Neuron-Glia-Immune Triad and Cortico-Limbic System in Pathology of Pain
Abstract
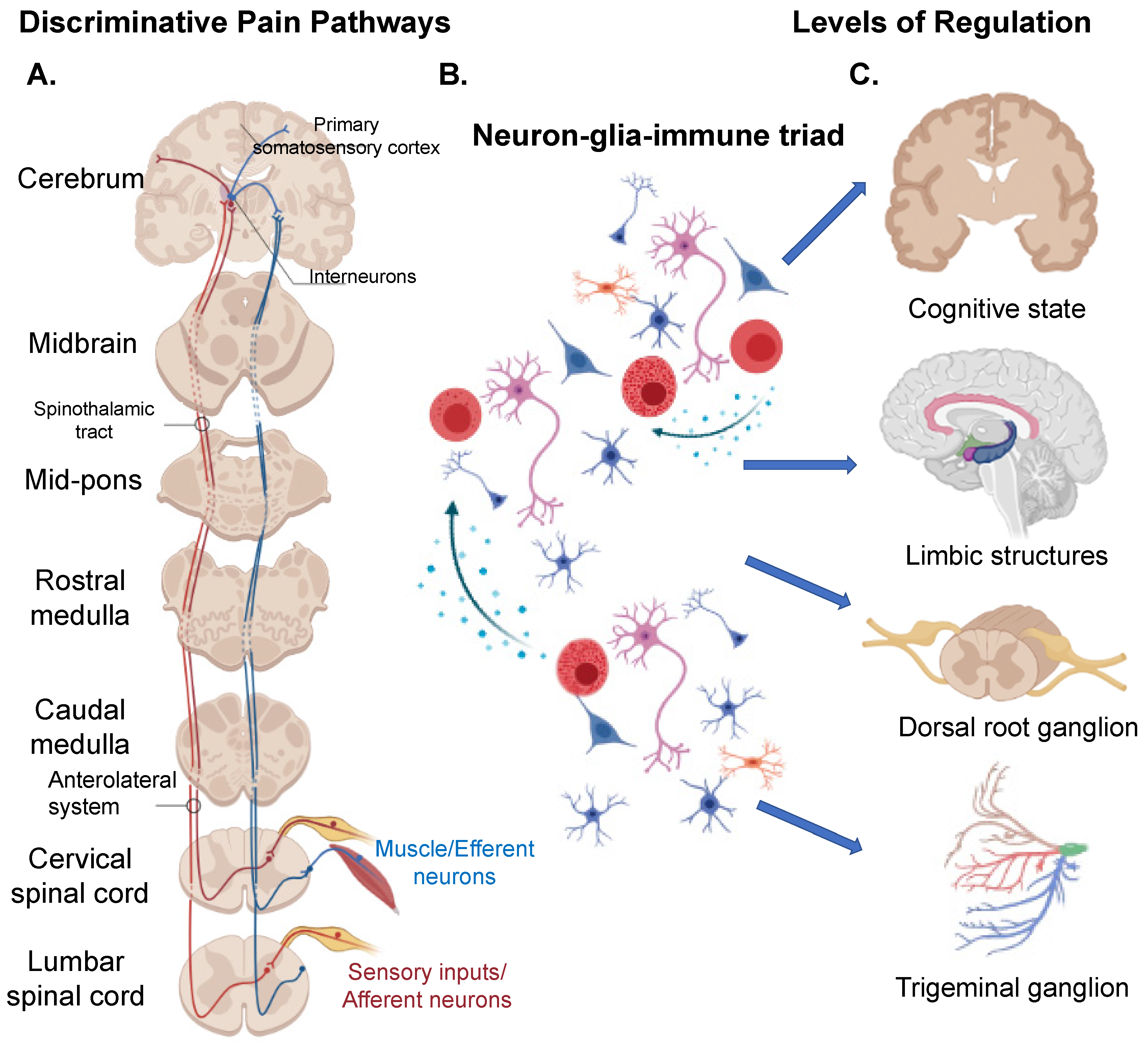
1. Introduction
1.1. “Central” Structures Orchestrating Pain
1.2. Peripheral Nervous System (PNS)
1.3. Neuron Clusters/Ganglion Systems in Pain
1.4. Glial/Non-Neuronal Cells in Pain
1.4.1. Glial Cells
1.4.2. Satellite Glial Cells
1.4.3. Astrocytes
1.4.4. Schwann Cells
1.5. Immune Cells in Pain
1.5.1. Microglia
1.5.2. Natural Killer Cells
1.5.3. Neutrophils
1.5.4. Helper T Cells
1.5.5. Mast Cells
1.6. The Neuron-Glial-Immune Triad Interaction in Pain Pathology
1.7. The Cortico-Limbic System in Pain
1.8. Mental Health/Altered Cognitive States in Pain Perception
2. Sex Differences in Injuries and Pain Perception—An Emerging Area
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Wernsdorff, M.; Loef, M.; Tuschen-Caffier, B.; Schmidt, S. Effects of open-label placebos in clinical trials: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 3855. [Google Scholar] [CrossRef]
- Craggs, J.G.; Price, D.N.D.; Robinson, M.E. Enhancing the Placebo Response: Functional Magnetic Resonance Imaging Evidence of Memory and Semantic Processing in Placebo Analgesia. J. Pain 2014, 15, 435–446. [Google Scholar] [CrossRef]
- Hashish, I.; Haia, H.K.; Harvey, W.; Feinmann, C.; Harris, M. Reduction of postoperative pain and swelling by ultrasound treatment: A placebo effect. Pain 1988, 33, 303–311. [Google Scholar] [CrossRef]
- Hashmi, J.A. Placebo Effect: Theory, Mechanisms and Teleological Roots. Int. Rev. Neurobiol. 2018, 139, 233–253. [Google Scholar] [CrossRef]
- Horwitz, D.L. Endocrine disorders. Introduction. Compr. Ther. 1980, 6, 7–11. [Google Scholar]
- Kleine-Borgmann, J.; Schmidt, K.; Hellmann, A.; Bingel, U. Effects of open-label placebo on pain, functional disability, and spine mobility in patients with chronic back pain: A randomized controlled trial. Pain 2019, 160, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F.; Emerson, N.M.; Farris, S.R.; Ray, J.N.; Jung, Y.; McHaffie, J.G.; Coghill, R.C. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J. Neurosci. 2015, 35, 15307–15325. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons. One in Two Americans have a Musculoskeletal Condition: New Report Outlines the Prevalence, Scope, Cost and Projected Growth of Musculoskeletal Disorders in the U.S. ScienceDaily. 2016. Available online: www.sciencedaily.com/releases/2016/2003/160301114116.htm (accessed on 1 March 2016).
- Hsu, J.R.; Mir, H.; Wally, M.K.; Seymour, R.B.; The Orthopaedic Trauma Association Musculoskeletal Pain Task Force. Clinical Practice Guidelines for Pain Management in Acute Musculoskeletal Injury. J. Orthop. Trauma 2019, 33, e158–e182. [Google Scholar] [CrossRef] [PubMed]
- Ahimsadasan, N.; Reddy, V.; Kumar, A. Neuroanatomy, Dorsal Root Ganglion; Stat Pearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Jasmin, L.; Vit, J.-P.; Bhargava, A.; Ohara, P.T. Can satellite glial cells be therapeutic targets for pain control? Neuron. Glia Biol. 2010, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ohara, P.T.; Vit, J.-P.; Bhargava, A.; Romero, M.; Sundberg, C.; Charles, A.C.; Jasmin, L. Gliopathic Pain: When Satellite Glial Cells Go Bad. Neuroscientist 2009, 15, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Vit, J.-P.; Jasmin, L.; Bhargava, A.; Ohara, P.T. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron. Glia Biol. 2006, 2, 247–257. [Google Scholar] [CrossRef]
- Vit, J.-P.; Ohara, P.T.; Bhargava, A.; Kelley, K.; Jasmin, L. Silencing the Kir4.1 Potassium Channel Subunit in Satellite Glial Cells of the Rat Trigeminal Ganglion Results in Pain-Like Behavior in the Absence of Nerve Injury. J. Neurosci. 2008, 28, 4161–4171. [Google Scholar] [CrossRef]
- Liem, L.; Van Dongen, E.; Huygen, F.J.; Staats, P.; Kramer, J. The Dorsal Root Ganglion as a Therapeutic Target for Chronic Pain. Reg. Anesth. Pain Med. 2016, 41, 511–519. [Google Scholar] [CrossRef]
- Chancellor-Freeland, C.; Zhu, G.F.; Kage, R.; Beller, D.I.; Leeman, S.E.; Black, P.H. Substance P and Stress-Induced Changes in Macrophages. Ann. N. Y. Acad. Sci. 1995, 771, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, G.S.; Anogeianaki, A.; Orso, C.; Tete, S.; Salini, V.; Antinolfi, P.L.; Sabatino, G. Impact of substance P on cellular immunity. J. Biol. Regul. Homeost. Agents 2008, 22, 93–98. [Google Scholar] [PubMed]
- Nicoll, R.A.; Schenker, C.; Leeman, S.E. Substance P as a Transmitter Candidate. Annu. Rev. Neurosci. 1980, 3, 227–268. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.S. The Role of the Dorsal Root Ganglion in the Development of Neuropathic Pain. Pain Med. 2014, 15, 1669–1685. [Google Scholar] [CrossRef] [PubMed]
- Fricke, B.; Andres, K.H.; Von Düring, M. Nerve fibers innervating the cranial and spinal meninges: Morphology of nerve fiber terminals and their structural integration. Microsc. Res. Tech. 2001, 53, 96–105. [Google Scholar] [CrossRef]
- Costa, Y.M.; Ariji, Y.; Ferreira, D.M.A.O.; Bonjardim, L.R.; Conti, P.C.R.; Ariji, E.; Svensson, P. Muscle hardness and masticatory myofascial pain: Assessment and clinical relevance. J. Oral Rehabil. 2018, 45, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Ohara, P.T.; Vit, J.-P.; Bhargava, A.; Jasmin, L. Evidence for a Role of Connexin 43 in Trigeminal Pain Using RNA Interference In Vivo. J. Neurophysiol. 2008, 100, 3064–3073. [Google Scholar] [CrossRef]
- Abati, E.; Citterio, G.; Bresolin, N.; Comi, G.P.; Corti, S. Glial cells involvement in spinal muscular atrophy: Could SMA be a neuroinflammatory disease? Neurobiol. Dis. 2020, 140, 104870. [Google Scholar] [CrossRef]
- Gosselin, R.-D.; Suter, M.R.; Ji, R.-R.; Decosterd, I. Glial Cells and Chronic Pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef]
- Gegelashvili, G.; Bjerrum, O.J. Glutamate Transport System as a Novel Therapeutic Target in Chronic Pain: Molecular Mechanisms and Pharmacology. Adv. Neurobiol. 2017, 16, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.M.; Rojas, C.; Wu, Y.W.; Slusher, B.S. The Role of Glutamate Signaling in Pain Processes and its Regulation by GCP II Inhibition. Curr. Med. Chem. 2012, 19, 1323–1334. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Cebrian-Silla, A.; Thomson, M.; Parraguez, J.I.; Anderson, R.; Guinto, C.; Rodriguez, J.R.; Garcia-Verdugo, J.-M.; Alvarez-Buylla, A. Adult Neurogenesis Is Sustained by Symmetric Self-Renewal and Differentiation. Cell Stem Cell 2018, 22, 221–234 e228. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin–N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell. Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Maftei, D.; Marconi, V.; Florenzano, F.; Franchi, S.; Borsani, E.; Rodella, L.F.; Balboni, G.; Salvadori, S.; Sacerdote, P.; et al. Prokineticin 2 Upregulation in the Peripheral Nervous System Has a Major Role in Triggering and Maintaining Neuropathic Pain in the Chronic Constriction Injury Model. BioMed Res. Int. 2015, 2015, 301292. [Google Scholar] [CrossRef]
- Poplawski, G.; Ishikawa, T.; Brifault, C.; Lee-Kubli, C.; Regestam, R.; Henry, K.W.; Shiga, Y.; Kwon, H.; Ohtori, S.; Gonias, S.L.; et al. Schwann cells regulate sensory neuron gene expression before and after peripheral nerve injury. Glia 2018, 66, 1577–1590. [Google Scholar] [CrossRef]
- Iwasaki, H.; Sakai, A.; Maruyama, M.; Ito, T.; Sakamoto, A.; Suzuki, H. Increased H19 Long Non-coding RNA Expression in Schwann Cells in Peripheral Neuropathic Pain. J. Nippon. Med. Sch. 2019, 86, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Barcelon, E.E.; Cho, W.-H.; Jun, S.B.; Lee, S.J. Brain Microglial Activation in Chronic Pain-Associated Affective Disorder. Front. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.J.; Kim, H.W.; Gonzalez-Cano, R.; Choi, J.; Back, S.K.; Roh, S.E.; Johnson, E.; Gabriac, M.; Kim, M.-S.; Lee, J.; et al. Natural Killer Cells Degenerate Intact Sensory Afferents following Nerve Injury. Cell 2019, 176, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.C.; Graham, M.A.; Triano, J.J.; Hondras, M.A.; Anderson, R.J. Lymphocyte profiles in patients with chronic low back pain enrolled in a clinical trial. J. Manip. Physiol. Ther. 1994, 17, 219–227. [Google Scholar]
- Kanashiro, A.; Hiroki, C.H.; da Fonseca, D.M.; Birbrair, A.; Ferreira, R.G.; Bassi, G.S.; Fonseca, M.D.; Kusuda, R.; Cebinelli, G.C.M.; da Silva, K.P.; et al. The role of neutrophils in neuro-immune modulation. Pharmacol. Res. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Cunha, T.M.; Verri, W.A., Jr.; Schivo, I.R.; Napimoga, M.H.; Parada, C.A.; Poole, S.; Teixeira, M.M.; Ferreira, S.H.; Cunha, F.Q. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 2008, 83, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Laumet, G.; Ma, J.; Robison, A.J.; Kumari, S.; Heijnen, C.J.; Kavelaars, A. T Cells as an Emerging Target for Chronic Pain Therapy. Front. Mol. Neurosci. 2019, 12, 216. [Google Scholar] [CrossRef]
- Massart, R.; Dymov, S.; Millecamps, M.; Suderman, M.; Gregoire, S.; Koenigs, K.; Alvarado, S.; Tajerian, M.; Stone, L.S.; Szyf, M. Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Sci. Rep. 2016, 6, 19615. [Google Scholar] [CrossRef]
- Singh, L.K.; Boucher, W.; Pang, X.; Letourneau, R.; Seretakis, D.; Green, M.; Theoharides, T.C. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J. Pharmacol. Exp. Ther. 1999, 288, 1349–1356. [Google Scholar]
- Koyuncu Irmak, D.; Kilinc, E.; Tore, F. Shared Fate of Meningeal Mast Cells and Sensory Neurons in Migraine. Front. Cell. Neurosci. 2019, 13, 136. [Google Scholar] [CrossRef]
- Cordner, Z.A.; Li, Q.; Liu, L.; Tamashiro, K.L.; Bhargava, A.; Moran, T.H.; Pasricha, P.J. Vagal gut-brain signaling mediates amygdaloid plasticity, affect, and pain in a functional dyspepsia model. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.-I.; Hasdemir, B.; Heyman, M.B.; Chang, L.; Bhargava, A. Plasma Corticotropin-Releasing Factor Receptors and B7-2+ Extracellular Vesicles in Blood Correlate with Irritable Bowel Syndrome Disease Severity. Cells 2019, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.-I.; Kaushal, E.; Paruthiyil, S.; Pasricha, P.J.; Hasdemir, B.; Bhargava, A. Gastric corticotropin-releasing factor influences mast cell infiltration in a rat model of functional dyspepsia. PLoS ONE 2018, 13, e0203704. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Sapolsky, R.; Liao, M.; Mehta, K.; Bhargava, A.; Pasricha, P.J. Transient Gastric Irritation in the Neonatal Rats Leads to Changes in Hypothalamic CRF Expression, Depression- and Anxiety-Like Behavior as Adults. PLoS ONE 2011, 6, e19498. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.E.; Lacroix, S.; Avilés-Trigueros, M.; David, S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 2005, 128, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Angert, M.; Dolkas, J.; Campana, W.M.; Palenscar, K.; Myers, R.R. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol. Cell. Neurosci. 2006, 31, 407–415. [Google Scholar] [CrossRef]
- Cureton, E.L.; Ereso, A.Q.; Victorino, G.P.; Curran, B.; Poole, D.P.; Liao, M.; Harken, A.H.; Bhargava, A. Local Secretion of Urocortin 1 Promotes Microvascular Permeability during Lipopolysaccharide-Induced Inflammation. Endocrinology 2009, 150, 5428–5437. [Google Scholar] [CrossRef]
- Costigan, M.; Befort, K.; Karchewski, L.; Griffin, R.S.; D’Urso, D.; Allchorne, A.; Sitarski, J.; Mannion, J.W.; Pratt, R.E.; Woolf, C.J. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Arruda, J.L.; Sweitzer, S.; Rutkowski, M.D.; DeLeo, J.A. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: Possible immune modulation in neuropathic pain. Brain Res. 2000, 879, 216–225. [Google Scholar] [CrossRef]
- Wolf, G.; Gabay, E.; Tal, M.; Yirmiya, R.; Shavit, Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain 2006, 120, 315–324. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Bonaccini, L.; Zanardelli, M.; Mello, T.; Ghelardini, C. Morphologic Features and Glial Activation in Rat Oxaliplatin-Dependent Neuropathic Pain. J. Pain 2013, 14, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Warwick, R.A.; Hanani, M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur. J. Pain 2013, 17, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K. Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science 2016, 354, 584–587. [Google Scholar] [CrossRef]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef]
- Thompson, J.M.; Neugebauer, V. Amygdala Plasticity and Pain. Pain Res. Manag. 2017, 8296501. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Inslicht, S.S.; Metzler, T.J.; Garcia, N.M.; Pineles, S.L.; Milad, M.R.; Orr, S.P.; Marmar, C.R.; Neylan, T.C. Sex differences in fear conditioning in posttraumatic stress disorder. J. Psychiatr. Res. 2013, 47, 64–71. [Google Scholar] [CrossRef]
- McCarberg, B.; Peppin, J. Pain Pathways and Nervous System Plasticity: Learning and Memory in Pain. Pain Med. 2019, 20, 2421–2437. [Google Scholar] [CrossRef]
- Malfliet, A.; Coppieters, I.; Van Wilgen, P.; Kregel, J.; De Pauw, R.; Dolphens, M.; Ickmans, K. Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. Eur. J. Pain 2017, 21, 769–786. [Google Scholar] [CrossRef]
- Bouffard, J.; Weber, Z.; Pearsall, L.; Emery, K.; Côté, J.N. Similar effects of fatigue induced by a repetitive pointing task on local and remote light touch and pain perception in men and women. PLoS ONE 2020, 15, e0244321. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Emery, K.; Cote, J.N. Sex differences in perceptual responses to experimental pain before and after an experimental fatiguing arm task. Biol. Sex Differ. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Behan, F.P.; Maden-Wilkinson, T.M.; Pain, M.T.G.; Folland, J.P. Sex differences in muscle morphology of the knee flexors and knee extensors. PLoS ONE 2018, 13, e0190903. [Google Scholar] [CrossRef]
- Bhargava, A.; Arnold, A.P.; Bangasser, D.A.; Denton, K.M.; Gupta, A.; Hilliard Krause, L.M.; Mayer, E.A.; McCarthy, M.; Miller, W.L.; Raznahan, A.; et al. Considering Sex as a Biological Variable in Basic and Clinical Studies: An Endocrine Society Scientific Statement. Endocr. Rev. 2021, 42, 219–258. [Google Scholar] [CrossRef] [PubMed]
- Vuppaladhadiam, L.; Ehsan, C.; Akkati, M.; Bhargava, A. Corticotropin-Releasing Factor Family: A Stress Hormone-Receptor System’s Emerging Role in Mediating Sex-Specific Signaling. Cells 2020, 9, 839. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Done, J.D.; Rudick, C.N.; Quick, M.L.; Schaeffer, A.J.; Thumbikat, P. Role of Mast Cells in Male Chronic Pelvic Pain. J. Urol. 2012, 187, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; McCarthy, M.M. A Starring Role for Microglia in Brain Sex Differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef]
- Chowen, J.A.; Argente-Arizon, P.; Freire-Regatillo, A.; Argente, J. Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front. Neuroendocr. 2018, 48, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.W.; Filosa, J.A. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience 2016, 339, 85–99. [Google Scholar] [CrossRef] [PubMed]
| Neurotransmitter and Neuromodulators |
|---|
| Adenosine Triphosphate (ATP) |
| Algogen, Bradykinin (BK) inflammatory mediator |
| Amino Acids (Includes glutamate: most abundant excitatory neurotransmitter, Serine, Glycine, etc.) |
| Artemin and glial cell-line derived neurotrophic factors (GDNF) |
| Brain-derived nerve growth factor (BDNF) |
| Calcitonin Gene-Related Peptide (CGRP) |
| Cytokines and chemokines |
| Gamma aminobutyric acid (GABA) |
| Nerve Growth Factor |
| Neuropeptides (includes corticotropin-releasing hormone, urocortins 1–3) |
| Norepinephrine |
| Tachykinins (includes Substance P, neurokinin A, neurokinin B) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, I.; Bhanot, G.; Bhargava, A. Neuron-Glia-Immune Triad and Cortico-Limbic System in Pathology of Pain. Cells 2021, 10, 1553. https://doi.org/10.3390/cells10061553
Murray I, Bhanot G, Bhargava A. Neuron-Glia-Immune Triad and Cortico-Limbic System in Pathology of Pain. Cells. 2021; 10(6):1553. https://doi.org/10.3390/cells10061553
Chicago/Turabian StyleMurray, Isabella, Gayatri Bhanot, and Aditi Bhargava. 2021. "Neuron-Glia-Immune Triad and Cortico-Limbic System in Pathology of Pain" Cells 10, no. 6: 1553. https://doi.org/10.3390/cells10061553
APA StyleMurray, I., Bhanot, G., & Bhargava, A. (2021). Neuron-Glia-Immune Triad and Cortico-Limbic System in Pathology of Pain. Cells, 10(6), 1553. https://doi.org/10.3390/cells10061553







