NLRP3 Triggers Attenuate Lipocalin-2 Expression Independent with Inflammasome Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culturing and Treatment
2.2. Animal Study
2.3. Detection of LCN2, IL-1β, TNFα, IL-6 Using ELISA
2.4. Western Blot Analysis
2.5. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Statistical Analyses
3. Results
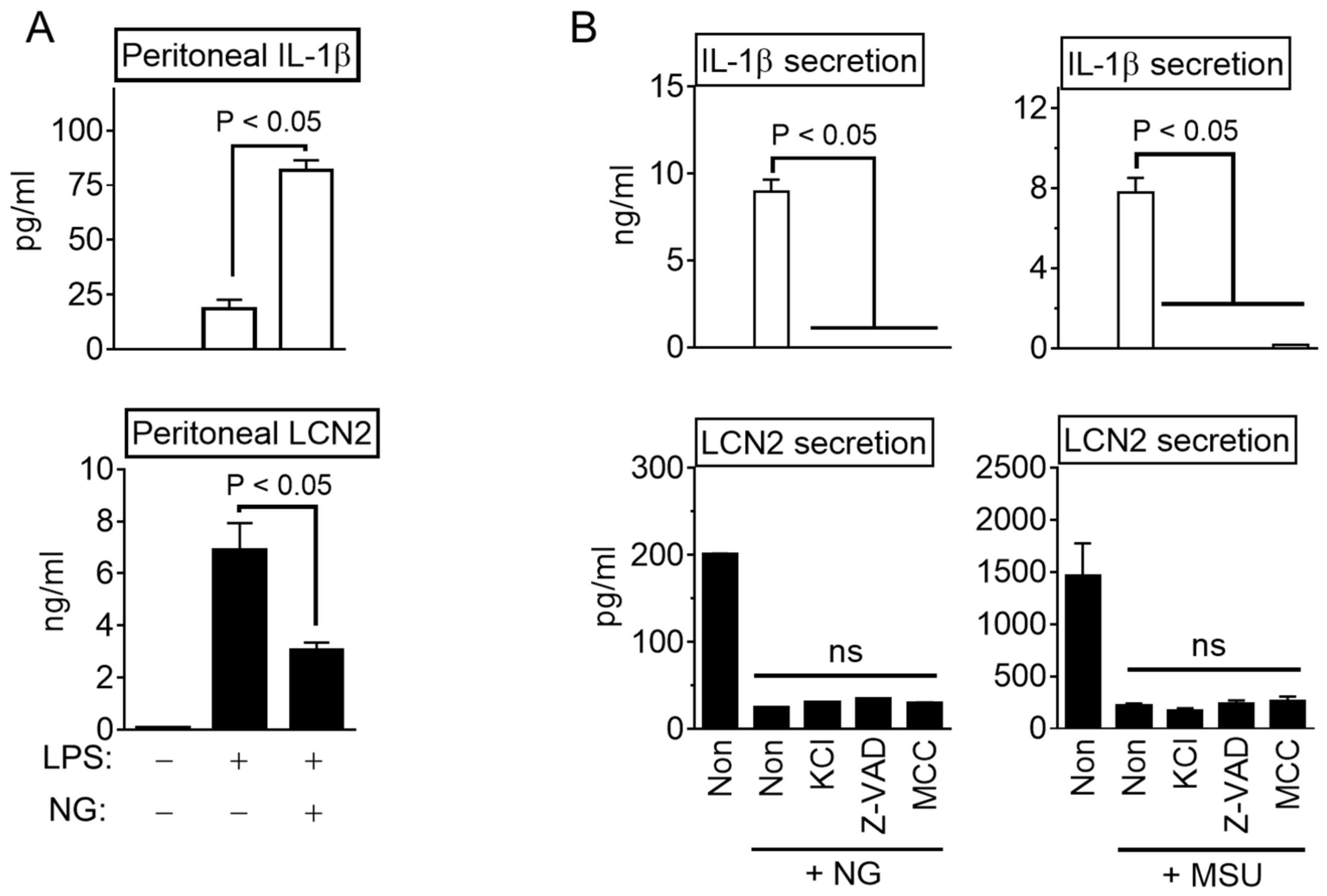
3.1. NLRP3 Inflammasome Activation Attenuate LCN2 Secretion
3.2. NLRP3 Triggers Inhibit LCN2 Secretion Independent Inflammasome Activation
3.3. NLRP3 Triggers Block LCN2 Transcription
3.4. NLRP3 Triggers Inhibit LCN2 Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, T.; Togawa, A.; Duncan, G.S.; Elia, A.J.; You-Ten, A.; Wakeham, A.; Fong, H.E.; Cheung, C.C.; Mak, T.W. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2006, 103, 1834–1839. [Google Scholar] [CrossRef] [Green Version]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab. TEM 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Moschen, A.R.; Gerner, R.R.; Wang, J.; Klepsch, V.; Adolph, T.E.; Reider, S.J.; Hackl, H.; Pfister, A.; Schilling, J.; Moser, P.L.; et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe 2016, 19, 455–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Reba, S.; Chen, W.D.; Porwal, S.K.; Boom, W.H.; Petersen, R.B.; Rojas, R.; Viswanathan, R.; Devireddy, L. Regulation of mammalian siderophore 2,5-DHBA in the innate immune response to infection. J. Exp. Med. 2014, 211, 1197–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Jin, D.; Chen, X. Lipocalin 2 is a regulator of macrophage polarization and NF-kappaB/STAT3 pathway activation. Mol. Endocrinol. 2014, 28, 1616–1628. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Kwon, H.M.; Lee, E.; Kim, P.H.; Jeung, E.B.; Lee, G.S. Role of inflammasome regulation on immune modulators. J. Biomed. Res. 2018, 32, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S. Inflammasomes, multi-cellular protein complex in myeloid cells, induce several metabolic diseases via interleukin-1β maturation. J. Biomed. Res. 2013, 14, 195–200. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Munoz-Planillo, R.; Kuffa, P.; Martinez-Colon, G.; Smith, B.L.; Rajendiran, T.M.; Nunez, G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.; Han, B.C.; Hong, E.J.; An, B.S.; Lee, E.; Lee, S.H.; Lee, G.S. Korean Red Ginseng attenuates ultraviolet-mediated inflammasome activation in keratinocytes. J. Ginseng Res. 2021, 45, 456–463. [Google Scholar] [CrossRef]
- Cowland, J.B.; Muta, T.; Borregaard, N. IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J. Immunol. 2006, 176, 5559–5566. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Jahng, J.W.; Chong, L.P.; Sung, H.K.; Han, M.; Luo, C.; Wu, D.; Boo, S.; Hinz, B.; Cooper, M.A.; et al. Lipocalin-2 induces NLRP3 inflammasome activation via HMGB1 induced TLR4 signaling in heart tissue of mice under pressure overload challenge. Am. J. Transl. Res. 2017, 9, 2723–2735. [Google Scholar]
- Kim, J.; Ahn, H.; Han, B.C.; Shin, H.; Kim, J.C.; Jung, E.M.; Kim, J.; Yang, H.; Lee, J.; Kang, S.G.; et al. Obovatol inhibits NLRP3, AIM2, and non-canonical inflammasome activation. Phytomed. Int. J. Phytother. Phytopharm. 2019, 63, 153019. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Han, B.C.; Lee, S.H.; Lee, G.S. Fructose-arginine, a non-saponin molecule of Korean Red Ginseng, attenuates AIM2 inflammasome activation. J. Ginseng Res. 2020, 44, 808–814. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, T.C.; Schattgen, S.; Monks, B.; Wang, D.; Cerny, A.; Latz, E.; Fitzgerald, K.; Golenbock, D.T. A Fluorescent Reporter Mouse for Inflammasome Assembly Demonstrates an Important Role for Cell-Bound and Free ASC Specks during In Vivo Infection. Cell Rep. 2016, 16, 571–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.; Han, B.C.; Kim, J.; Kang, S.G.; Kim, P.H.; Jang, K.H.; So, S.H.; Lee, S.H.; Lee, G.S. Nonsaponin fraction of Korean Red Ginseng attenuates cytokine production via inhibition of TLR4 expression. J. Ginseng Res. 2019, 43, 291–299. [Google Scholar] [CrossRef]
- Zhang, P.X.; Chang, J.X.; Xie, J.J.; Yuan, H.M.; Du, Z.P.; Zhang, F.R.; Lu, Z.; Xu, L.Y.; Li, E.M. Regulation of neutrophil gelatinase-associated lipocalin expression by C/EBPbeta in lung carcinoma cells. Oncol. Lett. 2012, 4, 919–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.; Weigert, A.; Tausendschon, M.; Mora, J.; Oren, B.; Sola, A.; Hotter, G.; Muta, T.; Brune, B. Interleukin-10-induced neutrophil gelatinase-associated lipocalin production in macrophages with consequences for tumor growth. Mol. Cell. Biol. 2012, 32, 3938–3948. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Stephens, J.M. STAT1, NF-kappaB and ERKs play a role in the induction of lipocalin-2 expression in adipocytes. Mol. Metab. 2013, 2, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, M.; Du, Z.; Fang, Z.; Wu, B.; Wu, J.; Xie, W.; Shen, J.; Zhu, T.; Xu, X.; et al. Cell Signaling Pathway in 12-O-Tetradecanoylphorbol-13-acetate-Induced LCN2 Gene Transcription in Esophageal Squamous Cell Carcinoma. BioMed Res. Int. 2017, 2017, 9592501. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Ahn, H.; Yu, S.; Ahn, J.H.; Ko, H.J.; Kweon, M.N.; Hong, E.J.; An, B.S.; Lee, E.; Lee, G.S. IkappaBzeta controls NLRP3 inflammasome activation via upregulation of the Nlrp3 gene. Cytokine 2020, 127, 154983. [Google Scholar] [CrossRef]
- Karlsen, J.R.; Borregaard, N.; Cowland, J.B. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J. Biol. Chem. 2010, 285, 14088–14100. [Google Scholar] [CrossRef] [Green Version]
- Myskiw, C.; Piper, J.; Huzarewich, R.; Booth, T.F.; Cao, J.; He, R. Nigericin is a potent inhibitor of the early stage of vaccinia virus replication. Antivir. Res. 2010, 88, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, G.; Liu, F.; Han, Y.; Dai, C.; Wang, S.; Wei, G.; Kuang, Y.; Wan, D.; Zhi, Q.; et al. Molecular Screening for Nigericin Treatment in Pancreatic Cancer by High-Throughput RNA Sequencing. Front. Oncol. 2020, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Warszawska, J.M.; Gawish, R.; Sharif, O.; Sigel, S.; Doninger, B.; Lakovits, K.; Mesteri, I.; Nairz, M.; Boon, L.; Spiel, A.; et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J. Clin. Investig. 2013, 123, 3363–3372. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, Y.; Zhang, Y.; Leroith, D.; Bernlohr, D.A.; Chen, X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 2008, 22, 1416–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.; Lee, G.; Kim, J.; Park, J.; Kang, S.G.; Yoon, S.-I.; Lee, E.; Lee, G.-S. NLRP3 Triggers Attenuate Lipocalin-2 Expression Independent with Inflammasome Activation. Cells 2021, 10, 1660. https://doi.org/10.3390/cells10071660
Ahn H, Lee G, Kim J, Park J, Kang SG, Yoon S-I, Lee E, Lee G-S. NLRP3 Triggers Attenuate Lipocalin-2 Expression Independent with Inflammasome Activation. Cells. 2021; 10(7):1660. https://doi.org/10.3390/cells10071660
Chicago/Turabian StyleAhn, Huijeong, Gilyoung Lee, Jeongeun Kim, Jeongho Park, Seung Goo Kang, Sung-Il Yoon, Eunsong Lee, and Geun-Shik Lee. 2021. "NLRP3 Triggers Attenuate Lipocalin-2 Expression Independent with Inflammasome Activation" Cells 10, no. 7: 1660. https://doi.org/10.3390/cells10071660
APA StyleAhn, H., Lee, G., Kim, J., Park, J., Kang, S. G., Yoon, S.-I., Lee, E., & Lee, G.-S. (2021). NLRP3 Triggers Attenuate Lipocalin-2 Expression Independent with Inflammasome Activation. Cells, 10(7), 1660. https://doi.org/10.3390/cells10071660






