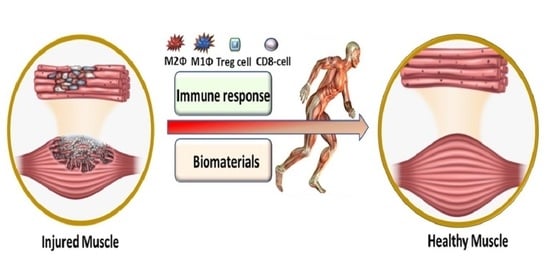
Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss
Abstract
1. Introduction
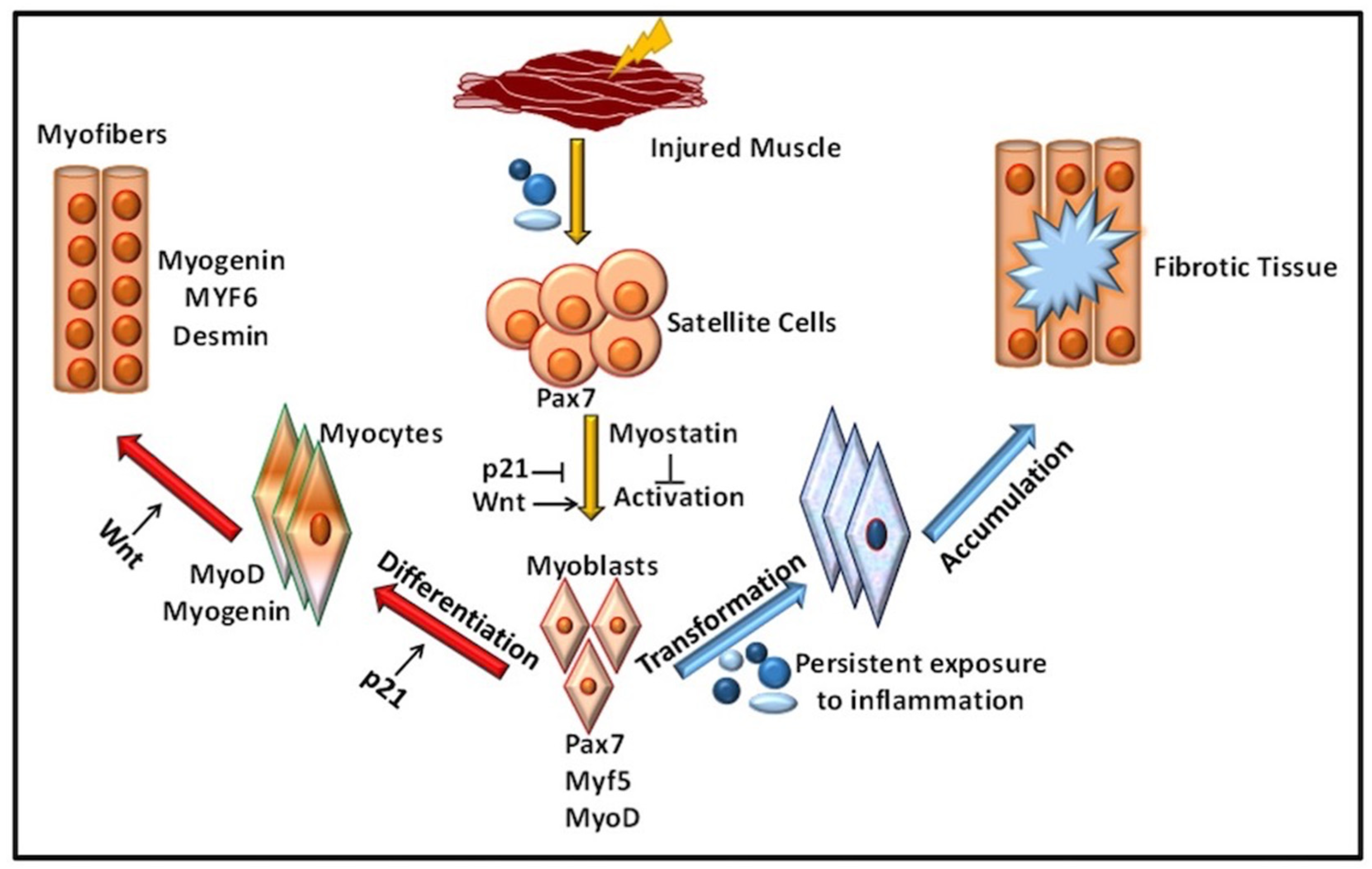
2. Role of Immune Response in VML
Inflammation in Immune-Mediated VML
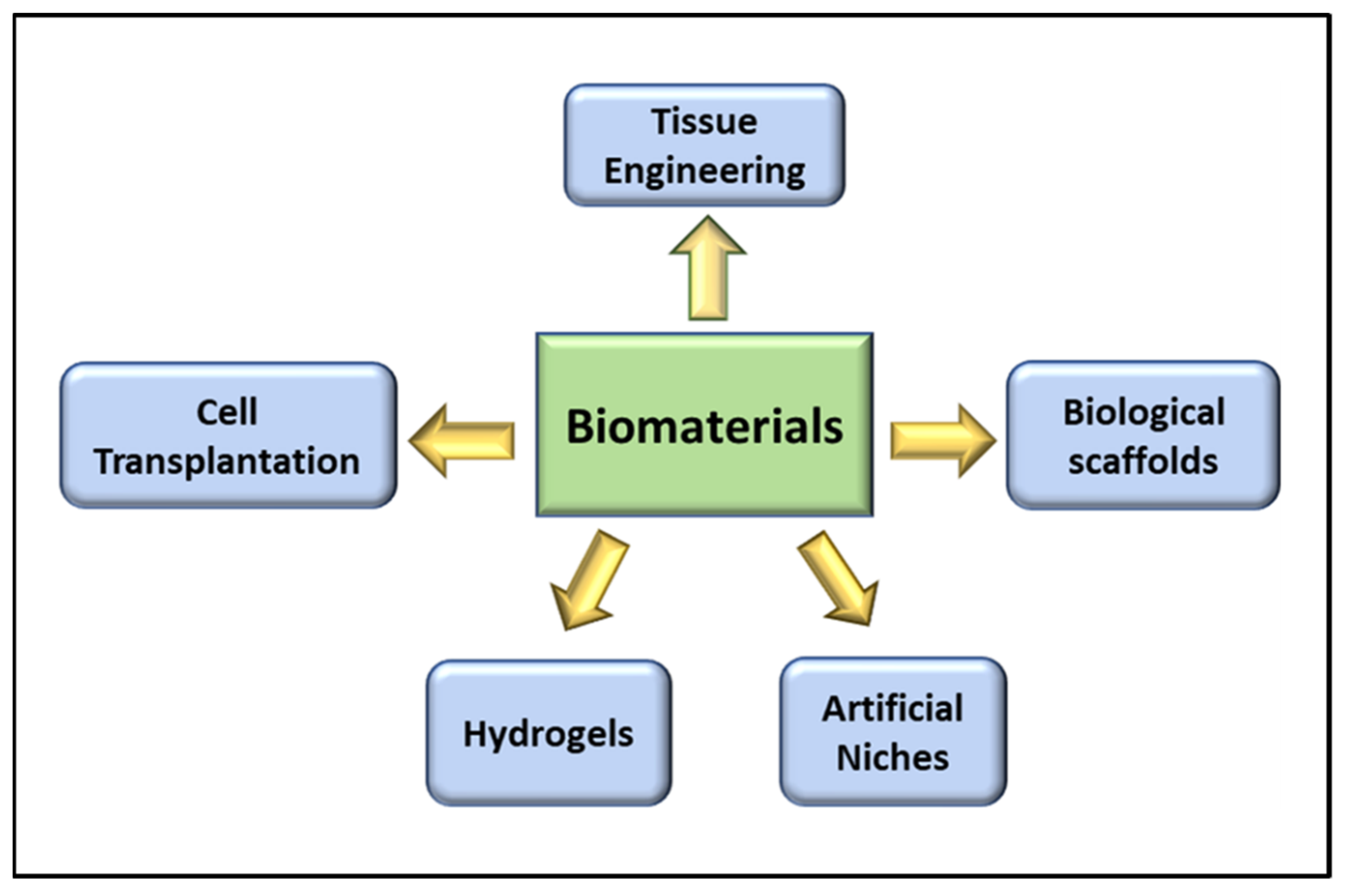
3. Role of Biomaterials in VML
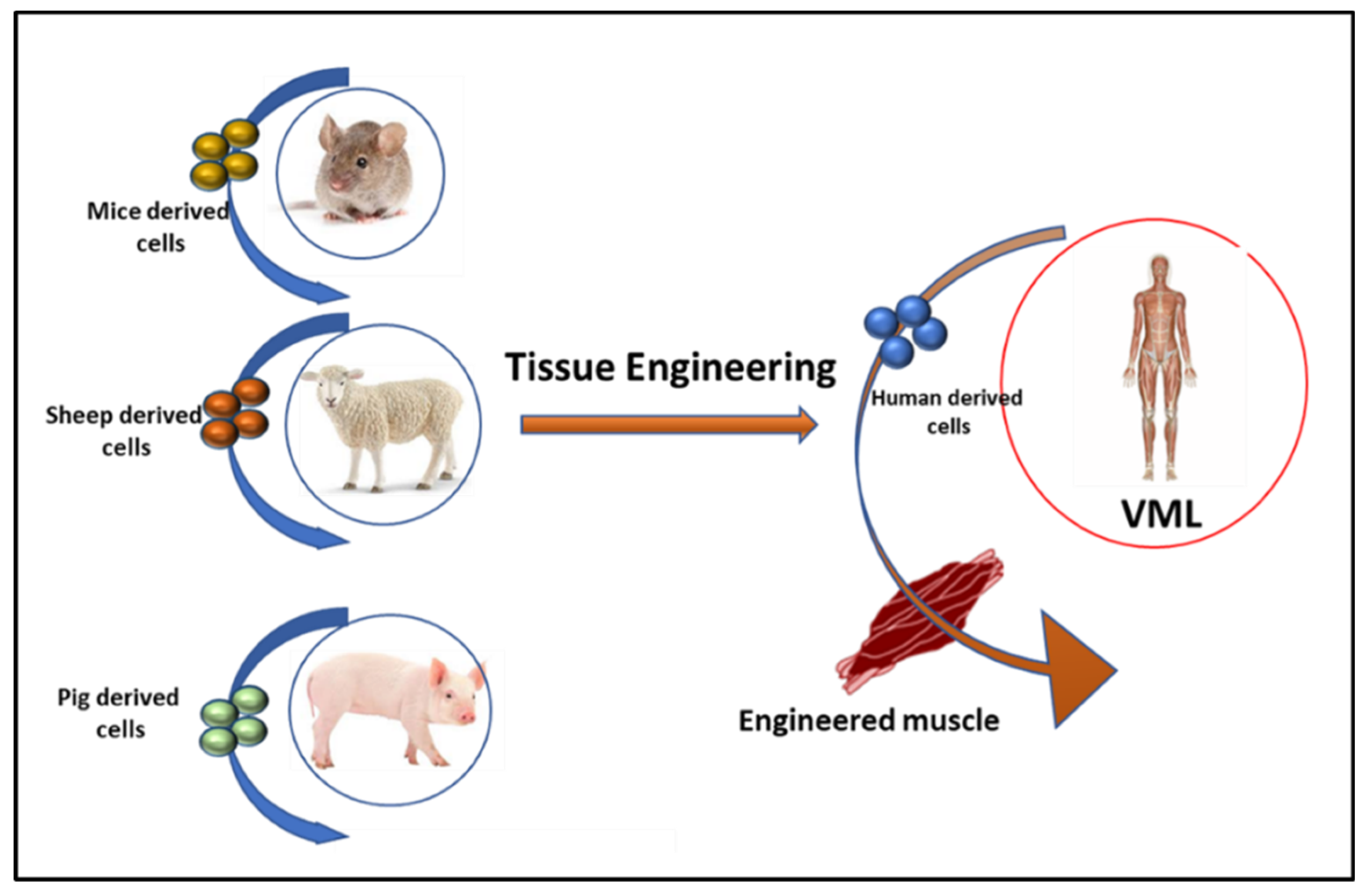
3.1. Tissue Engineering in VML
3.2. Cell Transplantation in VML
3.3. Biological Scaffolds in VML
3.4. Role of Hydrogels in VML Repair
3.5. The Artificial Niche
3.6. Non-Invasive Therapies for VML
4. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar]
- Song, T.; Manoharan, P.; Millay, D.P.; Koch, S.E.; Rubinstein, J.; Heiny, J.A.; Sadayappan, S. Dilated cardiomyopathy-mediated heart failure induces a unique skeletal muscle myopathy with inflammation. Skelet. Muscle 2019, 9, 4. [Google Scholar] [CrossRef]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Grogan, B.F.; Hsu, J.R. Volumetric Muscle Loss. J. Am. Acad. Orthop. Surg. 2011, 19, S35–S37. [Google Scholar] [CrossRef]
- Schnall, B.L.; Chen, M.Y.-T.; Bell, E.M.; Wolf, E.J.; Wilken, J.M. Functional Outcomes of Service Members With Bilateral Transfemoral and Knee Disarticulation Amputations Resulting From Trauma. Mil. Med. 2016, 181, 55–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greising, S.M.; Dearth, C.L.; Corona, B.T. Regenerative and Rehabilitative Medicine: A Necessary Synergy for Functional Recovery from Volumetric Muscle Loss Injury. Cells Tissues Organs 2016, 202, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M.; Valerio, M.S.; Janakiram, N.B.; Dearth, C.L. COX-2 inhibition does not alter wound healing outcomes of a volumetric muscle loss injury treated with a biologic scaffold. J. Tissue Eng. Regen. Med. 2020, 14, 1929–1938. [Google Scholar] [CrossRef]
- Magarotto, F.; Sgro, A.; Dorigo Hochuli, A.H.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.M.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653. [Google Scholar] [CrossRef]
- Goldman, S.M.; Janakiram, N.B.; Valerio, M.S.; Dearth, C.L. Evaluation of licofelone as an adjunct anti-inflammatory therapy to biologic scaffolds in the treatment of volumetric muscle loss. Cell Tissue Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Rodriguez, B.L.; Vega-Soto, E.E.; Kennedy, C.S.; Nguyen, M.H.; Cederna, P.S.; Larkin, L.M. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLOS ONE 2020, 15, e0239152. [Google Scholar] [CrossRef]
- Nuge, T.; Tshai, K.Y.; Lim, S.S.; Nordin, N.; Hoque, E. Characterization and optimization of the mechanical properties of electrospun gelatin nanofibrous scaffolds. World J. Eng. 2020, 17, 12–20. [Google Scholar] [CrossRef]
- Shayan, M.; Huang, N.F. Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioeng. 2020, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Collins, R.A.; Grounds, M.D. The Role of Tumor Necrosis Factor-alpha (TNF-α) in Skeletal Muscle Regeneration: Studies in TNF-α(-/-) and TNF-α(-/-)/LT-α(-/-) Mice. J. Histochem. Cytochemi. 2001, 49, 989–1001. [Google Scholar] [CrossRef]
- Frenette, J.; Cai, B.; Tidball, J.G. Complement Activation Promotes Muscle Inflammation during Modified Muscle Use. Am. J. Pathol. 2000, 156, 2103–2110. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Bazgir, B.; Fathi, R.; Valojerdi, M.R.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J 2016, 18, 473–484. [Google Scholar]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Etienne, J.; Liu, C.; Skinner, C.M.; Conboy, M.J.; Conboy, I.M. Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skelet. Muscle 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z. The skeletal muscle satellite cell: Still young and fascinating at 50. J. Histochem. Cytochem. 2011, 59, 1041–1059. [Google Scholar] [CrossRef]
- Saclier, M.; Theret, M.; Mounier, R.; Chazaud, B. Effects of Macrophage Conditioned-Medium on Murine and Human Muscle Cells: Analysis of Proliferation, Differentiation, and Fusion. Methods Mol. Biol. 2017, 1556, 317–327. [Google Scholar]
- Bencze, M.; Negroni, E.; Vallese, D.; Yacoub-Youssef, H.; Chaouch, S.; Wolff, A.; Aamiri, A.; Di Santo, J.; Chazaud, B.; Butler-Browne, G.; et al. Proinflammatory Macrophages Enhance the Regenerative Capacity of Human Myoblasts by Modifying Their Kinetics of Proliferation and Differentiation. Mol. Ther. 2012, 20, 2168–2179. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Dort, J.; Fabre, P.; Molina, T.; Dumont, N.A. Macrophages Are Key Regulators of Stem Cells during Skeletal Muscle Regeneration and Diseases. Stem Cells Int. 2019, 2019, 1–20. [Google Scholar] [CrossRef]
- Christ, G.J.; Saul, J.M.; Furth, M.E.; Andersson, K.-E. The Pharmacology of Regenerative Medicine. Pharmacol. Rev. 2013, 65, 1091–1133. [Google Scholar] [CrossRef] [PubMed]
- Cezar, C.A.; Mooney, D.J. Biomaterial-based delivery for skeletal muscle repair. Adv. Drug Deliv. Rev. 2015, 84, 188–197. [Google Scholar] [CrossRef]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Inflammation research sails through the sea of immunology to reach immunometabolism. Int. Immunopharmacol. 2019, 73, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.; Gilroy, D. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Schiaffino, S.; Pereira, M.G.; Ciciliot, S.; Rovere-Querini, P. Regulatory T cells and skeletal muscle regeneration. FEBS J. 2016, 284, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, A.; Pereira, T.; Bartolo, P.; Santos, J.; Luís, A.L.; Maurício, A.C.; Santos, A.R.C. Mesenchymal Stem Cells and Biomaterials Systems—Perspectives for Skeletal Muscle Tissue Repair and Regeneration. Procedia Eng. 2015, 110, 90–97. [Google Scholar] [CrossRef]
- Kim, H.; Bae, C.; Kook, Y.-M.; Koh, W.-G.; Lee, K.; Park, M.H. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Sakthivel, D.; Kruse, C.; Tran, P.; Giatsidis, G.; Sinha, I. Gene expression profiling of skeletal muscle after volumetric muscle loss. Wound Repair Regen. 2017, 25, 408–413. [Google Scholar] [CrossRef]
- Corona, B.T.; Rivera, J.C.; Greising, S.M. Inflammatory and Physiological Consequences of Debridement of Fibrous Tissue after Volumetric Muscle Loss Injury. Clin. Transl. Sci. 2017, 11, 208–217. [Google Scholar] [CrossRef]
- Kim, J.T.; Kasukonis, B.; Dunlap, G.; Perry, R.; Washington, T.; Wolchok, J.C.; Wolchok, J. Regenerative Repair of Volumetric Muscle Loss Injury is Sensitive to Age. Tissue Eng. Part A 2020, 26, 3–14. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Nakatsuji, M.; Seno, H.; Ishizu, S.; Akitake-Kawano, R.; Kanda, K.; Ueo, T.; Komekado, H.; Kawada, M.; Minami, M.; et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 2011, 32, 1333–1339. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef] [PubMed]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengeneering 2020, 7, 85. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Nutter, G.P.; VanDusen, K.W.; Florida, S.E.; Syverud, B.C.; Larkin, L.M. The Effects of Engineered Skeletal Muscle on Volumetric Muscle Loss in The Tibialis Anterior Of Rat After Three Months In Vivo. Regen. Eng. Transl. Med. 2020, 6, 365–372. [Google Scholar] [CrossRef]
- Wroblewski, O.M.; Vega-Soro, E.E.; Nguyen, M.H.; Cederna, P.S.; Larkin, L.M. Impact of Human Epidermal Growth Factor on Tissue-Engineered Skeletal Muscle Structure and Function. Tissue Eng. Part A 2021. preprint. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Ghiacci, G.; Macaluso, G.M. Tailoring the Interface of Biomaterials to Design Effective Scaffolds. J. Funct. Biomater. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Pantelic, M.N.; Larkin, L.M. Stem Cells for Skeletal Muscle Tissue Engineering. Tissue Eng. Part B Rev. 2018, 24, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T.V. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015, 21, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Wilson, A.; Hodgson-Garms, M.; Frith, J.E.; Genever, P. Multiplicity of Mesenchymal Stromal Cells: Finding the Right Route to Therapy. Front. Immunol. 2019, 10, 1112. [Google Scholar] [CrossRef] [PubMed]
- Maesner, C.C.; Almada, A.E.; Wagers, A.J. Established cell surface markers efficiently isolate highly overlapping populations of skeletal muscle satellite cells by fluorescence-activated cell sorting. Skelet. Muscle 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Francetic, T.; Li, Q. Skeletal myogenesis andMyf5activation. Transcription 2011, 2, 109–114. [Google Scholar] [CrossRef]
- Rogers, R.G.; Li, L.; Peck, K.; Sanchez, L.; Liu, W.; Ciullo, A.; Alfaro, J.; Rannou, A.; Fournier, M.; Lee, Y.; et al. Cardiosphere-derived cells, with and without a biological scaffold, stimulate myogenesis and recovery of muscle function in mice with volumetric muscle loss. Biomaterials 2021, 274, 120852. [Google Scholar] [CrossRef]
- Nalbandian, M.; Zhao, M.; Sasaki-Honda, M.; Jonouchi, T.; Lucena-Cacace, A.; Mizusawa, T.; Yasuda, M.; Yoshida, Y.; Hotta, A.; Sakurai, H. Characterization of hiPSC-Derived Muscle Progenitors Reveals Distinctive Markers for Myogenic Cell Purification Toward Cell Therapy. Stem Cell Rep. 2021, 16, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tazumi, A.; Takayama, S.; Takenaka-Ninagawa, N.; Nalbandian, M.; Nagai, M.; Nakamura, Y.; Nakasa, M.; Watanabe, A.; Ikeya, M.; et al. Induced Fetal Human Muscle Stem Cells with High Therapeutic Potential in a Mouse Muscular Dystrophy Model. Stem Cell Rep. 2020, 15. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; Mccool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Haas, G.J.; Dunn, A.J.; Marcinczyk, M.; Talovic, M.; Schwartz, M.; Scheidt, R.; Patel, A.D.; Hixon, K.R.; Elmashhady, H.; Mcbride-Gagyi, S.H.; et al. Biomimetic sponges for regeneration of skeletal muscle following trauma. J. Biomed. Mater. Res. Part A 2018, 107, 92–103. [Google Scholar] [CrossRef]
- Haas, G.; Dunn, A.; Madsen, J.; Genovese, P.; Chauvin, H.; Au, J.; Ziemkiewicz, N.; Johnson, D.; Paoli, A.; Lin, A.; et al. Biomimetic sponges improve muscle structure and function following volumetric muscle loss. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.D. FK506, An Immunosuppressant Targeting Calcineurin Function. Curr. Med. Chem. 2000, 7, 731–748. [Google Scholar]
- Rizzi, R.; Bearzi, C.; Mauretti, A.; Bernardini, S.; Cannata, S.; Gargioli, C. Tissue engineering for skeletal muscle regeneration. Muscle Ligaments Tendons J. 2012, 2, 230–234. [Google Scholar]
- Urciuolo, A.; De Coppi, P. Decellularized Tissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.; Blau, H.M. Designing materials to direct stem-cell fate. Nat. Cell Biol. 2009, 462, 433–441. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Alcazar, C.; Yang, G.; Quarta, M.; Paine, P.; Doan, L.; Davies, A.; Rando, T.A.; Huang, N.F. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. Npj Regen. Med. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachex Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Herberts, C.A.; Kwa, M.S.G.; Hermsen, H.P.H. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pr. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; García, A.J. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. J. Int. Soc. Matrix Biol. 2017, 57–58, 324–333. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Vega, M.E.; Pastino, A.K.; Singh, S.; Guvendiren, M.; Kohn, J.; Murthy, N.S.; Schwarzbauer, J.E. Development of hybrid scaffolds with natural extracellular matrix deposited within synthetic polymeric fibers. J. Biomed. Mater. Res. Part A 2017, 105, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Rao Kummara, M.; Kamal, T.; Alghyamah, A.-a.A.; Jan Iftikhar, F.; Bano, B.; Khan, N.; Amjid Afridi, M.; Soo Han, S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Grasman, J.M.; Zayas, M.J.; Page, R.L.; Pins, G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef]
- Briggs, D.; Morgan, J.E. Recent progress in satellite cell/myoblast engraftment—Relevance for therapy. FEBS J. 2013, 280, 4281–4293. [Google Scholar] [CrossRef] [PubMed]
- Kasukonis, B.; Kim, J.; Brown, L.; Jones, J.; Ahmadi, S.; Washington, T.; Wolchok, J. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng. Part A 2016, 22, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Hai, Z.; Ding, Z.; Wang, L.; Liu, Y.; Zhang, H.; Liang, G. Alkaline phosphatase-triggered assembly of etoposide enhances its anticancer effect. Chem. Commun. 2018, 54, 1853–1856. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.P.; Elliott, R.M.; Kozin, S.H.; Levin, L.S. Free Function Muscle Transfers for Upper Extremity Reconstruction: A Review of Indications, Techniques, and Outcomes. J. Hand Surg. 2013, 38, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2017, 106, 672–679. [Google Scholar] [CrossRef]
- Han, W.M.; Anderson, S.E.; Mohiuddin, M.; Barros, D.; Nakhai, S.A.; Shin, E.; Amaral, I.F.; Pêgo, A.P.; García, A.J.; Jang, Y.C. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci. Adv. 2018, 4, eaar4008. [Google Scholar] [CrossRef]
- Dienes, J.; Browne, S.; Farjun, B.; Amaral Passipieri, J.; Mintz, E.L.; Killian, G.; Healy, K.E.; Christ, G.J. Semisynthetic Hyaluronic Acid-Based Hydrogel Promotes Recovery of the Injured Tibialis Anterior Skeletal Muscle Form and Function. ACS Biomater. Sci. Eng. 2021, 7, 1587–1599. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, I.G.; Piao, S.; Jung, A.R.; Lee, J.Y.; Park, K.D. Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials 2013, 34, 6037–6045. [Google Scholar] [CrossRef]
- Chu, H.; Wang, Y. Therapeutic angiogenesis: Controlled delivery of angiogenic factors. Ther. Deliv. 2012, 3, 693–714. [Google Scholar] [CrossRef]
- Emerich, D.F.; Silva, E.; Ali, O.; Mooney, D.; Bell, W.; Yu, S.J.; Kaneko, Y.; Borlongan, C. Injectable VEGF Hydrogels Produce Near Complete Neurological and Anatomical Protection following Cerebral Ischemia in Rats. Cell Transplant. 2010, 19, 1063–1071. [Google Scholar] [CrossRef]
- Jones, N.C.; Tyner, K.J.; Nibarger, L.; Stanley, H.M.; Cornelison, D.D.W.; Fedorov, Y.V.; Olwin, B.B. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 2005, 169, 105–116. [Google Scholar] [CrossRef]
- Cosgrove, B.D.; Gilbert, P.M.; Porpiglia, E.; Mourkioti, F.; Lee, S.P.; Corbel, S.Y.; Llewellyn, M.E.; Delp, S.L.; Blau, H.M. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014, 20, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Paul, A.; Vrana, N.E.; Zhao, X.; Memic, A.; Hwang, Y.-S.; Dokmeci, M.R.; Khademhosseini, A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 2014, 35, 7308–7325. [Google Scholar] [CrossRef] [PubMed]
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated Biomaterials for Engineering 3D Tissues. Adv. Mater. 2012, 24, 1782–1804. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Prüller, J.; Mannhardt, I.; Eschenhagen, T.; Zammit, P.S.; Figeac, N. Satellite cells delivered in their niche efficiently generate functional myotubes in three-dimensional cell culture. PLOS ONE 2018, 13, e0202574. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, C.; Salvatori, M.L.; Biondo, A.; Shapira-Schweitzer, K.; Santoleri, S.; Antonini, S.; Bernardini, S.; Tedesco, F.S.; Cannata, S.; Seliktar, D.; et al. Injectable polyethylene glycol-fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal-muscle degeneration. Skelet. Muscle 2012, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-X.; Xu, X.-Y.; Wang, J.; He, X.-T.; Sun, H.-H.; Chen, F.-M. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl. Mater. Today 2018, 11, 144–165. [Google Scholar] [CrossRef]
- Ferraro, F.; Celso, C.L.; Scadden, D. Adult stem cels and their niches. Adv. Exp. Med. Biol. 2010, 695, 155–168. [Google Scholar] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.A.; Pozzobon, M.; De Coppi, P. Advances in musculoskeletal tissue engineering: Moving towards therapy. Organogenesis 2010, 6, 167–172. [Google Scholar] [CrossRef]
- Thomas, K.; Engler, A.; Meyer, G.A. Extracellular matrix regulation in the muscle satellite cell niche. Connect. Tissue Res. 2014, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 2018, 15, 20180388. [Google Scholar] [CrossRef]
- Pacelli, S.; Basu, S.; Whitlow, J.; Chakravarti, A.; Acosta, F.; Varshney, A.; Modaresi, S.; Berkland, C.; Paul, A. Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug Deliv. Rev. 2017, 120, 50–70. [Google Scholar] [CrossRef]
- Patel, A.; Vendrell-Gonzalez, S.; Haas, G.; Marcinczyk, M.; Ziemkiewicz, N.; Talovic, M.; Fisher, J.S.; Garg, K. Regulation of Myogenic Activity by Substrate and Electrical Stimulation In Vitro. BioRes. Open Access 2019, 8, 129–138. [Google Scholar] [CrossRef]
- Stölting, M.N.; Arnold, A.S.; Haralampieva, D.; Handschin, C.; Sulser, T.; Eberli, D. Magnetic stimulation supports muscle and nerve regeneration after trauma in mice. Muscle Nerve 2015, 53, 598–607. [Google Scholar] [CrossRef]
- Ferraresi, C.; Bertucci, D.; Schiavinato, J.; Reiff, R.; Araújo, A.; Panepucci, R.; Matheucci, E., Jr.; Cunha, A.F.; Arakelian, V.M.; Hamblin, M.R.; et al. Effects of Light-Emitting Diode Therapy on Muscle Hypertrophy, Gene Expression, Performance, Damage, and Delayed-Onset Muscle Soreness: Case-control Study with a Pair of Identical Twins. Am. J. Phys. Med. Rehab. 2016, 9, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Borsa, P.A.; Larkin, K.A.; True, J.M. Does Phototherapy Enhance Skeletal Muscle Contractile Function and Postexercise Recovery? A Systematic Review. J. Athl. Train. 2013, 48, 57–67. [Google Scholar] [CrossRef]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Boil. Med. 2012, 85, 201–215. [Google Scholar]
- Fujita, N.; Murakami, S.; Arakawa, T.; Miki, A.; Fujino, H. The combined effect of electrical stimulation and resistance isometric contraction on muscle atrophy in rat tibialis anterior muscle. Bosn. J. Basic Med Sci. 2011, 11, 74–79. [Google Scholar] [CrossRef]
- Larkin-Kaiser, K.A.; Christou, E.; Tillman, M.; George, S.; Borsa, P.A. Near-Infrared Light Therapy to Attenuate Strength Loss After Strenuous Resistance Exercise. J. Athl. Train. 2015, 50, 45–50. [Google Scholar] [CrossRef] [PubMed]

| Advantages | Disadvantages | |
|---|---|---|
| Tissue Engineering |
|
|
| Cell Therapy |
|
|
| Biological Scaffolds |
|
|
| Hydrogels |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiran, S.; Dwivedi, P.; Kumar, V.; Price, R.L.; Singh, U.P. Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss. Cells 2021, 10, 2016. https://doi.org/10.3390/cells10082016
Kiran S, Dwivedi P, Kumar V, Price RL, Singh UP. Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss. Cells. 2021; 10(8):2016. https://doi.org/10.3390/cells10082016
Chicago/Turabian StyleKiran, Sonia, Pankaj Dwivedi, Vijay Kumar, Robert L. Price, and Udai P. Singh. 2021. "Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss" Cells 10, no. 8: 2016. https://doi.org/10.3390/cells10082016
APA StyleKiran, S., Dwivedi, P., Kumar, V., Price, R. L., & Singh, U. P. (2021). Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss. Cells, 10(8), 2016. https://doi.org/10.3390/cells10082016