Enzymatic Spermine Metabolites Induce Apoptosis Associated with Increase of p53, caspase-3 and miR-34a in Both Neuroblastoma Cells, SJNKP and the N-Myc-Amplified Form IMR5
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Purification and Determination of Catalytic Properties of BSAO
2.3. Neuroblastoma Cell Culture
2.4. Isolation of Neurons from Rat Brain Cortex
2.5. Treatments, Clonogenic and MTT Cytotoxicity Assays
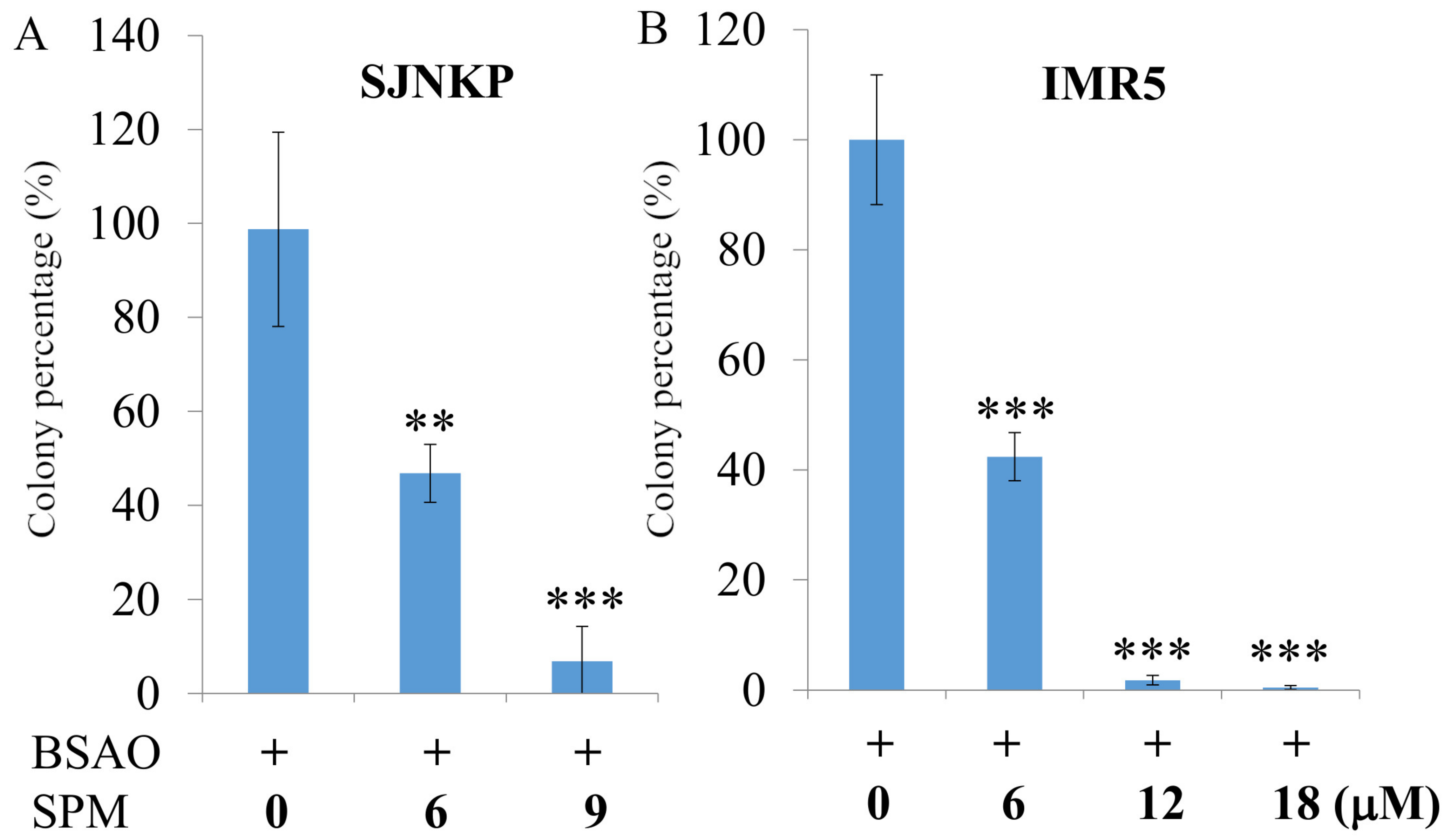
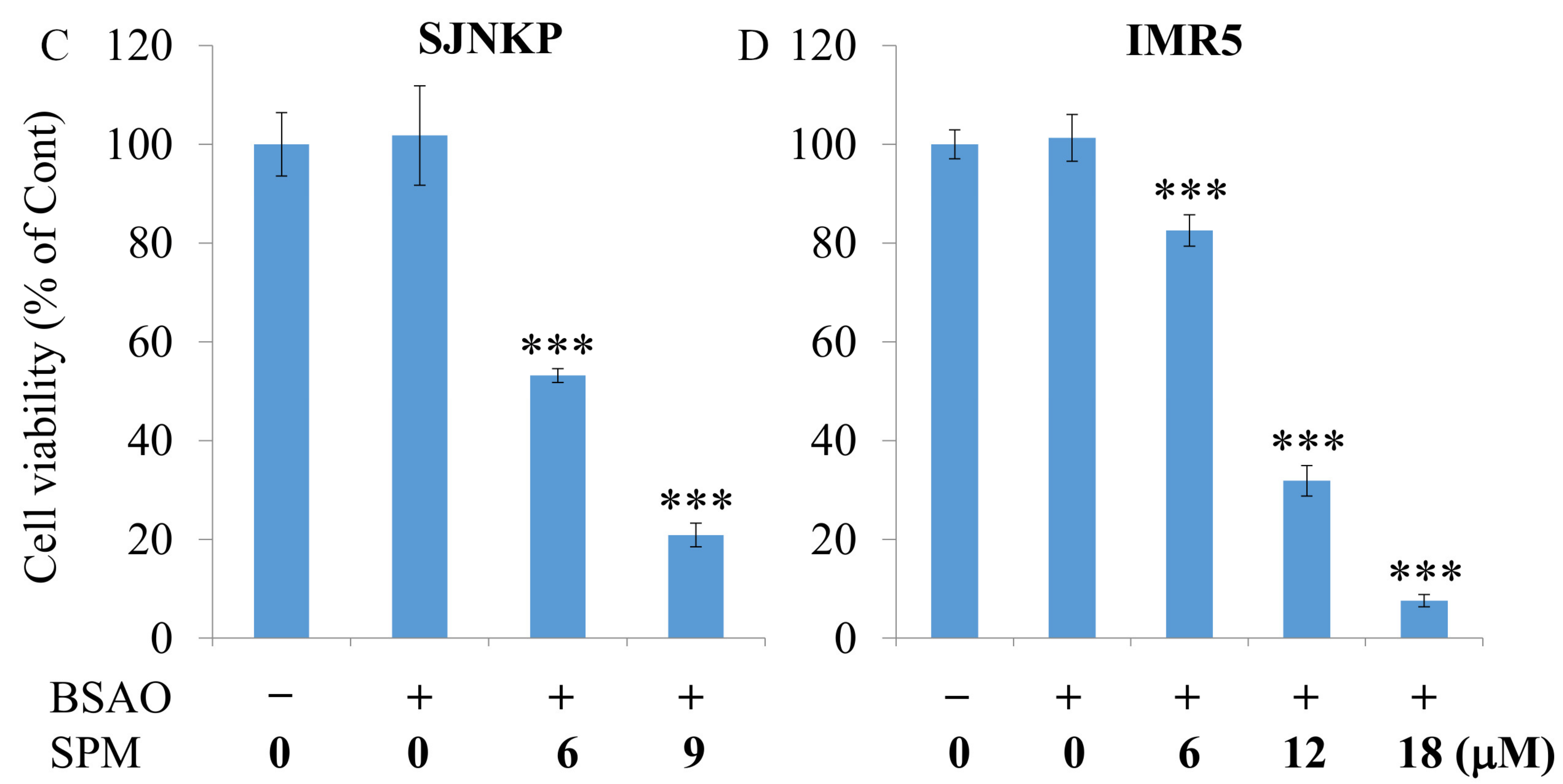
2.5.1. Clonogenic Assay
2.5.2. MTT Assay
2.6. Measurements “In Situ” of Mitochondrial Membrane Potential (Δψm)
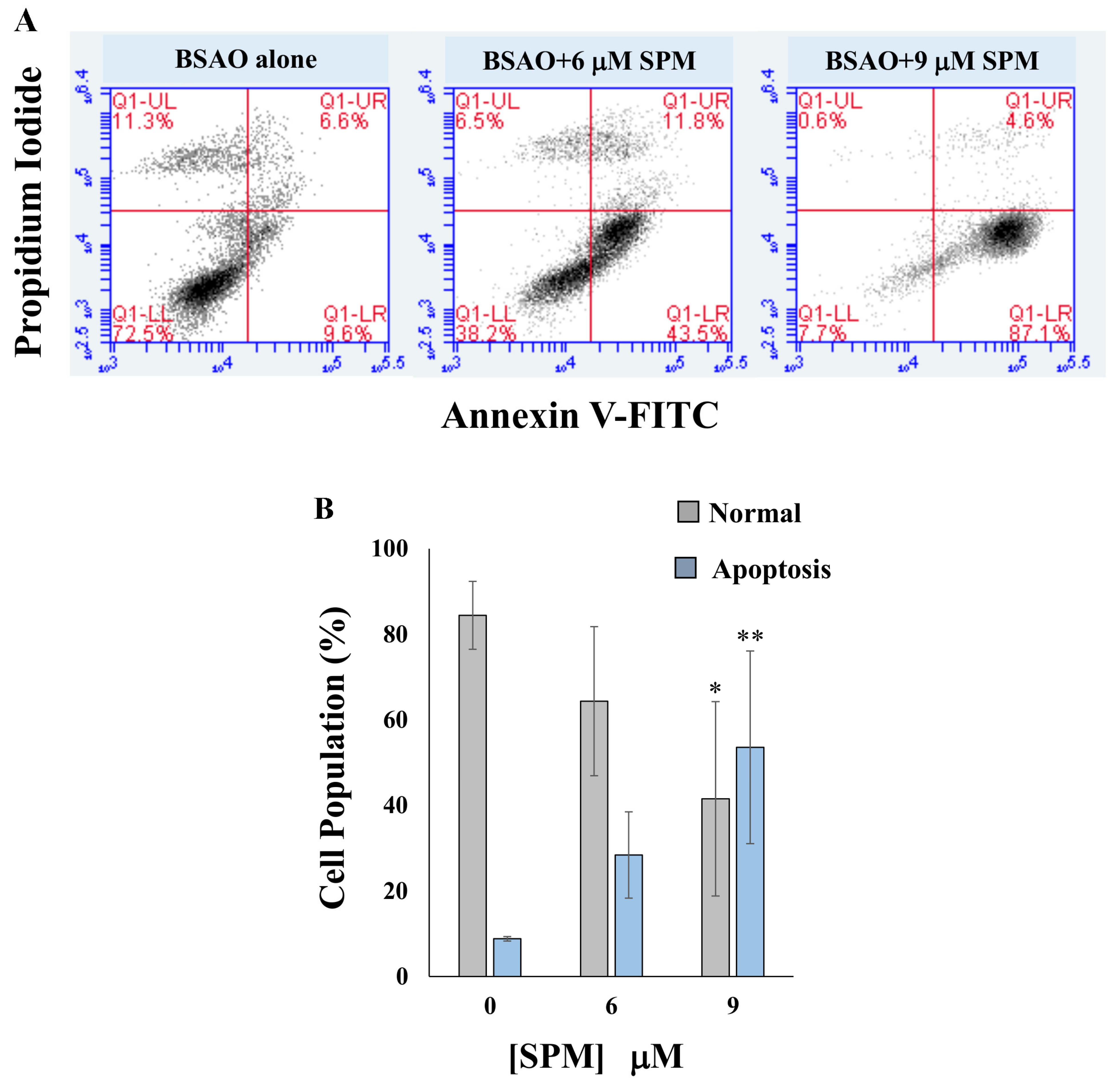
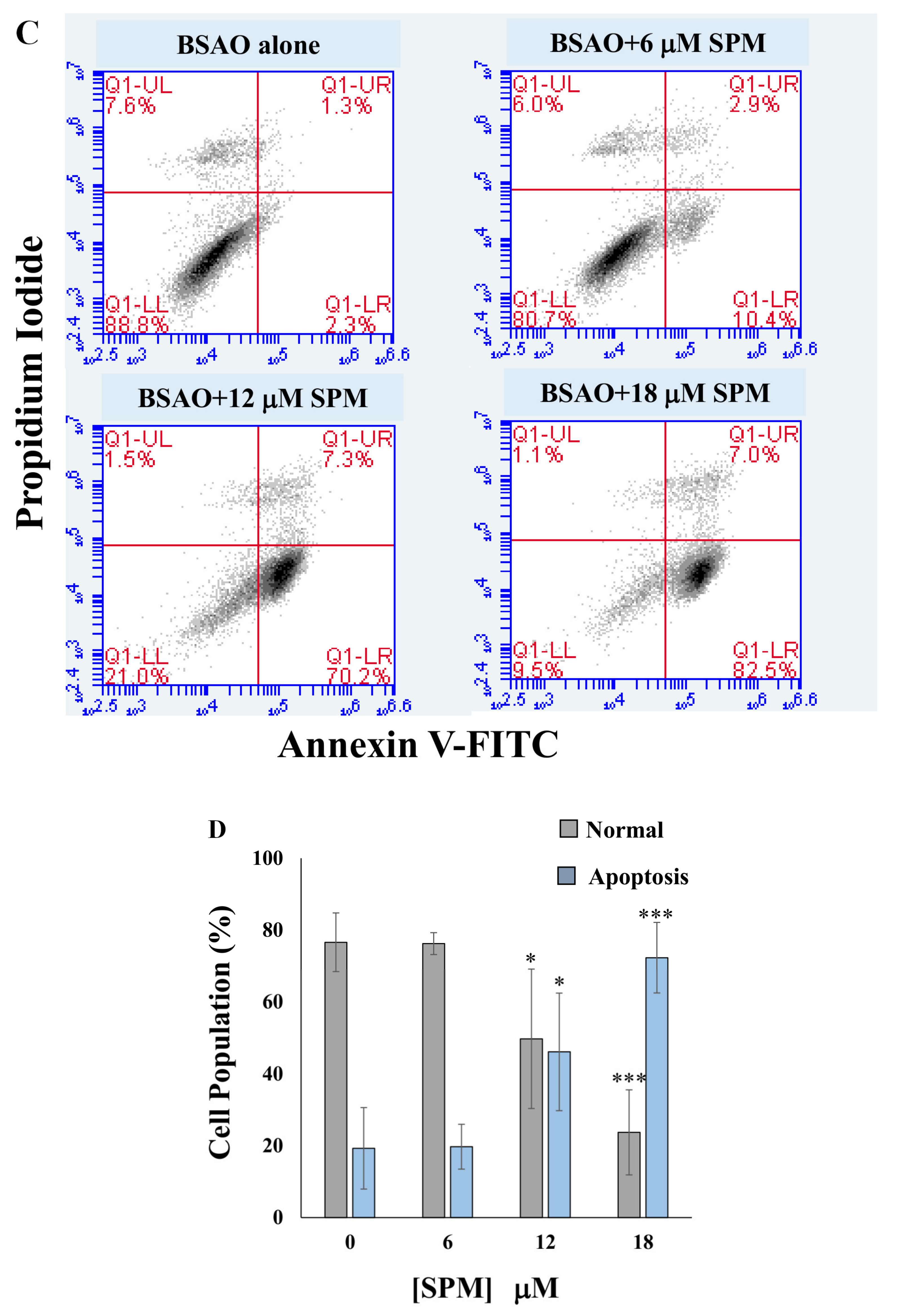
2.7. Determination of Apoptotic Cell Death by Annexin V-FITC Staining
2.8. Cell Cycle Analysis
2.9. RNA Isolation and Analysis
2.10. mRNA Reverse Transcription and Quantitative Real Time PCR (RT-qPCR)
2.11. miRNA Reverse Transcription and Quantitative Real Time PCR (RT-qPCR)
2.12. Western Blotting Analysis
2.13. Vitality Assay
2.14. Statistical Analysis
3. Results
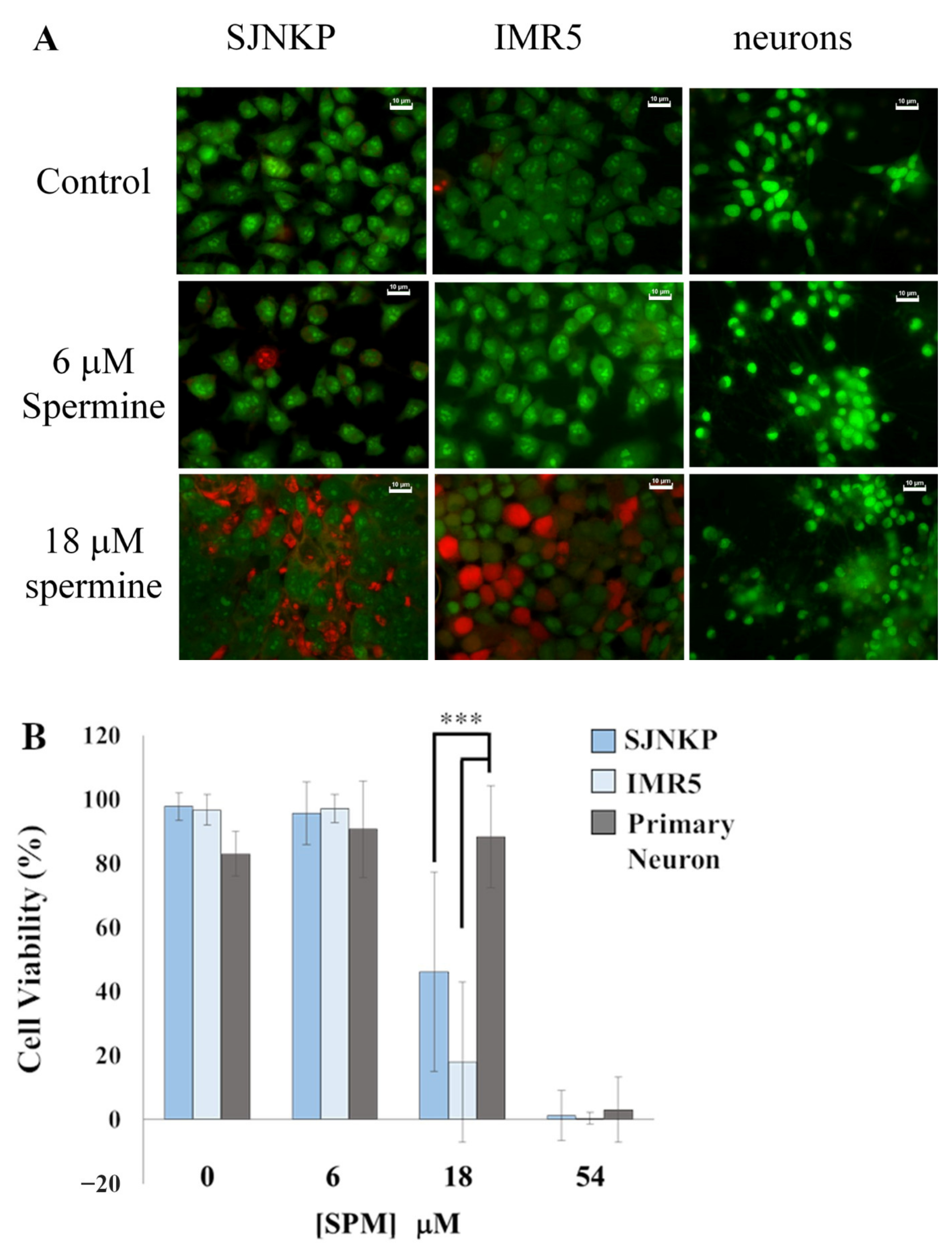
3.1. Cytotoxic Effect Induced by BSAO in the Presence of SPM on Neuroblastoma Cell Lines
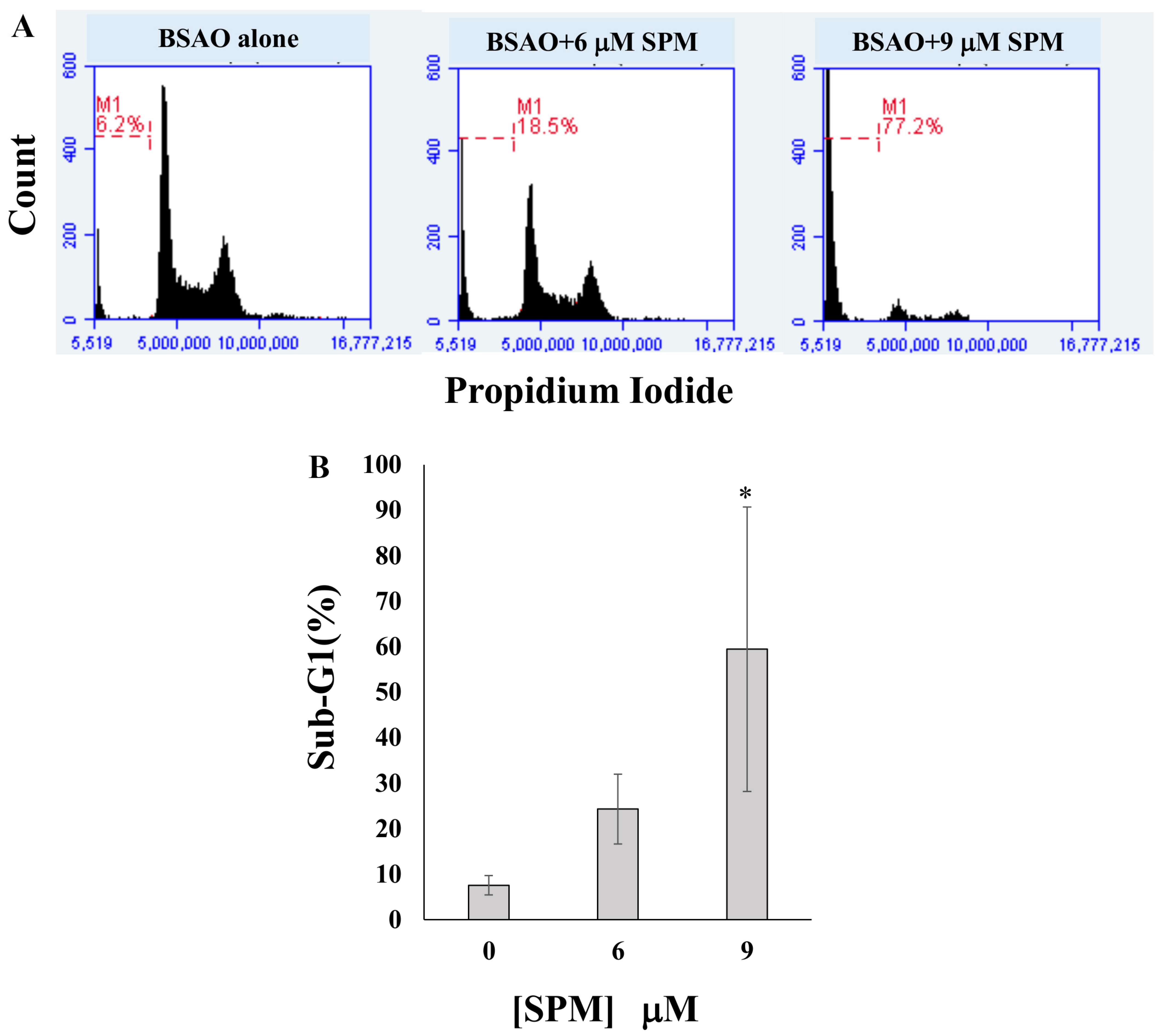
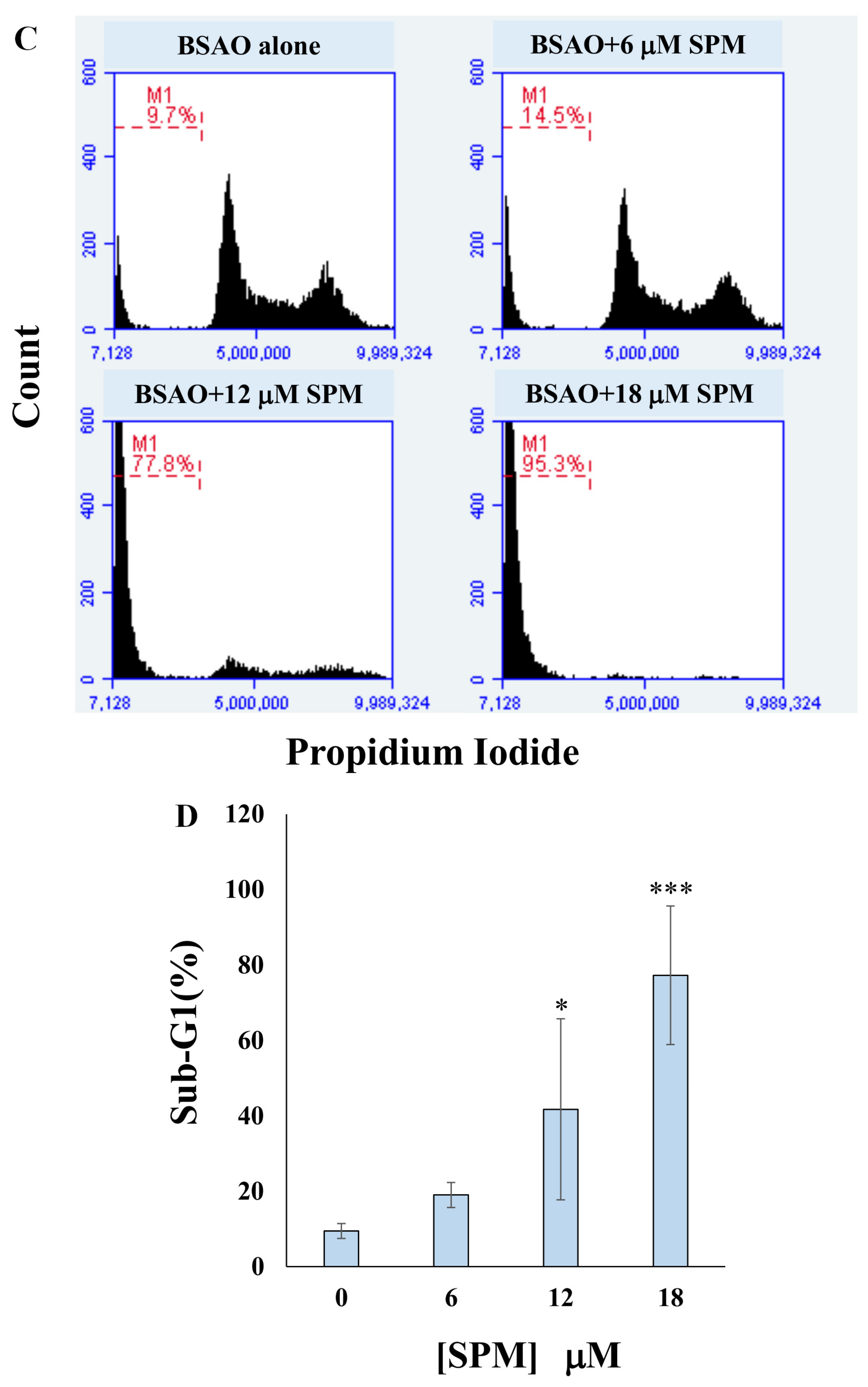
3.2. Proapoptotic Effects on Neuroblastoma Cell Lines: Annexin V-FITC/PI Assay and Cell Cycle Analysis by Flow Cytometry
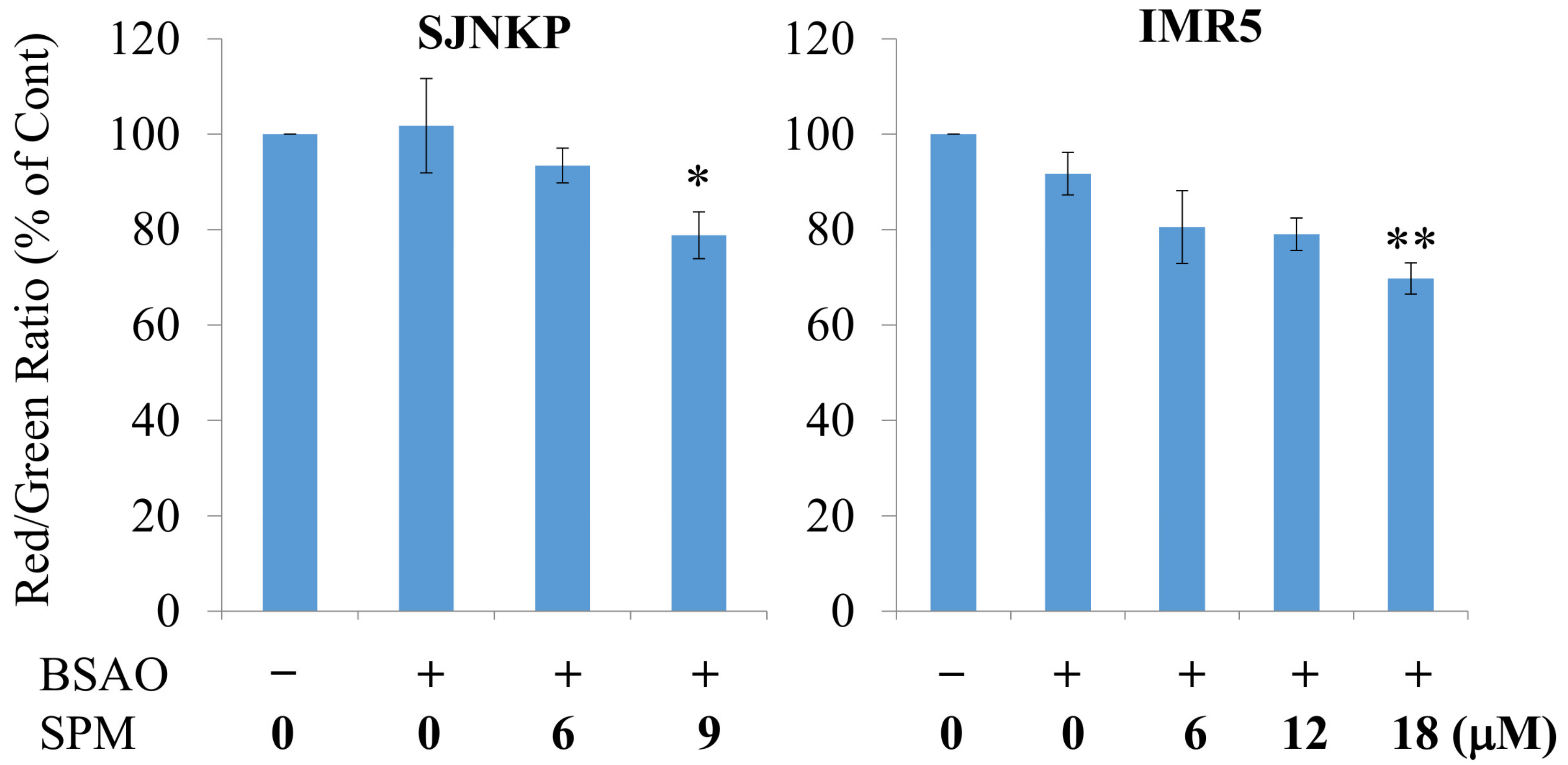
3.3. Effect of BSAO and SPM on Mitochondrial Membrane Potential in Neuroblastoma Cells
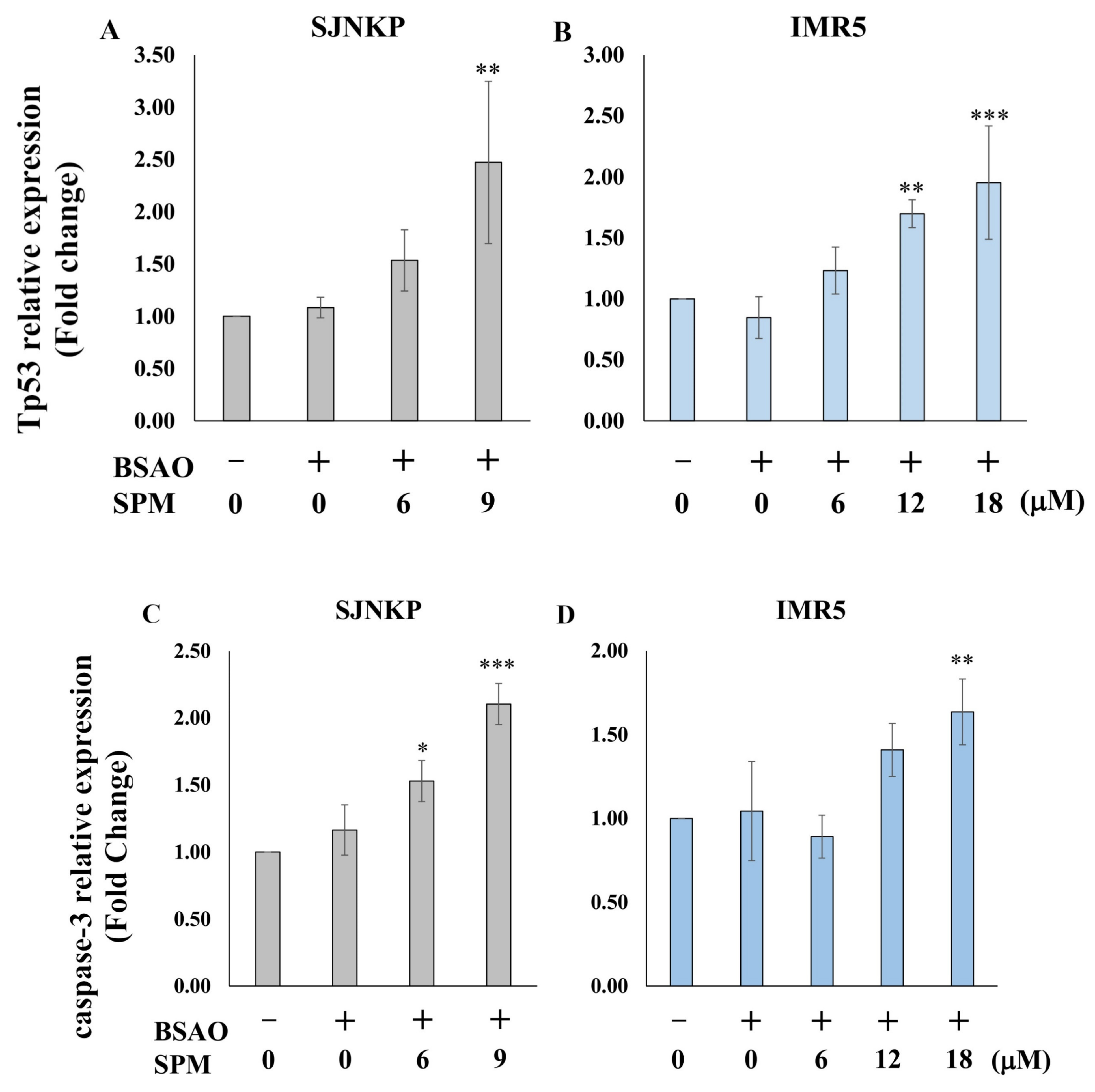
3.4. Analysis of Mitochondrial Apoptosis-Related mRNAs
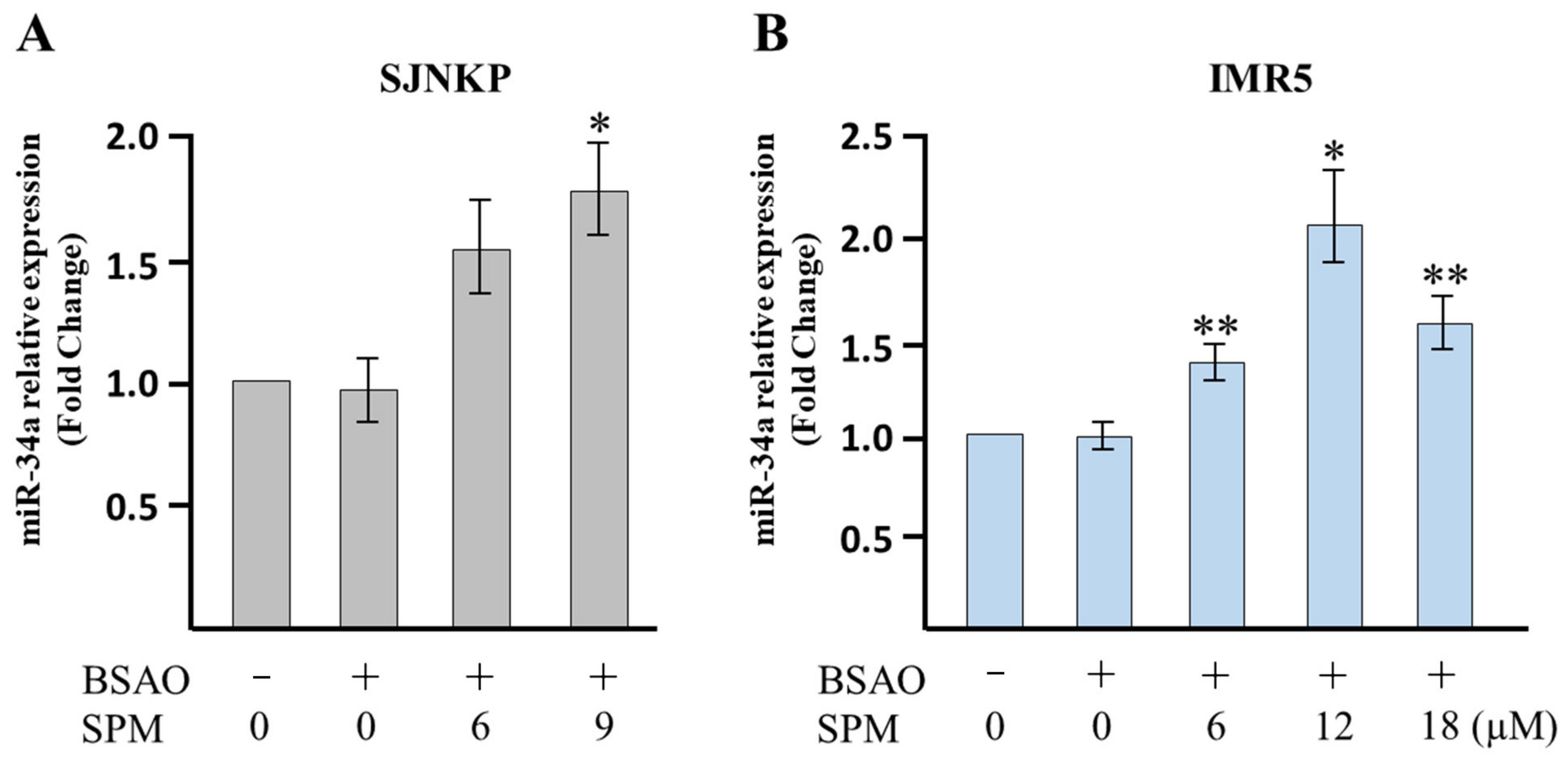
3.5. Analysis of Apoptosis-Related miR-34a
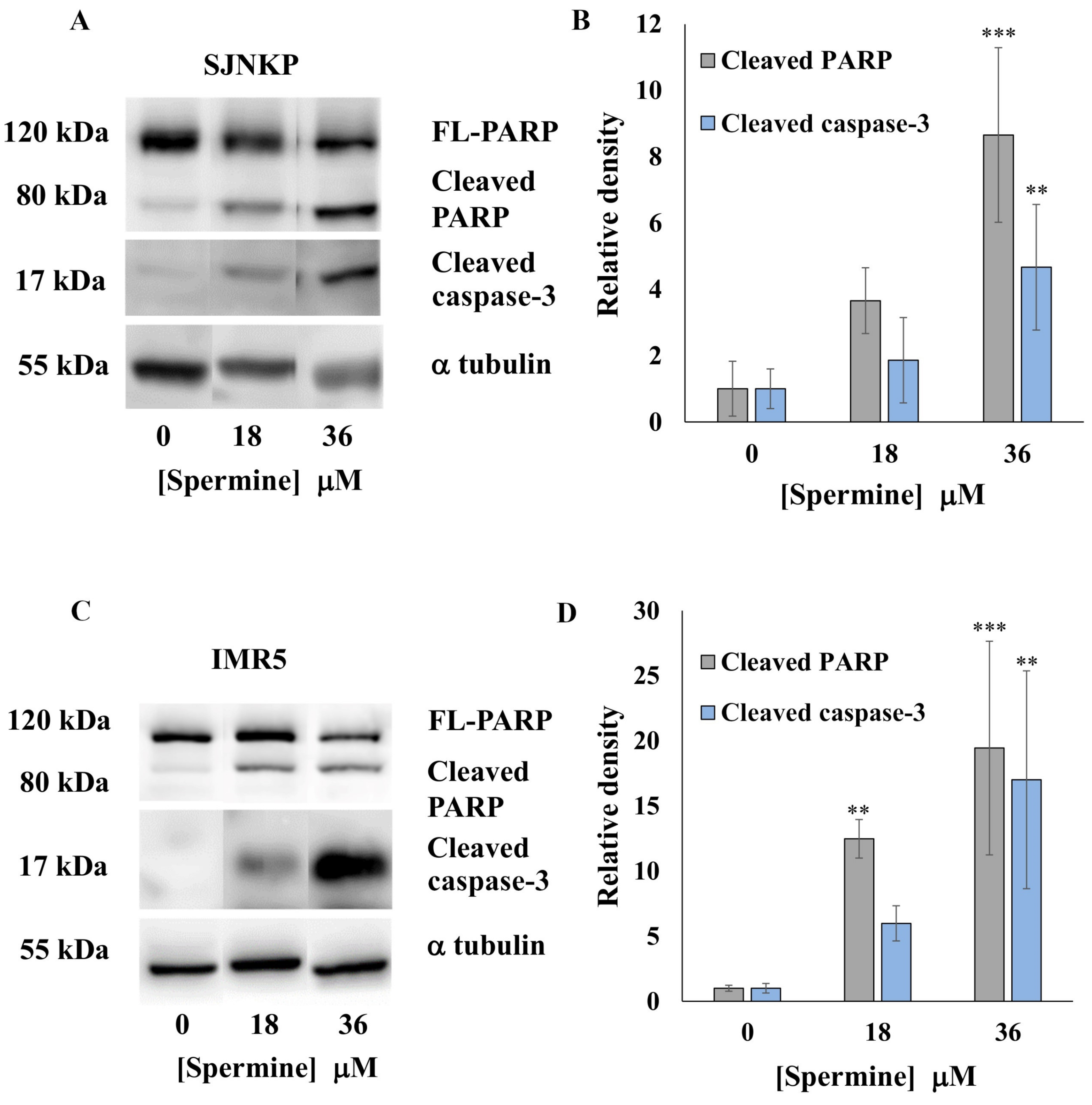
3.6. Effect of BSAO and SPM on Proapoptotic Protein Expression
3.7. Comparison of the Effects of BSAO/SPM on Both Neuroblastoma and Neurons
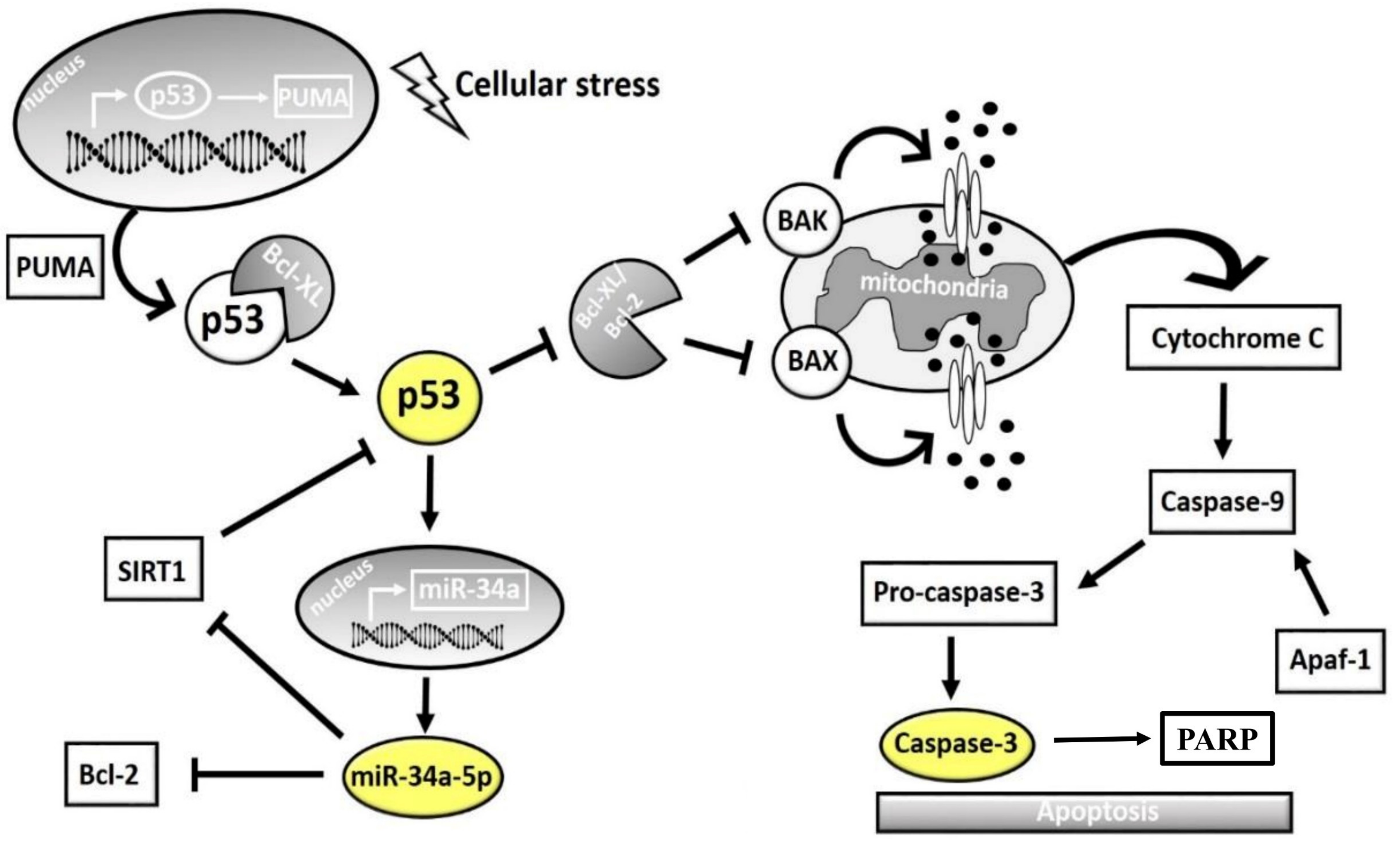
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostinelli, E.; Condello, M.; Tempera, G.; Macone, A.; Bozzuto, G.; Ohkubo, S.; Calcabrini, A.; Arancia, G.; Molinari, A. The combined treatment with chloroquine and the enzymatic oxidation products of spermine overcomes multidrug resistance of melanoma M14 ADR2 cells: A new therapeutic approach. Int. J. Oncol. 2014, 45, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Thompson, T.D.; Miller, J.W.; Pollack, L.A.; Stewart, S.L. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics 2008, 121, e1470–e1477. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Maris, J.M. Neuroblastoma. In Principles and Practice of Pediatric Oncology, 5th ed.; Pizzo, D.A., Poplack, D.G., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 933–970. [Google Scholar]
- Fritsch, P.; Kerbl, R.; Lackner, H.; Urban, C. “Wait and see” strategy in localized neuroblastoma in infants: An option not only for cases detected by mass screening. Pediatr. Blood Cancer 2004, 43, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Luksch, R.; Castellani, M.R.; Collini, P.; De Bernardi, B.; Conte, M.; Gambini, C.; Gandola, L.; Garaventa, A.; Biasoni, D.; Podda, M.; et al. Neuroblastoma (Peripheral neuroblastic tumours). Crit. Rev. Oncol. Hematol. 2016, 107, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, A.; Li, Y.; Izumi, H.; Muramori, K.; Inada, H.; Nishi, M. Neuroblastoma. Jpn. J. Clin. Oncol. 2018, 48, 214–241. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef]
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. 2015, 33, 3008–3017. [Google Scholar] [CrossRef]
- Coughlan, D.; Gianferante, M.; Lynch, C.F.; Stevens, J.L.; Harlan, L.C. Treatment and survival of childhood neuroblastoma: Evidence from a population-based study in the United States. Pediatr. Hematol. Oncol. 2017, 34, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A.; Abdessalam, S.; Aldrink, J.H.; Austin, M.; Heaton, T.E.; Bruny, J.; Ehrlich, P.; Dasgupta, R.; Baertschiger, R.M.; Lautz, T.B.; et al. Update on neuroblastoma. J. Pediatr. Surg. 2019, 54, 383–389. [Google Scholar] [CrossRef]
- Moreno, L.; Barone, G.; DuBois, S.G.; Molenaar, J.; Fischer, M.; Schulte, J.; Eggert, A.; Schleiermacher, G.; Speleman, F.; Chesler, L.; et al. Accelerating drug development for neuroblastoma: Summary of the Second Neuroblastoma Drug Development Strategy forum from Innovative Therapies for Children with Cancer and International Society of Paediatric Oncology Europe Neuroblastoma. Eur. J. Cancer 2020, 136, 52–68. [Google Scholar] [CrossRef]
- Ohkubo, S.; Mancinelli, R.; Miglietta, S.; Cona, A.; Angelini, R.; Canettieri, G.; Spandidos, D.A.; Gaudio, E.; Agostinelli, E. Maize polyamine oxidase in the presence of spermine/spermidine induces the apoptosis of LoVo human colon adenocarcinoma cells. Int. J. Oncol. 2019, 54, 2080–2094. [Google Scholar] [CrossRef]
- Pegg, A.E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988, 48, 759–774. [Google Scholar]
- Bachrach, U.; Wang, Y.C.; Tabib, A. Polyamines: New cues in cellular signal transduction. News Physiol. Sci. 2001, 16, 106–109. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Minocha, S.C.; Minocha, R.; Handa, A.K. Polyamines and cellular metabolism in plants: Transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 2010, 38, 405–413. [Google Scholar] [CrossRef]
- Shukla, V.; Fatima, T.; Goyal, R.K.; Handa, A.K.; Mattoo, A.K. Engineered Ripening-Specific Accumulation of Polyamines Spermidine and Spermine in Tomato Fruit Upregulates Clustered C/D Box snoRNA Gene Transcripts in Concert with Ribosomal RNA Biogenesis in the Red Ripe Fruit. Plants 2020, 9, 1710. [Google Scholar] [CrossRef]
- Migliaccio, N.; Martucci, N.M.; Ruggiero, I.; Sanges, C.; Ohkubo, S.; Lamberti, A.; Agostinelli, E.; Arcari, P. Ser/Thr kinases and polyamines in the regulation of non-canonical functions of elongation factor 1A. Amino Acids 2016, 48, 2339–2352. [Google Scholar] [CrossRef]
- Schuber, F. Influence of polyamines on membrane functions. Biochem. J. 1989, 260, 1–10. [Google Scholar] [CrossRef]
- Ficker, E.; Taglialatela, M.; Wible, B.A.; Henley, C.M.; Brown, A.M. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science 1994, 266, 1068–1072. [Google Scholar] [CrossRef]
- Neel, B.G.; Tonks, N.K. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 1997, 9, 193–204. [Google Scholar] [CrossRef]
- Grancara, S.; Dalla Via, L.; Garcia-Argaez, A.N.; Ohkubo, S.; Pacella, E.; Manente, S.; Bragadin, M.; Toninello, A.; Agostinelli, E. Spermine cycling in mitochondria is mediated by adenine nucleotide translocase activity: Mechanism and pathophysiological implications. Amino Acids 2016, 48, 2327–2337. [Google Scholar] [CrossRef]
- Pottosin, I.; Olivas-Aguirre, M.; Dobrovinskaya, O.; Zepeda-Jazo, I.; Shabala, S. Modulation of Ion Transport Across Plant Membranes by Polyamines: Understanding Specific Modes of Action Under Stress. Front. Plant. Sci. 2020, 11, 616077. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359. [Google Scholar] [CrossRef]
- Lee, C.Y.; Su, G.C.; Huang, W.Y.; Ko, M.Y.; Yeh, H.Y.; Chang, G.D.; Lin, S.J.; Chi, P. Promotion of homology-directed DNA repair by polyamines. Nat. Commun. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Zammit, V.; Baron, B.; Ayers, D. MiRNA Influences in Neuroblast Modulation: An Introspective Analysis. Genes 2018, 9, 26. [Google Scholar] [CrossRef]
- Valencia-Sanchez, M.A.; Liu, J.; Hannon, G.J.; Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006, 20, 515–524. [Google Scholar] [CrossRef]
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Nuew trends in the development of miRNA therapeutic strategies in oncology (Review). Int. J. Oncol. 2016, 49, 5–32. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Brognara, E.; Fabbri, E.; Montagner, G.; Gasparello, J.; Manicardi, A.; Corradini, R.; Bianchi, N.; Finotti, A.; Breveglieri, G.; Borgatti, M.; et al. High levels of apoptosis are induced in human glioma cell lines by co-administration of peptide nucleic acids targeting miR-221 and miR-222. Int. J. Oncol. 2016, 48, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.A.; Hald, O.H.; Fuchs, S.; Lokke, C.; Mikkola, I.; Flaegstad, T.; Schulte, J.; Einvik, C. MicroRNA-193b-3p represses neuroblastoma cell growth via downregulation of Cyclin D1, MCL-1 and MYCN. Oncotarget 2018, 9, 18160–18179. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Lin, H.; Wang, Q.; Chen, W.; Liu, Z.; Chen, H.; Zhang, H.; Chen, W. miR34a suppresses proliferation and induces apoptosis of human lens epithelial cells by targeting E2F3. Mol. Med. Rep. 2016, 14, 5049–5056. [Google Scholar] [CrossRef]
- Maugeri, M.; Barbagallo, D.; Barbagallo, C.; Banelli, B.; Di Mauro, S.; Purrello, F.; Magro, G.; Ragusa, M.; Di Pietro, C.; Romani, M.; et al. Altered expression of miRNAs and methylation of their promoters are correlated in neuroblastoma. Oncotarget 2016, 7, 83330–83341. [Google Scholar] [CrossRef]
- De Antonellis, P.; Carotenuto, M.; Vandenbussche, J.; De Vita, G.; Ferrucci, V.; Medaglia, C.; Boffa, I.; Galiero, A.; Di Somma, S.; Magliulo, D.; et al. Early targets of miR-34a in neuroblastoma. Mol. Cell Proteom. 2014, 13, 2114–2131. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Carter, D.R.; Liu, B.; Mayoh, C.; Beckers, A.; Lalwani, A.; Nagy, Z.; De Brouwer, S.; Decaesteker, B.; Hung, T.T.; et al. Network Modeling of microRNA-mRNA Interactions in Neuroblastoma Tumorigenesis Identifies miR-204 as a Direct Inhibitor of MYCN. Cancer Res. 2018, 78, 3122–3134. [Google Scholar] [CrossRef]
- Soriano, A.; Paris-Coderch, L.; Jubierre, L.; Martinez, A.; Zhou, X.; Piskareva, O.; Bray, I.; Vidal, I.; Almazan-Moga, A.; Molist, C.; et al. MicroRNA-497 impairs the growth of chemoresistant neuroblastoma cells by targeting cell cycle, survival and vascular permeability genes. Oncotarget 2016, 7, 9271–9287. [Google Scholar] [CrossRef]
- Voigt, A.; Hartmann, P.; Zintl, F. Differentiation, proliferation and adhesion of human neuroblastoma cells after treatment with retinoic acid. Cell Adhes. Commun. 2000, 7, 423–440. [Google Scholar] [CrossRef]
- Tonelli, R.; Purgato, S.; Camerin, C.; Fronza, R.; Bologna, F.; Alboresi, S.; Franzoni, M.; Corradini, R.; Sforza, S.; Faccini, A.; et al. Anti-gene peptide nucleic acid specifically inhibits MYCN expression in human neuroblastoma cells leading to cell growth inhibition and apoptosis. Mol. Cancer Ther. 2005, 4, 779–786. [Google Scholar] [CrossRef]
- Timeus, F.; Crescenzio, N.; Fandi, A.; Doria, A.; Foglia, L.; Cordero di Montezemolo, L. In vitro antiproliferative and antimigratory activity of dasatinib in neuroblastoma and Ewing sarcoma cell lines. Oncol. Rep. 2008, 19, 353–359. [Google Scholar] [CrossRef][Green Version]
- Timeus, F.; Crescenzio, N.; Doria, A.; Foglia, L.; Pagliano, S.; Ricotti, E.; Fagioli, F.; Tovo, P.A.; Cordero di Montezemolo, L. In vitro anti-neuroblastoma activity of saquinavir and its association with imatinib. Oncol. Rep. 2012, 27, 734–740. [Google Scholar] [CrossRef]
- Turini, P.; Sabatini, S.; Befani, O.; Chimenti, F.; Casanova, C.; Riccio, P.L.; Mondovi, B. Purification of bovine plasma amine oxidase. Anal. Biochem. 1982, 125, 294–298. [Google Scholar] [CrossRef]
- Janes, S.M.; Mu, D.; Wemmer, D.; Smith, A.J.; Kaur, S.; Maltby, D.; Burlingame, A.L.; Klinman, J.P. A new redox cofactor in eukaryotic enzymes: 6-hydroxydopa at the active site of bovine serum amine oxidase. Science 1990, 248, 981–987. [Google Scholar] [CrossRef]
- Angelini, R.; Cona, A.; Tavladoraki, P. Determination of Copper Amine Oxidase Activity in Plant Tissues. Methods Mol. Biol. 2018, 1694, 129–139. [Google Scholar] [CrossRef]
- Cestelli, A.; Savettieri, G.; Ferraro, D.; Vitale, F. Formulation of a novel synthetic medium for selectively culturing rat CNS neurons. Brain Res. 1985, 354, 219–227. [Google Scholar] [CrossRef]
- Savettieri, G.; Licata, L.; Catania, C.; Raneri, R.; Di Liegro, I.; Cestelli, A. Synergistic effects of laminin and thyroid hormones on neuron polarity in culture. Neuroreport 1999, 10, 1269–1272. [Google Scholar] [CrossRef]
- Caradonna, F.; Schiera, G.; Di Liegro, C.M.; Vitale, V.; Cruciata, I.; Ferrara, T.; D’Oca, P.; Mormino, R.; Rizzo, S.M.A.; Di Liegro, I. Establishment and Preliminary Characterization of Three Astrocytic Cells Lines Obtained from Primary Rat Astrocytes by Sub-Cloning. Genes 2020, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, A.; Arancia, G.; Marra, M.; Crateri, P.; Befani, O.; Martone, A.; Agostinelli, E. Enzymatic oxidation products of spermine induce greater cytotoxic effects on human multidrug-resistant colon carcinoma cells (LoVo) than on their wild-type counterparts. Int. J. Cancer 2002, 99, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, Y.; Via, L.D.; Macone, A.; Canettieri, G.; Greco, A.; Toninello, A.; Agostinelli, E. Aged garlic extract and its constituent, S-allyl-L-cysteine, induce the apoptosis of neuroblastoma cancer cells due to mitochondrial membrane depolarization. Exp. Ther. Med. 2020, 19, 1511–1521. [Google Scholar] [CrossRef]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Moll, U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta 2009, 1787, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Di Magno, L.; Manni, S.; Di Pastena, F.; Coni, S.; Macone, A.; Cairoli, S.; Sambucci, M.; Infante, P.; Moretti, M.; Petroni, M.; et al. Phenformin Inhibits Hedgehog-Dependent Tumor Growth through a Complex I-Independent Redox/Corepressor Module. Cell Rep. 2020, 30, 1735–1752.e1737. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Agostinelli, E.; Bates, D.; Przybytkowski, E.; Mateescu, M.; Mondovi, B. Cytotoxicity of polyamines in Chinese hamster ovary (CHO) cells in the presence of purified serum amine oxidase. Life Chem. Rep. 1991, 9, 193–204. [Google Scholar]
- Tivnan, A.; Tracey, L.; Buckley, P.G.; Alcock, L.C.; Davidoff, A.M.; Stallings, R.L. MicroRNA-34a is a potent tumor suppressor molecule in vivo in neuroblastoma. BMC Cancer 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zheng, H.; Li, D.; Xue, Y.; Wang, Q.; Yan, S.; Zhu, Y.; Deng, M. MicroRNA-34a regulates proliferation and apoptosis of gastric cancer cells by targeting silent information regulator 1. Exp. Ther. Med. 2018, 15, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lee, J.S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 2012, 21, 362–373. [Google Scholar] [CrossRef]
- Palmer, A.J.; Wallace, H.M. The polyamine transport system as a target for anticancer drug development. Amino Acids 2010, 38, 415–422. [Google Scholar] [CrossRef]
- Arancia, G.; Calcabrini, A.; Marra, M.; Crateri, P.; Artico, M.; Martone, A.; Martelli, F.; Agostinelli, E. Mitochondrial alterations induced by serum amine oxidase and spermine on human multidrug resistant tumor cells. Amino Acids 2004, 26, 273–282. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Grancara, S.; Zonta, F.; Ohkubo, S.; Brunati, A.M.; Agostinelli, E.; Toninello, A. Pathophysiological implications of mitochondrial oxidative stress mediated by mitochondriotropic agents and polyamines: The role of tyrosine phosphorylation. Amino Acids 2015, 47, 869–883. [Google Scholar] [CrossRef]
- Agostinelli, E.; Arancia, G.; Vedova, L.D.; Belli, F.; Marra, M.; Salvi, M.; Toninello, A. The biological functions of polyamine oxidation products by amine oxidases: Perspectives of clinical applications. Amino Acids 2004, 27, 347–358. [Google Scholar] [CrossRef]
- Agostinelli, E. Polyamines and transglutaminases: Future perspectives. Amino Acids 2016, 48, 2273–2281. [Google Scholar] [CrossRef]
- Amendola, R.; Cervelli, M.; Fratini, E.; Sallustio, D.E.; Tempera, G.; Ueshima, T.; Mariottini, P.; Agostinelli, E. Reactive oxygen species spermine metabolites generated from amine oxidases and radiation represent a therapeutic gain in cancer treatments. Int. J. Oncol. 2013, 43, 813–820. [Google Scholar] [CrossRef]
- Rihani, A.; Van Goethem, A.; Ongenaert, M.; De Brouwer, S.; Volders, P.J.; Agarwal, S.; De Preter, K.; Mestdagh, P.; Shohet, J.; Speleman, F.; et al. Genome wide expression profiling of p53 regulated miRNAs in neuroblastoma. Sci. Rep. 2015, 5, 9027. [Google Scholar] [CrossRef]
- Yang, F.; Li, Q.J.; Gong, Z.B.; Zhou, L.; You, N.; Wang, S.; Li, X.L.; Li, J.J.; An, J.Z.; Wang, D.S.; et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol. Cancer Res. Treat. 2014, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Gasparello, J.; Gambari, L.; Papi, C.; Rozzi, A.; Manicardi, A.; Corradini, R.; Gambari, R.; Finotti, A. High Levels of Apoptosis Are Induced in the Human Colon Cancer HT-29 Cell Line by Co-Administration of Sulforaphane and a Peptide Nucleic Acid Targeting miR-15b-5p. Nucleic Acid Ther. 2020, 30, 164–174. [Google Scholar] [CrossRef]
- Averill-Bates, D.A.; Cherif, A.; Agostinelli, E.; Tanel, A.; Fortier, G. Anti-tumoral effect of native and immobilized bovine serum amine oxidase in a mouse melanoma model. Biochem. Pharmacol. 2005, 69, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A.; Ke, Q.; Tanel, A.; Roy, J.; Fortier, G.; Agostinelli, E. Mechanism of cell death induced by spermine and amine oxidase in mouse melanoma cells. Int. J. Oncol. 2008, 32, 79–88. [Google Scholar] [CrossRef] [PubMed]
| Assays for Transcripts Expression Analysis | ||
|---|---|---|
| Gene Name | Probe Sequences | |
| Tp53 | Probe | 5′-/56FAM/TCCCAGAAT/ZEN/GCAAGAAGCCCAGA/3IABkFQ/-3′ |
| Primer FW | 5′-AACCCACAGCTGCACAG-3′ | |
| Primer RW | 5′-CCTTCCCAGAAAACCTACCAG-3′ | |
| caspase-3 RPL13A | Probe | 5′-/56-FAM/AGTTTCGTG/ZEN/AGTGCTCGCAGCTC/3IABkFQ/-3′ |
| Primer FW | 5′-CACGGATACACAGCCACAG-3′ | |
| Primer RW | 5′-CGGATGGGTGCTATTGTGAG-3′ | |
| Probe | 5′-/5HEX/CGCACGGTC/ZEN/CGCCAGAAGAT/3IABkFQ/-3′ | |
| Primer FW | 5′-GGCAATTTCTACAGAAACAAGTTG-3′ | |
| Primer RW | 5′-GTTTTGTGGGGCAGCATACC-3′ | |
| Assays for miRNAs Expression Analysis | ||
|---|---|---|
| miRNA Name | Cat. Number | Assay ID |
| miR-34a-5p | 4427975 | 000426 |
| miR-let-7c-5p | 4427975 | 000379 |
| snRNA U6 | 4427975 | 001973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanamori, Y.; Finotti, A.; Di Magno, L.; Canettieri, G.; Tahara, T.; Timeus, F.; Greco, A.; Tirassa, P.; Gasparello, J.; Fino, P.; et al. Enzymatic Spermine Metabolites Induce Apoptosis Associated with Increase of p53, caspase-3 and miR-34a in Both Neuroblastoma Cells, SJNKP and the N-Myc-Amplified Form IMR5. Cells 2021, 10, 1950. https://doi.org/10.3390/cells10081950
Kanamori Y, Finotti A, Di Magno L, Canettieri G, Tahara T, Timeus F, Greco A, Tirassa P, Gasparello J, Fino P, et al. Enzymatic Spermine Metabolites Induce Apoptosis Associated with Increase of p53, caspase-3 and miR-34a in Both Neuroblastoma Cells, SJNKP and the N-Myc-Amplified Form IMR5. Cells. 2021; 10(8):1950. https://doi.org/10.3390/cells10081950
Chicago/Turabian StyleKanamori, Yuta, Alessia Finotti, Laura Di Magno, Gianluca Canettieri, Tomoaki Tahara, Fabio Timeus, Antonio Greco, Paola Tirassa, Jessica Gasparello, Pasquale Fino, and et al. 2021. "Enzymatic Spermine Metabolites Induce Apoptosis Associated with Increase of p53, caspase-3 and miR-34a in Both Neuroblastoma Cells, SJNKP and the N-Myc-Amplified Form IMR5" Cells 10, no. 8: 1950. https://doi.org/10.3390/cells10081950
APA StyleKanamori, Y., Finotti, A., Di Magno, L., Canettieri, G., Tahara, T., Timeus, F., Greco, A., Tirassa, P., Gasparello, J., Fino, P., Di Liegro, C. M., Proia, P., Schiera, G., Di Liegro, I., Gambari, R., & Agostinelli, E. (2021). Enzymatic Spermine Metabolites Induce Apoptosis Associated with Increase of p53, caspase-3 and miR-34a in Both Neuroblastoma Cells, SJNKP and the N-Myc-Amplified Form IMR5. Cells, 10(8), 1950. https://doi.org/10.3390/cells10081950












