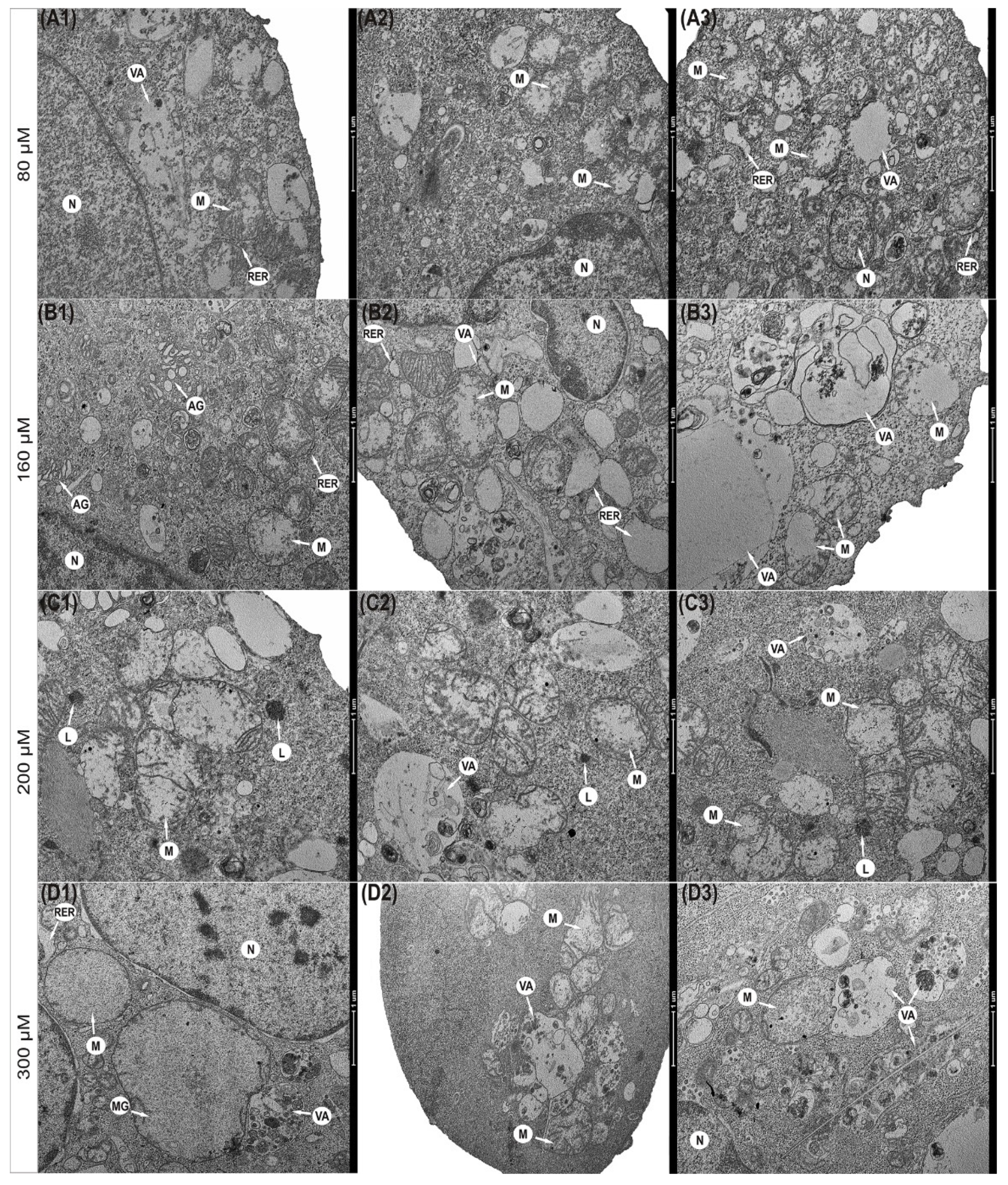
3.5. Progressive Mitochondrial Damage
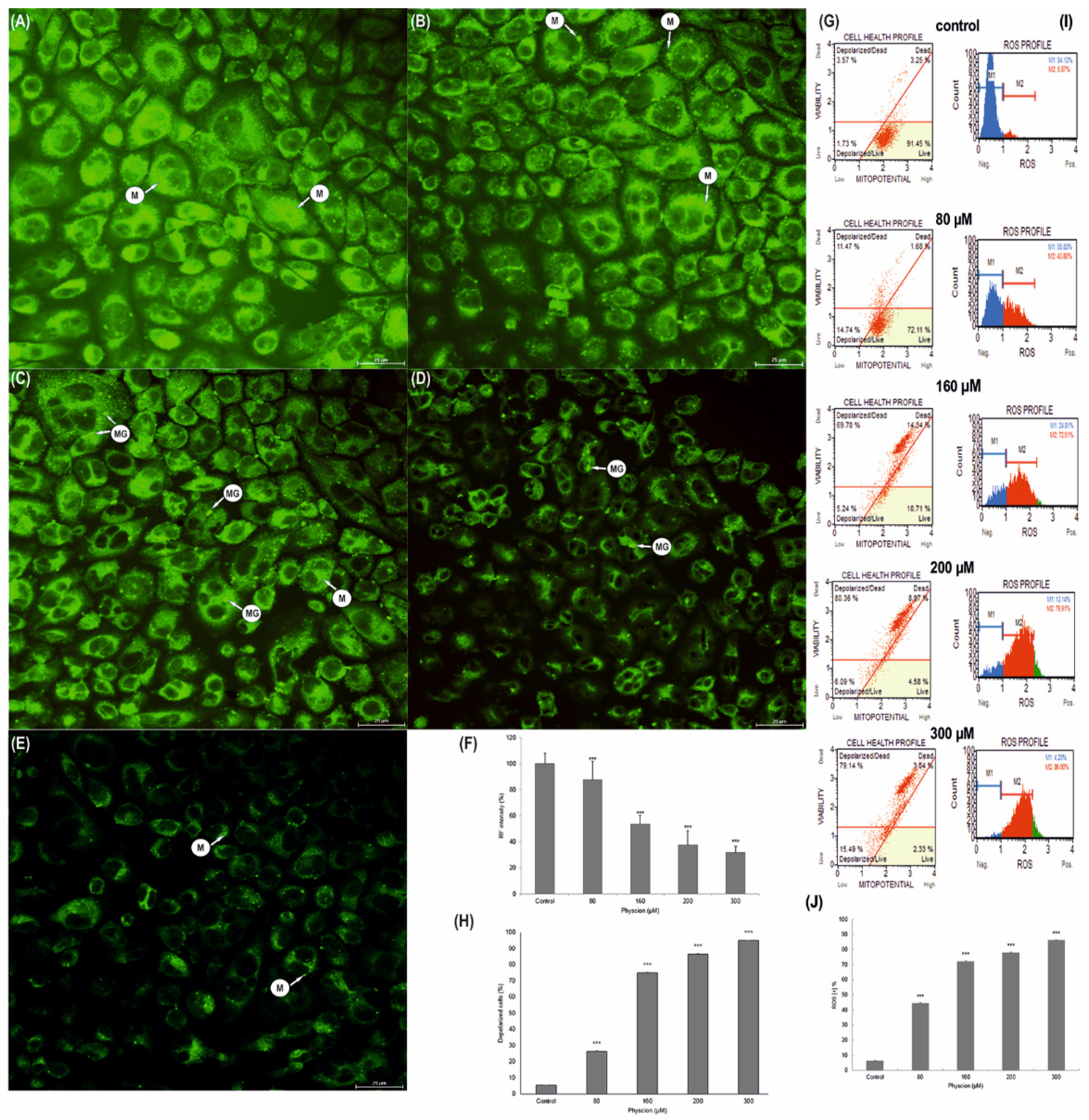
3.6. Change in the Membrane Potential (ΔΨm) of HeLa Mitochondria
3.7. Increase in Production of ROS in HeLa Cells
3.8. Induction of Autophagic Processes
3.9. Physcion Promotes Autophagy by Increasing the Level of LC3 Protein
3.10. Increased Vacuolization
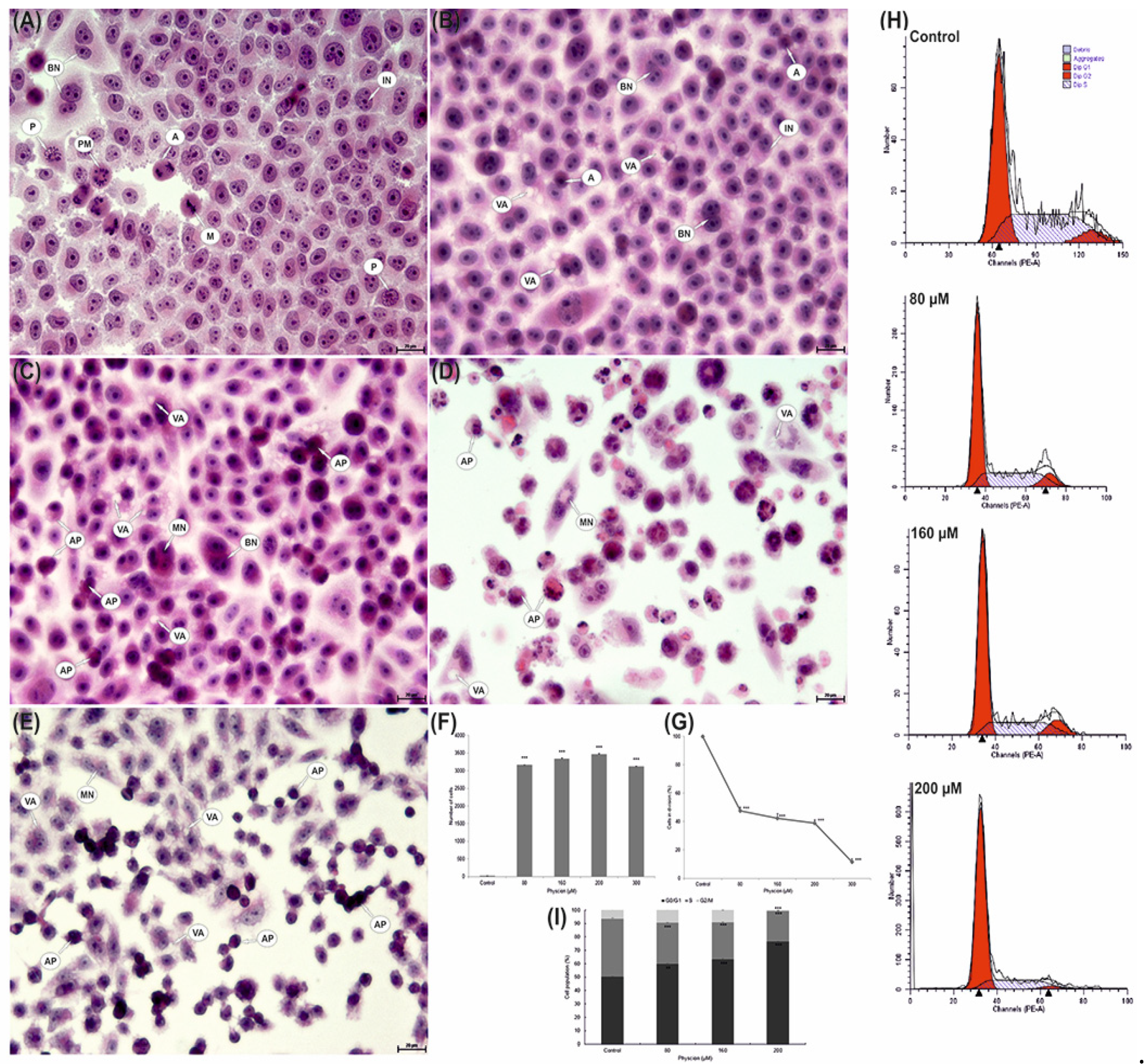
3.11. Reduction of the Mitotic Index of HeLa Cells
3.12. Changes in Cell Distribution in the Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fulda, S. Autophagy in cancer therapy. Front. Oncol. 2017, 7, 128. [Google Scholar] [CrossRef]
- Król, T.; Schmidt, M.; Kołątaj, A.; Witek, B. Vinblastine-induced autophagy in mouse liver. Comp. Biochem. Physiol. C Toxicol. Pharm. 1994, 107, 165–169. [Google Scholar] [CrossRef]
- Król, T. Vincristine-induced autophagy in mouse liver. Acta Biol. Cracoviensia. Ser. Zool. 1996, 38, 5–12. [Google Scholar]
- Krol, T. Activity of lysosomal system in mouse liver after taxol administration. Gen. Pharm. 1998, 30, 239–243. [Google Scholar] [CrossRef]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Karantza-Wadsworth, V. Role and regulation of autophagy in cancer. Biochim. Biophys. Acta 2009, 1793, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-inducing strategy in anticancer therapy. Oxidative Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Trybus, W.; Krol, G.; Trybus, E.; Stachurska, A.; Kopacz- Bednarska, A.; Krol, T. Aloe-Emodin Influence on the lysosomal compartment of Hela cells. Asian Pac. J. Cancer Prev. 2017, 18, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Trybus, W.; Krol, T.; Trybus, E.; Stachurska, A.; Kopacz-Bednarska, A.; Krol, G. Induction of mitotic catastrophe in human cervical cancer cells after administration of aloe-emodin. Anticancer Res. 2018, 38, 2037–2044. [Google Scholar] [CrossRef]
- Trybus, W.; Krol, T.; Trybus, E.; Stachurska, A.; Krol, G.; Kopacz-Bednarska, A. Emodin induces death in human cervical cancer cells through mitotic catastrophe. Anticancer Res. 2019, 39, 679–686. [Google Scholar] [CrossRef]
- Tamokou Jde, D.; Tala, M.F.; Wabo, H.K.; Kuiate, J.R.; Tane, P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J. Ethnopharmacol. 2009, 124, 571–575. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, J.B.; Zhou, G.D.; Shan, L.M.; Xiao, X.H. Investigations of free anthraquinones from rhubarb against alpha-naphthylisothiocyanate-induced cholestatic liver injury in rats. Basic Clin. Pharm. Toxicol 2009, 104, 463–469. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J. Pharm. Pharmacol. 2010, 62, 1158–1166. [Google Scholar] [CrossRef]
- Yu, J.; Xie, J.; Mao, X.J.; Wei, H.; Zhao, S.L.; Ma, Y.G.; Li, N.; Zhao, R.H. Comparison of laxative and antioxidant activities of raw, processed and fermented Polygoni Multiflori radix. Chin. J. Nat. Med. 2012, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Mazouri, S.E.; Aanniz, T.; Touhtouh, J.; Kandoussi, I.; Hakmi, M.; Belyamani, L.; Ibrahimi, A.; Ouadghir, M. Anthraquinones: A Promising Multi-target Therapeutic ScaffoldTo Treat Covid-19. Int J. Appl. Biol. Pharm. 2021, 12, 338–355. [Google Scholar] [CrossRef]
- Pang, M.J.; Yang, Z.; Zhang, X.L.; Liu, Z.F.; Fan, J.; Zhang, H.Y. Physcion, a naturally occurring anthraquinone derivative, induces apoptosis and autophagy in human nasopharyngeal carcinoma. Acta Pharm. Sin. 2016, 37, 1623–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.T.; Chen, X.H.; Gao, H.; Ye, J.L.; Wang, C.B. Physcion inhibits the metastatic potential of human colorectal cancer SW620 cells in vitro by suppressing the transcription factor SOX2. Acta Pharm. Sin. 2016, 37, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Tong, D.; Wang, C.; Sun, L.; Zhao, C.; Li, Y.; Zhu, L.; Wu, D. Physcion induces apoptosis in hepatocellular carcinoma by modulating miR-370. Am. J. Cancer Res. 2016, 6, 2919–2931. [Google Scholar]
- Hong, J.Y.; Chung, H.J.; Bae, S.Y.; Trung, T.N.; Bae, K.; Lee, S.K. Induction of Cell Cycle Arrest and Apoptosis by Physcion, an Anthraquinone Isolated From Rhubarb (Rhizomes of Rheum tanguticum), in MDA-MB-231 Human Breast Cancer Cells. J. Cancer Prev. 2014, 19, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Turzanski, J.; Daniels, I.; Haynes, A.P. Involvement of macroautophagy in the caspase-independent killing of Burkitt lymphoma cell lines by rituximab. Br. J. Haematol. 2009, 145, 137–140. [Google Scholar] [CrossRef]
- Wu, W.K.; Sakamoto, K.M.; Milani, M.; Aldana-Masankgay, G.; Fan, D.; Wu, K.; Lee, C.W.; Cho, C.H.; Yu, J.; Sung, J.J. Macroautophagy modulates cellular response to proteasome inhibitors in cancer therapy. Drug Resist. Updat. 2010, 13, 87–92. [Google Scholar] [CrossRef]
- Elmore, S.P.; Qian, T.; Grissom, S.F.; Lemasters, J.J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001, 15, 2286–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinsztein, D.C.; Gestwicki, J.E.; Murphy, L.O.; Klionsky, D.J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007, 6, 304–312. [Google Scholar] [CrossRef]
- Shimizu, S.; Kanaseki, T.; Mizushima, N.; Mizuta, T.; Arakawa-Kobayashi, S.; Thompson, C.B.; Tsujimoto, Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 2004, 6, 1221–1228. [Google Scholar] [CrossRef]
- Trybus, W.; Krol, T.; Trybus, E.; Stachurska, A.; Krol, G. The potential antitumor effect of chrysophanol in relation to cervical cancer cells. J. Cell. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.B.; Feng, L.X.; Yue, Q.X.; Wu, W.Y.; Guan, S.H.; Jiang, B.H.; Yang, M.; Liu, X.; Guo, D.A. Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J. Cell Physiol. 2012, 227, 2196–2206. [Google Scholar] [CrossRef] [PubMed]
- Guaman Ortiz, L.M.; Croce, A.L.; Aredia, F.; Sapienza, S.; Fiorillo, G.; Syeda, T.M.; Buzzetti, F.; Lombardi, P.; Scovassi, A.I. Effect of new berberine derivatives on colon cancer cells. Acta Biochim. Biophys. Sin. 2015, 47, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, G.; Venugopal, A.; Ramamoorthy, P.; Standing, D.; Subramaniam, D.; Umar, S.; Jensen, R.A.; Anant, S.; Mammen, J.M. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol. Carcinog. 2015, 54, 1710–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, A.N.; Ledbetter, D.J.; Gawriluk, T.R.; Rucker, E.B., 3rd. Autophagy: Regulation and role in development. Autophagy 2013, 9, 951–972. [Google Scholar] [CrossRef]
- Ozpolat, B.; Benbrook, D.M. Targeting autophagy in cancer management—Strategies and developments. Cancer Manag. Res. 2015, 7, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baehrecke, E.H. Autophagy: Dual roles in life and death? Nat. Rev. Mol. Cell Biol. 2005, 6, 505–510. [Google Scholar] [CrossRef]
- Gonzalez-Polo, R.A.; Boya, P.; Pauleau, A.L.; Jalil, A.; Larochette, N.; Souquere, S.; Eskelinen, E.L.; Pierron, G.; Saftig, P.; Kroemer, G. The apoptosis/autophagy paradox: Autophagic vacuolization before apoptotic death. J. Cell Sci. 2005, 118, 3091–3102. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trybus, W.; Król, T.; Trybus, E.; Stachurska, A. Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells. Cells 2021, 10, 2029. https://doi.org/10.3390/cells10082029
Trybus W, Król T, Trybus E, Stachurska A. Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells. Cells. 2021; 10(8):2029. https://doi.org/10.3390/cells10082029
Chicago/Turabian StyleTrybus, Wojciech, Teodora Król, Ewa Trybus, and Anna Stachurska. 2021. "Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells" Cells 10, no. 8: 2029. https://doi.org/10.3390/cells10082029
APA StyleTrybus, W., Król, T., Trybus, E., & Stachurska, A. (2021). Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells. Cells, 10(8), 2029. https://doi.org/10.3390/cells10082029





