The Timecourses of Functional, Morphological, and Molecular Changes Triggered by Light Exposure in Sprague–Dawley Rat Retinas
Abstract
:1. Introduction
- To characterize and correlate early morphological and biochemical changes occurring in the degenerating retina with in vivo functional analysis of retinal activity.
- To investigate the progression of neurodegenerative stages.
2. Materials and Methods
2.1. Animals
2.2. Light Exposure Protocols
2.3. Electrophysiological Recordings and Data Analysis
2.4. Tissue Processing, Morphological Analyses, and Immunostaining Protocols
2.5. Microscopy and Image Analysis
2.6. Statistical Analysis
3. Results
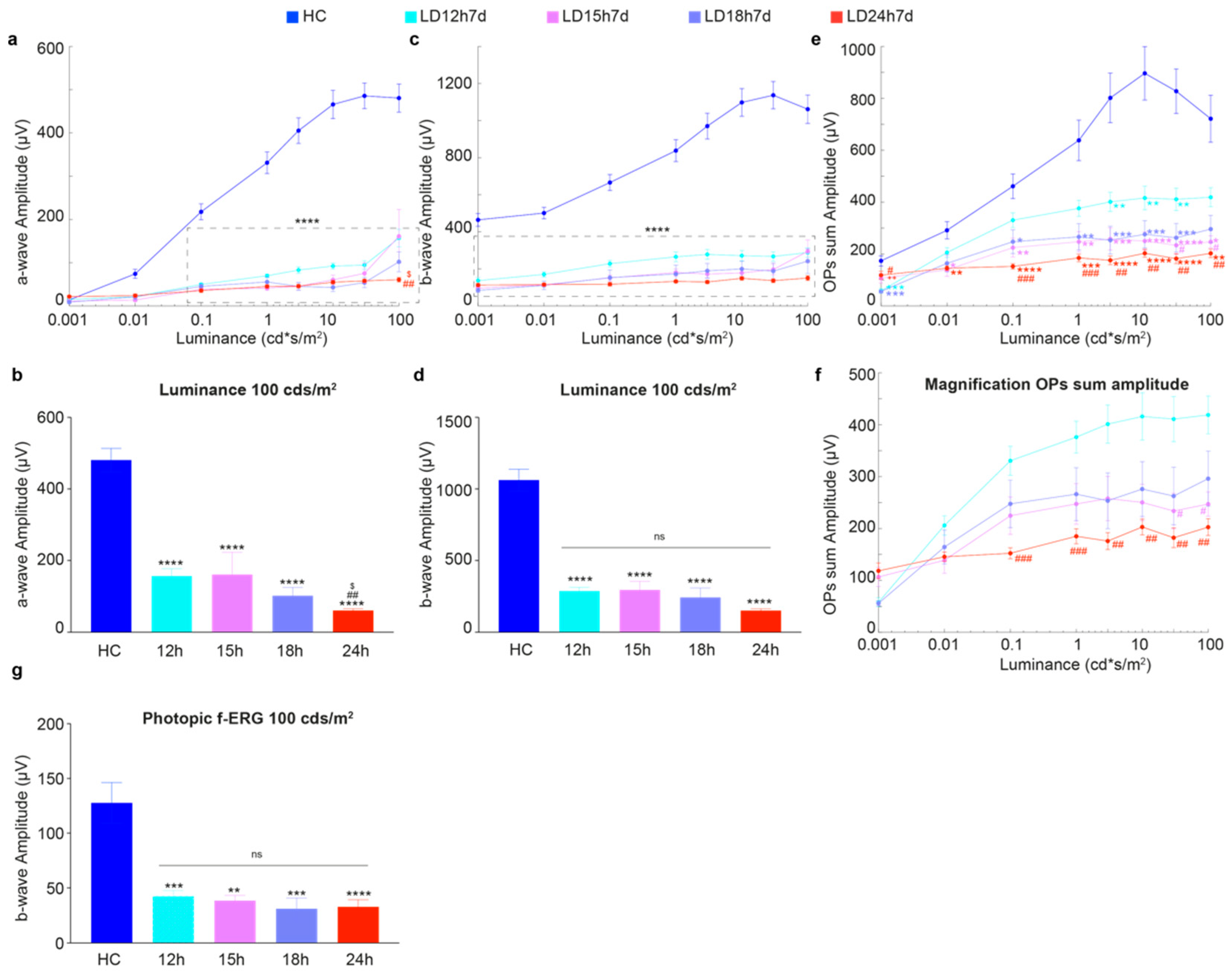
3.1. Different Durations of Light Exposure: Discrepancies between Functional Output and the Extent of Structural Damage
3.1.1. Exposure to Constant Light for 12 h Led to Functional but Not Severe Morphological Retinal Alterations
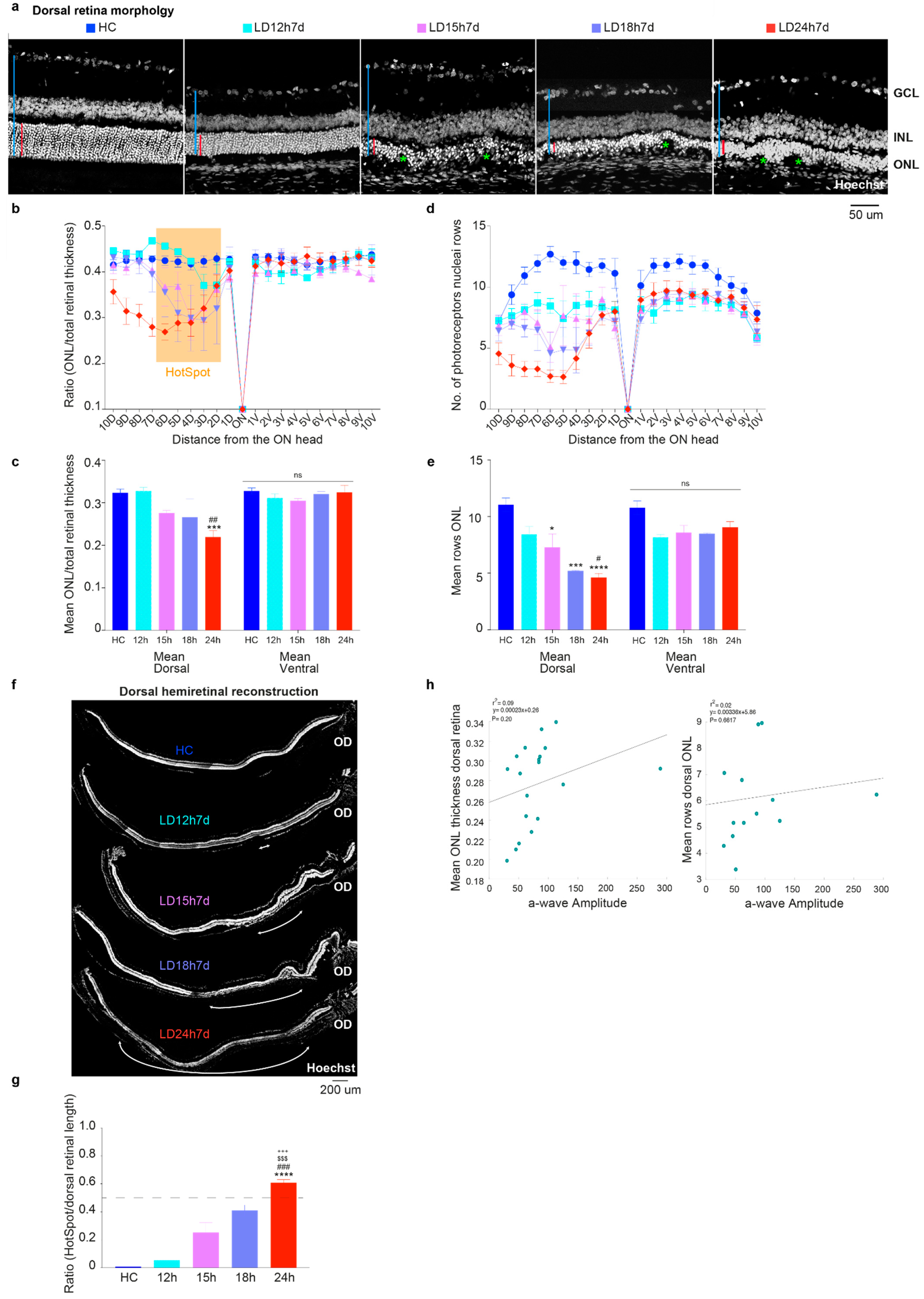
3.1.2. Outer Nuclear Layer (ONL) Disruption Does Not Correlate with Detectable Functional Alterations
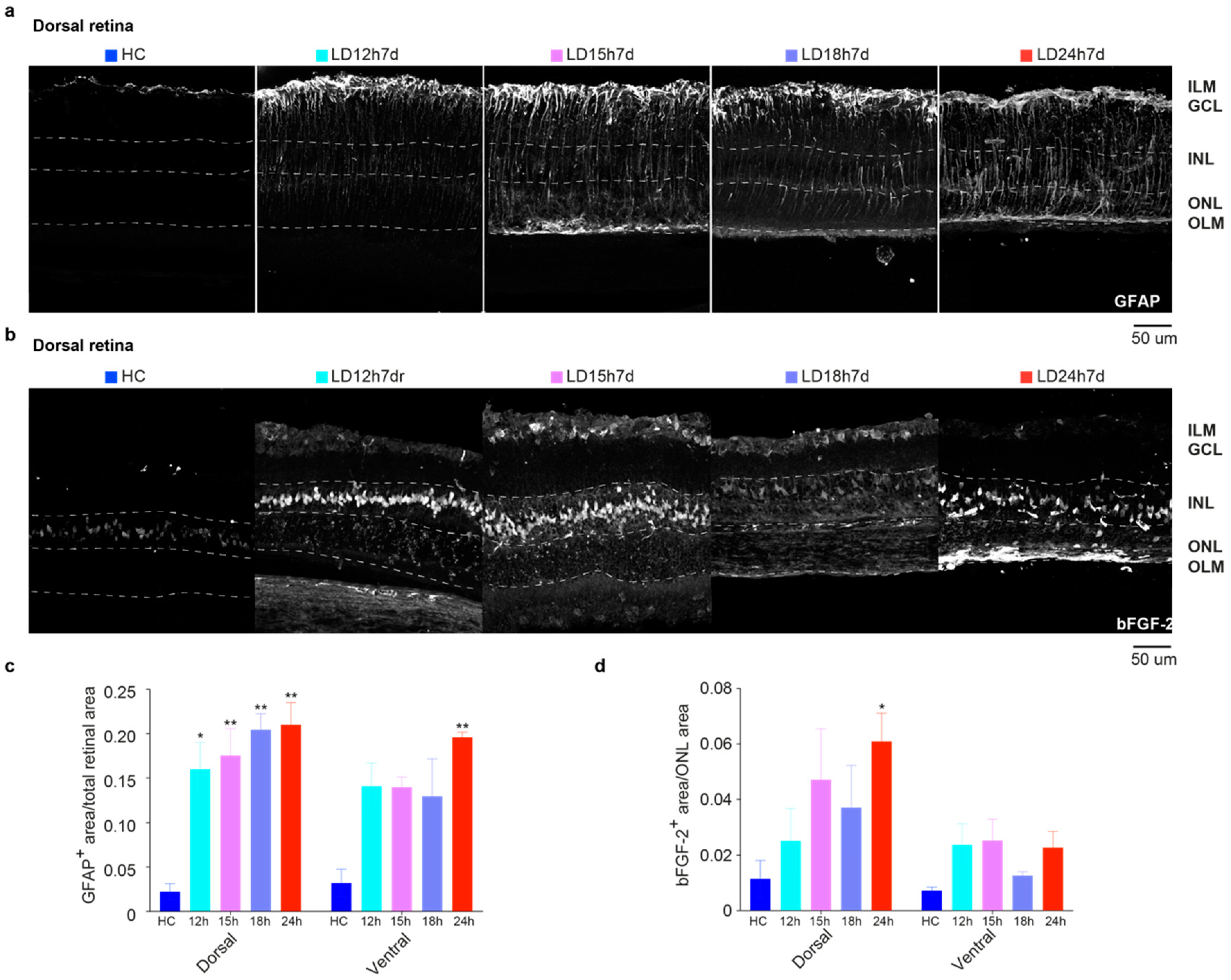
3.1.3. Upregulation of GFAP and bFGF-2 Was Triggered after Short Periods of Constant Light Exposure
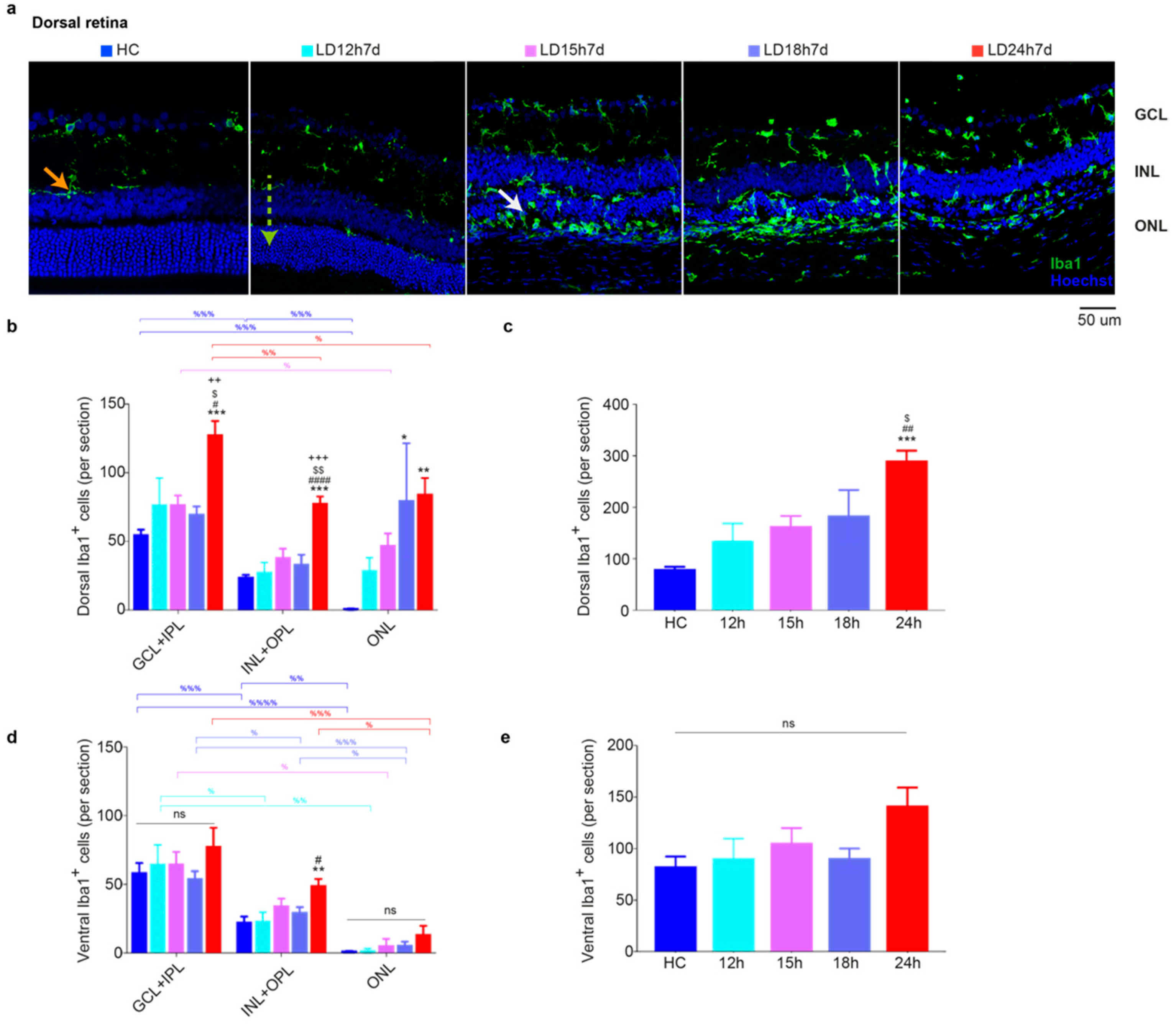
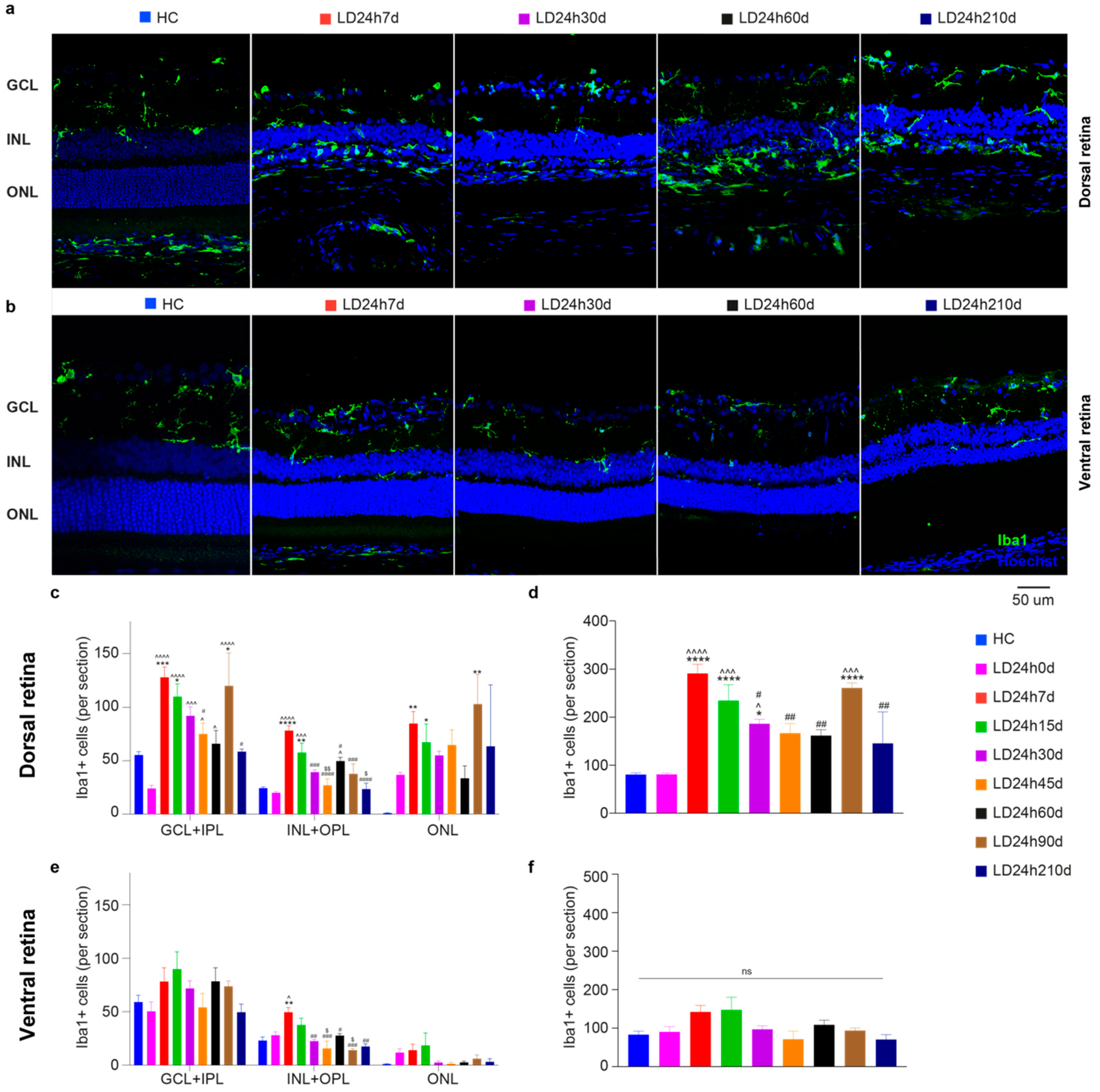
3.1.4. Microglia Increased in the Dorsal Retina after Exposure to Constant Light
3.2. Long-Term Morpho-Functional Changes in the Neurodegenerative Progression
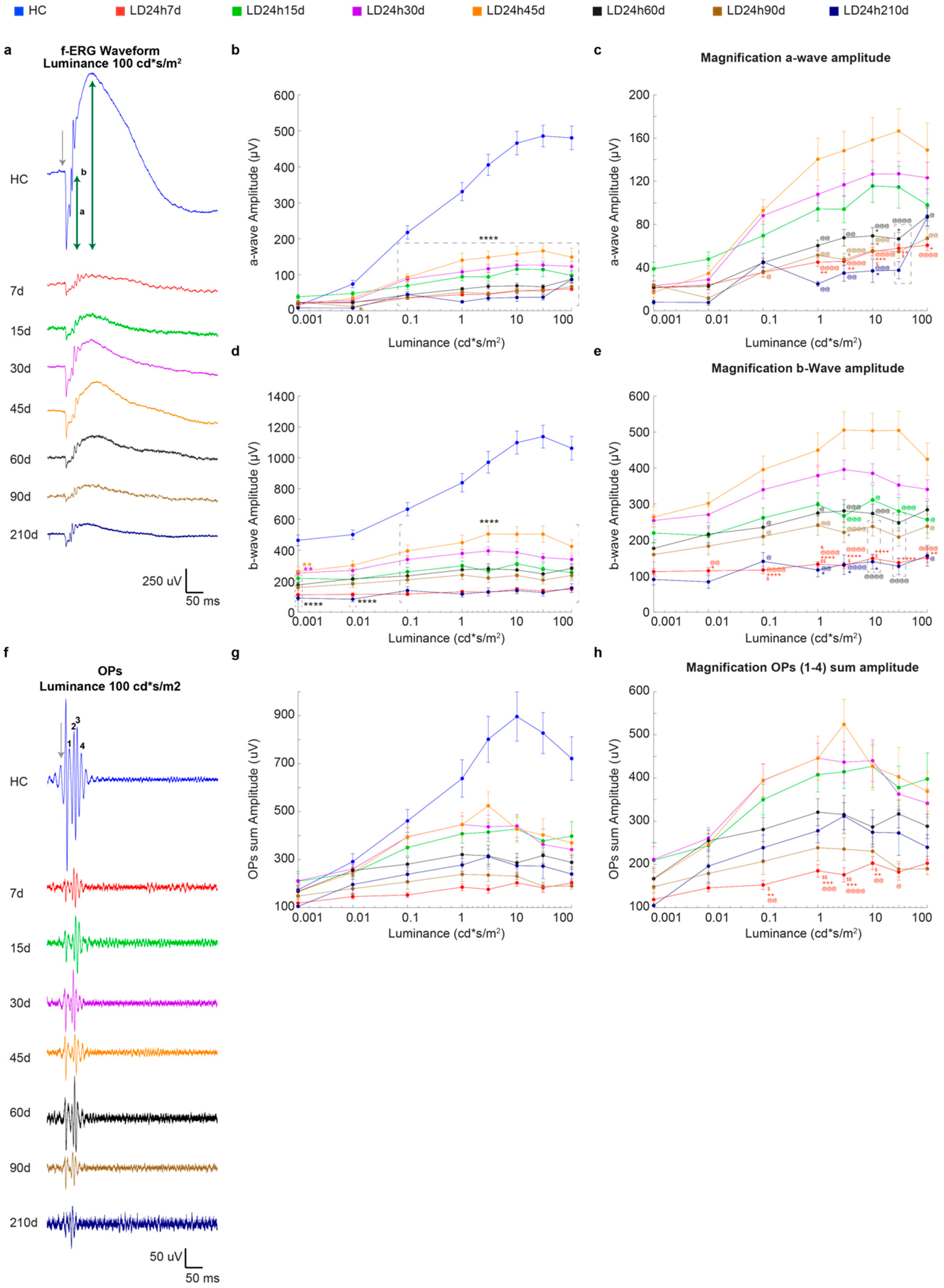
3.2.1. Timecourse Effects of 24 h of Bright Light Exposure Highlighted a Transient Functional Recovery
3.2.2. The Ventral Retinal Structural Layer Organization Was Better Preserved Compared to the Dorsal One
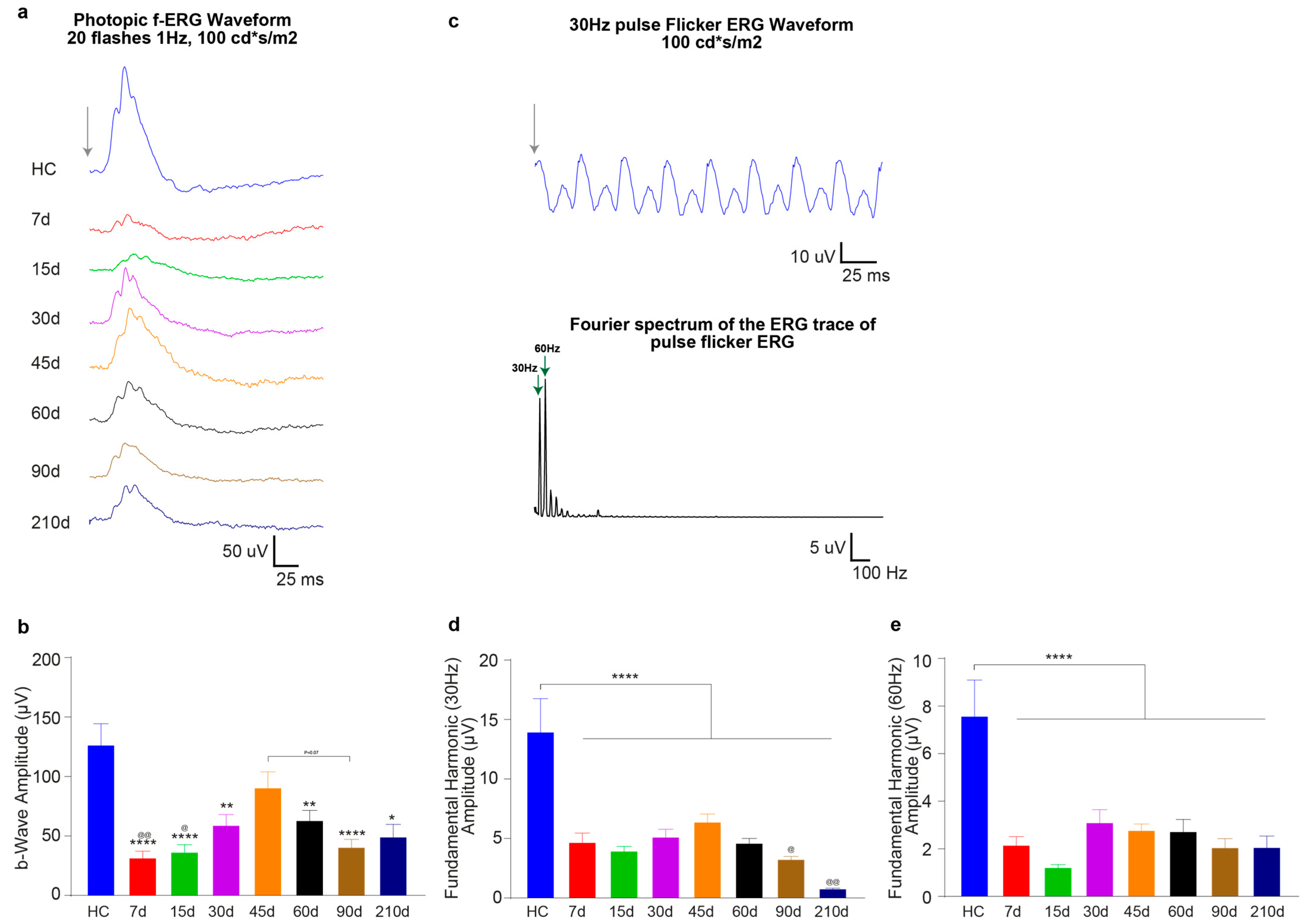
3.2.3. Light-Induced Cone-Photoreceptor Damage
3.2.4. Light Exposure Induced Modifications in the Inner Nuclear Layer (INL), Detectable at Least after 15 Days Following Light Exposure
3.2.5. Spatial Differences in Glial Cells’ Reaction, bFGF-2, and Microglia Modulation: Non-Neuronal Cells’ Involvement in Retinal Degeneration
4. Discussion and Conclusions
4.1. Different Durations of Bright Light Exposure Provided Insights into Early Events in the Pathology of Light-Induced Retinal Damage
4.2. Analyses Performed after Different Recovery Periods Following 24 h of Bright Light Exposure Showed Discrepancies between the Morphological and Functional Timecourses of the Disease
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lamoureux, E.; Pesudovs, K. Vision-specific quality-of-life research: A need to improve the quality. Am. J. Ophthalmol. 2011, 151, 195–197.e2. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguilà, M.; Bevilacqua, D.; Novoselov, S.S.; Parfitt, D.A.; Cheetham, M.E. The cell stress machinery and retinal degeneration. FEBS Lett. 2013, 587, 2008–2017. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.F.; Chakarova, C.F.; Abd El-Aziz, M.M.; Bhattacharya, S.S. Photoreceptor degeneration: Genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010, 11, 273–284. [Google Scholar] [CrossRef]
- Chacon-Camacho, O.F.; Zenteno, J.C. Review and update on the molecular basis of Leber congenital amaurosis. World J. Clin. Cases 2015, 3, 112–124. [Google Scholar] [CrossRef]
- Song, H.; Rossi, E.A.; Latchney, L.; Bessette, A.; Stone, E.; Hunter, J.J.; Williams, D.R.; Chung, M. Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA Ophthalmol. 2015, 133, 1198–1203. [Google Scholar] [CrossRef]
- Young, M.J.; Ray, J.; Whiteley, S.J.; Klassen, H.; Gage, F.H. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol. Cell. Neurosci. 2000, 16, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, G.; Ben M’Barek, K.; Goureau, O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog. Retin. Eye Res. 2019, 71, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Antognazza, M.R.; Maccarone, R.; Bellani, S.; Lanzarini, E.; Martino, N.; Mete, M.; Pertile, G.; Bisti, S.; Lanzani, G.; et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photonics 2013, 7, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Maya-Vetencourt, J.F.; Ghezzi, D.; Antognazza, M.R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S.; et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017, 16, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, B.S.; Marc, R.E.; Bernstein, P.S. Optogenetics for retinal disorders. J. Ophthalmic Vis. Res. 2014, 9, 374–382. [Google Scholar] [PubMed]
- Garita-Hernandez, M.; Lampič, M.; Chaffiol, A.; Guibbal, L.; Routet, F.; Santos-Ferreira, T.; Gasparini, S.; Borsch, O.; Gagliardi, G.; Reichman, S.; et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat. Commun. 2019, 10, 4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 2021. [Google Scholar]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, S.; Riccitelli, S.; Di Paolo, M.; Campos, E.; Buzzi, M.; Bisti, S.; Versura, P. Cord Blood Serum (CBS)-Based Eye Drops Modulate Light-Induced Neurodegeneration in Albino Rat Retinas. Biomolecules 2020, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Daien, V.; Eldem, B.M.; Talks, J.S.; Korobelnik, J.-F.; Mitchell, P.; Sakamoto, T.; Wong, T.Y.; Pantiri, K.; Carrasco, J. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration-a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020, 20, 294. [Google Scholar] [CrossRef] [PubMed]
- Kniggendorf, V.; Dreyfuss, J.L.; Regatieri, C.V. Age-related macular degeneration: A review of current therapies and new treatments. Arq. Bras. Oftalmol. 2020, 83, 552–561. [Google Scholar]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef]
- Marangoni, D.; Falsini, B.; Piccardi, M.; Ambrosio, L.; Minnella, A.M.; Savastano, M.C.; Bisti, S.; Maccarone, R.; Fadda, A.; Mello, E.; et al. Functional effect of Saffron supplementation and risk genotypes in early age-related macular degeneration: A preliminary report. J. Transl. Med. 2013, 11, 228. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.W.; Kondo, M.; Terasaki, H.; Lin, Y.; McCall, M.; Marc, R.E. Retinal remodeling. Jpn. J. Ophthalmol. 2012, 56, 289–306. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Cherukuri, P.; Poria, D.; Goel, M.; Dagar, S.; Dhingra, N.K. Retinal remodeling: Concerns, emerging remedies and future prospects. Front. Cell Neurosci. 2016, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2007, 500, 222–238. [Google Scholar] [CrossRef] [Green Version]
- Marc, R.E.; Jones, B.W. Retinal remodeling in inherited photoreceptor degenerations. Mol. Neurobiol. 2003, 28, 139–147. [Google Scholar] [CrossRef]
- Strettoi, E.; Porciatti, V.; Falsini, B.; Pignatelli, V.; Rossi, C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J. Neurosci. 2002, 22, 5492–5504. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.L.; Nag, T.C.; Jha, K.A.; Kathpalia, P.; Maurya, M.; Kumar, P.; Gupta, S.; Roy, T.S. Changes in the Inner Retinal Cells after Intense and Constant Light Exposure in Sprague-Dawley Rats. Photochem. Photobiol. 2020, 96, 1061–1073. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Anderson, J.R.; Kinard, K.; Marshak, D.W.; Wilson, J.H.; Wensel, T.; Lucas, R.J. Neural reprogramming in retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal ganglion cell death as a late remodeling effect of photoreceptor degeneration. Int. J. Mol. Sci. 2019, 20, 4649. [Google Scholar] [CrossRef] [Green Version]
- Marco-Gomariz, M.A.; Hurtado-Montalbán, N.; Vidal-Sanz, M.; Lund, R.D.; Villegas-Pérez, M.P. Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J. Comp. Neurol. 2006, 498, 163–179. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Salinas-Navarro, M.; Agudo-Barriuso, M.; Alarcón-Martínez, L.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal ganglion cell axonal compression by retinal vessels in light-induced retinal degeneration. Mol. Vis. 2011, 17, 1716–1733. [Google Scholar]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Stone, J.; Stowe, S.; Barnett, N.L.; Valter, K. The status of cones in the rhodopsin mutant P23H-3 retina: Light-regulated damage and repair in parallel with rods. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Demontis, G.C.; Longoni, B.; Marchiafava, P.L. Molecular steps involved in light-induced oxidative damage to retinal rods. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2421–2427. [Google Scholar]
- Stone, J.; Maslim, J.; Valter-Kocsi, K.; Mervin, K.; Bowers, F.; Chu, Y.; Barnett, N.; Provis, J.; Lewis, G.; Fisher, S.K.; et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Retin. Eye Res. 1999, 18, 689–735. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin. Eye Res. 2020, 74, 100771. [Google Scholar] [CrossRef] [PubMed]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Vazquez-Chona, F.; Vaughan, D.K.; Organisciak, D.T. Extreme retinal remodeling triggered by light damage: Implications for age related macular degeneration. Mol. Vis. 2008, 14, 782–806. [Google Scholar]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef]
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Investig. Ophthalmol. 1966, 5, 450–473. [Google Scholar]
- Chaychi, S.; Polosa, A.; Lachapelle, P. Differences in Retinal Structure and Function between Aging Male and Female Sprague-Dawley Rats are Strongly Influenced by the Estrus Cycle. PLoS ONE 2015, 10, e0136056. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Nicolás, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The aging rat retina: From function to anatomy. Neurobiol. Aging 2018, 61, 146–168. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef]
- Valter, K.; Bisti, S.; Gargini, C.; Di Loreto, S.; Maccarone, R.; Cervetto, L.; Stone, J. Time course of neurotrophic factor upregulation and retinal protection against light-induced damage after optic nerve section. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1748–1754. [Google Scholar] [CrossRef]
- Rutar, M.; Provis, J.M.; Valter, K. Brief exposure to damaging light causes focal recruitment of macrophages, and long-term destabilization of photoreceptors in the albino rat retina. Curr. Eye Res. 2010, 35, 631–643. [Google Scholar] [CrossRef]
- Fiorani, L.; Passacantando, M.; Santucci, S.; Di Marco, S.; Bisti, S.; Maccarone, R. Cerium oxide nanoparticles reduce microglial activation and neurodegenerative events in light damaged retina. PLoS ONE. 2015, 10, e0140387. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.-C.; Hsiao, Y.-T.; Kao, S.-Y.; Chen, C.-F.; Chen, Y.-C.; Chiang, C.-W.; Lee, C.-F.; Lu, J.-C.; Chern, Y.; Wang, C.-T. Adenosine A(2A) receptor up-regulates retinal wave frequency via starburst amacrine cells in the developing rat retina. PLoS ONE 2014, 9, e95090. [Google Scholar]
- Antognazza, M.R.; Di Paolo, M.; Ghezzi, D.; Mete, M.; Di Marco, S.; Maya-Vetencourt, J.F.; Maccarone, R.; Desii, A.; Di Fonzo, F.; Bramini, M.; et al. Characterization of a Polymer-Based, Fully Organic Prosthesis for Implantation into the Subretinal Space of the Rat. Adv. Healthc. Mater. 2016, 5, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Wachtmeister, L. Oscillatory potentials in the retina: What do they reveal. Prog. Retin. Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Ohira, A.; Anderson, R.E. Topography of retinal damage in light-exposed albino rats. Exp. Eye Res. 2008, 87, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflamm. 2012, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Eter, N.; Heiduschka, P. The microglia in healthy and diseased retina. Exp. Eye Res. 2015, 136, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Bruera, M.G.; Benedetto, M.M.; Guido, M.E.; Degano, A.L.; Contin, M.A. Glial Cell Responses to constant low Light exposure in Rat Retina. BioRxiv 2021. [Google Scholar]
- Noailles, A.; Fernández-Sánchez, L.; Lax, P.; Cuenca, N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J. Neuroinflamm. 2014, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Ortín-Martínez, A.; Jiménez-López, M.; Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Sauvé, Y.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Automated quantification and topographical distribution of the whole population of S- and L-cones in adult albino and pigmented rats. Investig. Ophthalmol. Vis. Sci. 2020, 51, 3171–3183. [Google Scholar] [CrossRef]
- Edward, D.P.; Lim, K.; Sawaguchi, S.; Tso, M.O. An immunohistochemical study of opsin in photoreceptor cells following light-induced retinal degeneration in the rat. Graefes Arch. Clin. Exp. Ophthalmol. 1993, 231, 289–294. [Google Scholar] [CrossRef] [PubMed]
- D’Orazi, F.D.; Suzuki, S.C.; Wong, R.O. Neuronal remodeling in retinal circuit assembly, disassembly, and reassembly. Trends Neurosci. 2014, 37, 594–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidday, J.M.; Zhang, L.; Chiang, C.-W.; Zhu, Y. Enhanced retinal ganglion cell survival in glaucoma by hypoxic postconditioning after disease onset. Neurotherapeutics 2015, 12, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Gidday, J.M. Adaptive plasticity in the retina: Protection against acute injury and neurodegenerative disease by conditioning stimuli. Cond. Med. 2018, 1, 85–97. [Google Scholar]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [Green Version]
- Noell, W.K. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 1980, 20, 1163–1171. [Google Scholar] [CrossRef]
- Tso, M.O.; Woodford, B.J. Effect of photic injury on the retinal tissues. Ophthalmology 1983, 90, 952–963. [Google Scholar] [CrossRef]
- Cicerone, C.M. Cones survive rods in the light-damaged eye of the albino rat. Science 1976, 194, 1183–1185. [Google Scholar] [CrossRef] [Green Version]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.M.; Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Triviño, A. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp. Eye Res. 2001, 73, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Puro, D.G. Growth factors and Müller cells. Prog. Retin. Eye Res. 1995, 15, 89–101. [Google Scholar] [CrossRef]
- Gargini, C.; Bisti, S.; Demontis, G.C.; Valter, K.; Stone, J.; Cervetto, L. Electroretinogram changes associated with retinal upregulation of trophic factors: Observations following optic nerve section. Neuroscience 2004, 126, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Montalbán-Soler, L.; Alarcón-Martínez, L.; Jiménez-López, M.; Salinas-Navarro, M.; Galindo-Romero, C.; Bezerra de Sá, F.; García-Ayuso, D.; Avilés-Trigueros, M.; Vidal-Sanz, M.; Agudo-Barriuso, M.; et al. Retinal compensatory changes after light damage in albino mice. Mol. Vis. 2012, 18, 675–693. [Google Scholar]
- Schremser, J.L.; Williams, T.P. Rod outer segment (ROS) renewal as a mechanism for adaptation to a new intensity environment. I. Rhodopsin levels and ROS length. Exp. Eye Res. 1995, 61, 17–23. [Google Scholar] [CrossRef]
- Sugawara, T.; Sieving, P.A.; Bush, R.A. Quantitative relationship of the scotopic and photopic ERG to photoreceptor cell loss in light damaged rats. Exp. Eye Res. 2000, 70, 693–705. [Google Scholar] [CrossRef]
- Cao, W.; Wen, R.; Li, F.; Lavail, M.M.; Steinberg, R.H. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp. Eye Res. 1997, 65, 241–248. [Google Scholar] [CrossRef]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen. Res. 2018, 13, 1885–1886. [Google Scholar] [CrossRef]




| Group | Treatment | Light Exposure Duration (hours) | Days (d) after Light Exposure |
|---|---|---|---|
| HC | Healthy Control | - | - |
| LD12h7d | LD 1000 lux | 12 (short-term) | 7 |
| LD15h7d | 15 | ||
| LD18h7d | 18 | ||
| LD24h0d | 24 (long-term) | 0 | |
| LD24h7d | 7 | ||
| LD24h15d | 15 | ||
| LD24h30d | 30 | ||
| LD24h45d | 45 | ||
| LD24h60d | 60 | ||
| LD24h90d | 90 | ||
| LD24h210d | 210 |
| Primary Antibodies | Supplier | Host Organism | Dilution | Product# | Ref |
| Anti-ionized calcium-binding adaptor molecule 1 (Iba1) | Wako | Rabbit | 1:1000 | 019–19741 | [42] |
| Anti-glial fibrillary acidic protein (GFAP) | Dako | Rabbit | 1:500 | Z0334 | [41] |
| Anti-choline acetyltransferase (ChAT) | Millipore (Chemicon) | Goat | 1:100 | AB144P | [43] |
| Anti-L/M Opsin | Millipore (Chemicon) | Rabbit | 1:100 | AB5405 | [41] |
| Anti-fibroblast growth factor (FGF)−2/basic FGF | Millipore | Mouse | 1:200 | 05–117 | [14] |
| Anti-synaptophysin (SYN) | Osenses | Rabbit | 1:300 | OSS00021W | |
| Secondary Antibodies | Supplier | Host Organism | Dilution | Product# | |
| Anti-rabbit IgG (H + L) Alexa Fluor 488 | Thermo Fisher Scientific | Goat | 1:500 | A−11008 | |
| Anti-rabbit IgG (H + L) Alexa Fluor 594 | Thermo Fisher Scientific | Goat | 1:500 | A−11012 | |
| Anti-goat IgG (H + L) biotinylated | Thermo Fisher Scientific | Rabbit | 1:300 | 31732 | |
| Anti-mouse IgG (H + L) Alexa Fluor 488 | Thermo Fisher Scientific | Goat | 1:500 | A−11001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccitelli, S.; Di Paolo, M.; Ashley, J.; Bisti, S.; Di Marco, S. The Timecourses of Functional, Morphological, and Molecular Changes Triggered by Light Exposure in Sprague–Dawley Rat Retinas. Cells 2021, 10, 1561. https://doi.org/10.3390/cells10061561
Riccitelli S, Di Paolo M, Ashley J, Bisti S, Di Marco S. The Timecourses of Functional, Morphological, and Molecular Changes Triggered by Light Exposure in Sprague–Dawley Rat Retinas. Cells. 2021; 10(6):1561. https://doi.org/10.3390/cells10061561
Chicago/Turabian StyleRiccitelli, Serena, Mattia Di Paolo, James Ashley, Silvia Bisti, and Stefano Di Marco. 2021. "The Timecourses of Functional, Morphological, and Molecular Changes Triggered by Light Exposure in Sprague–Dawley Rat Retinas" Cells 10, no. 6: 1561. https://doi.org/10.3390/cells10061561
APA StyleRiccitelli, S., Di Paolo, M., Ashley, J., Bisti, S., & Di Marco, S. (2021). The Timecourses of Functional, Morphological, and Molecular Changes Triggered by Light Exposure in Sprague–Dawley Rat Retinas. Cells, 10(6), 1561. https://doi.org/10.3390/cells10061561






