Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Tissues and Cells
2.2. Cell Culture
2.3. Flow Cytometry Analysis
2.4. Cell Sorting
2.5. Western Blotting
2.6. Immunofluorescence
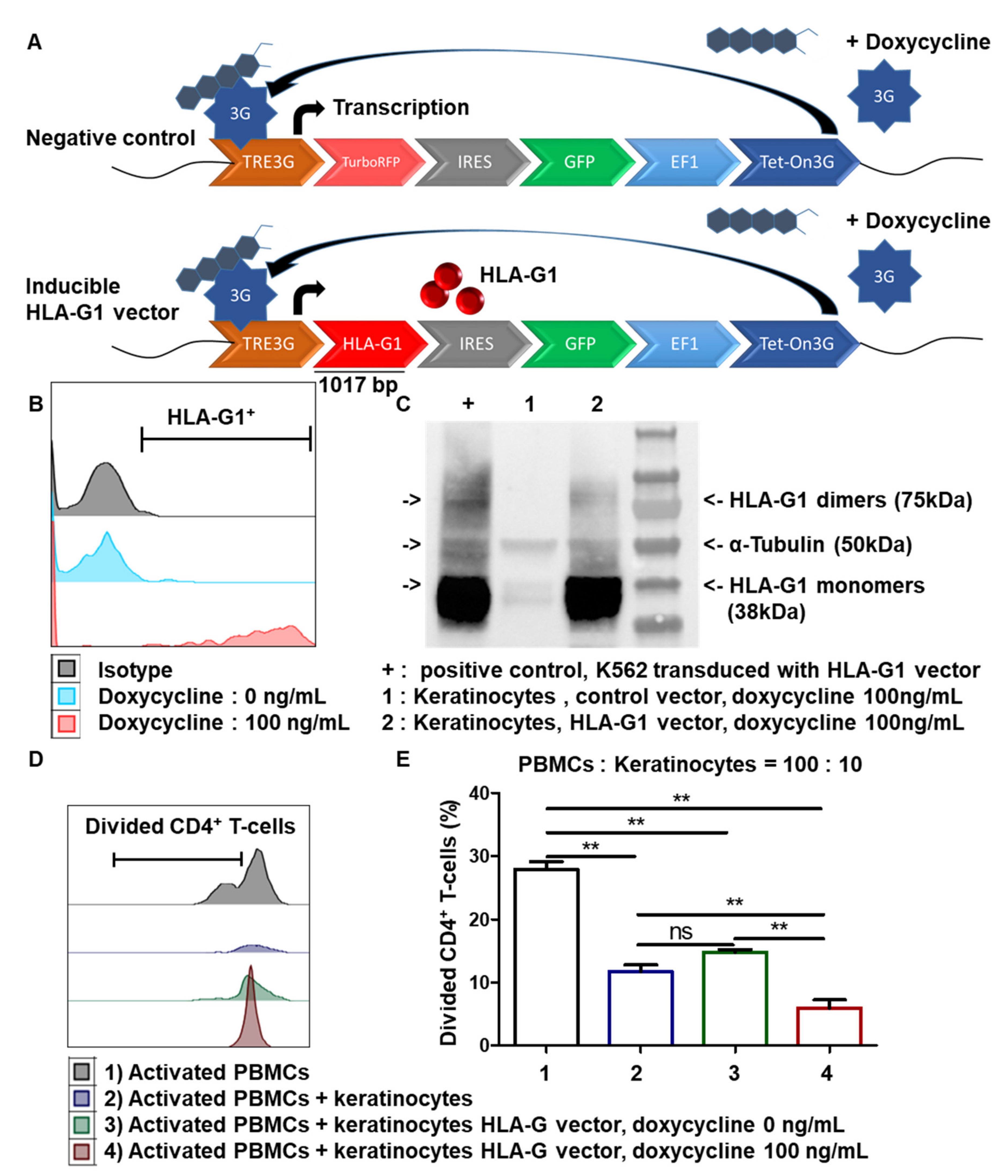
2.7. Cell Transduction for Inducible Expression of HLA-G1
2.8. PBMC Proliferation Measured by 3H-Thymidine Incorporation
2.9. Flow Cytometry-Based Analysis of CD4+ T-Cell Proliferation
2.10. Supernatant Collection
2.11. Antibody Blocking Experiments
2.12. Statistics
3. Results
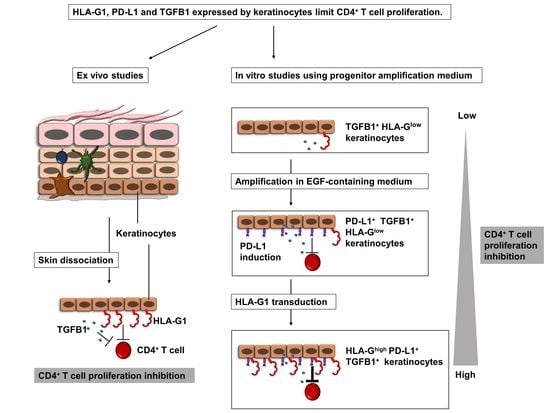
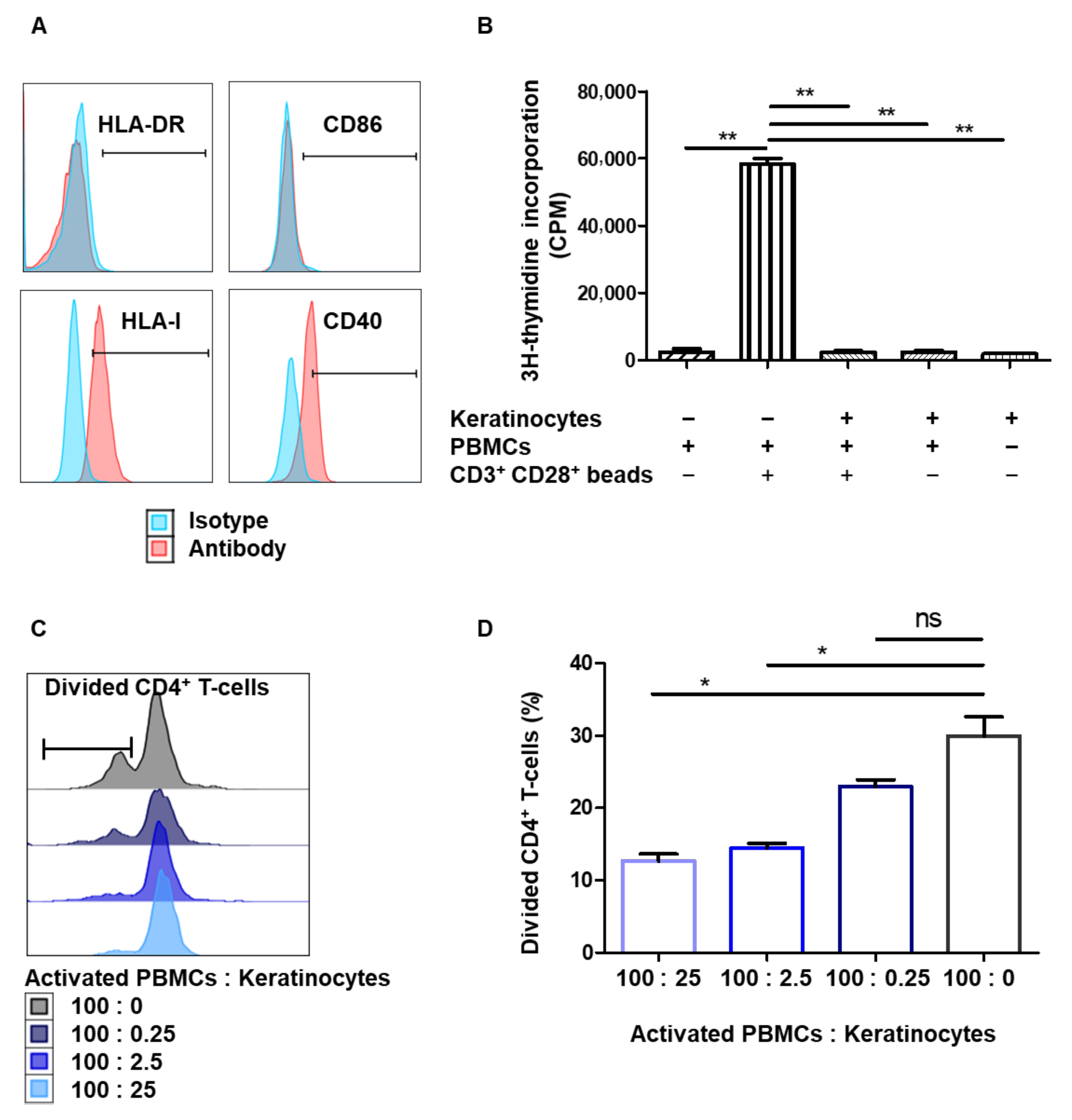
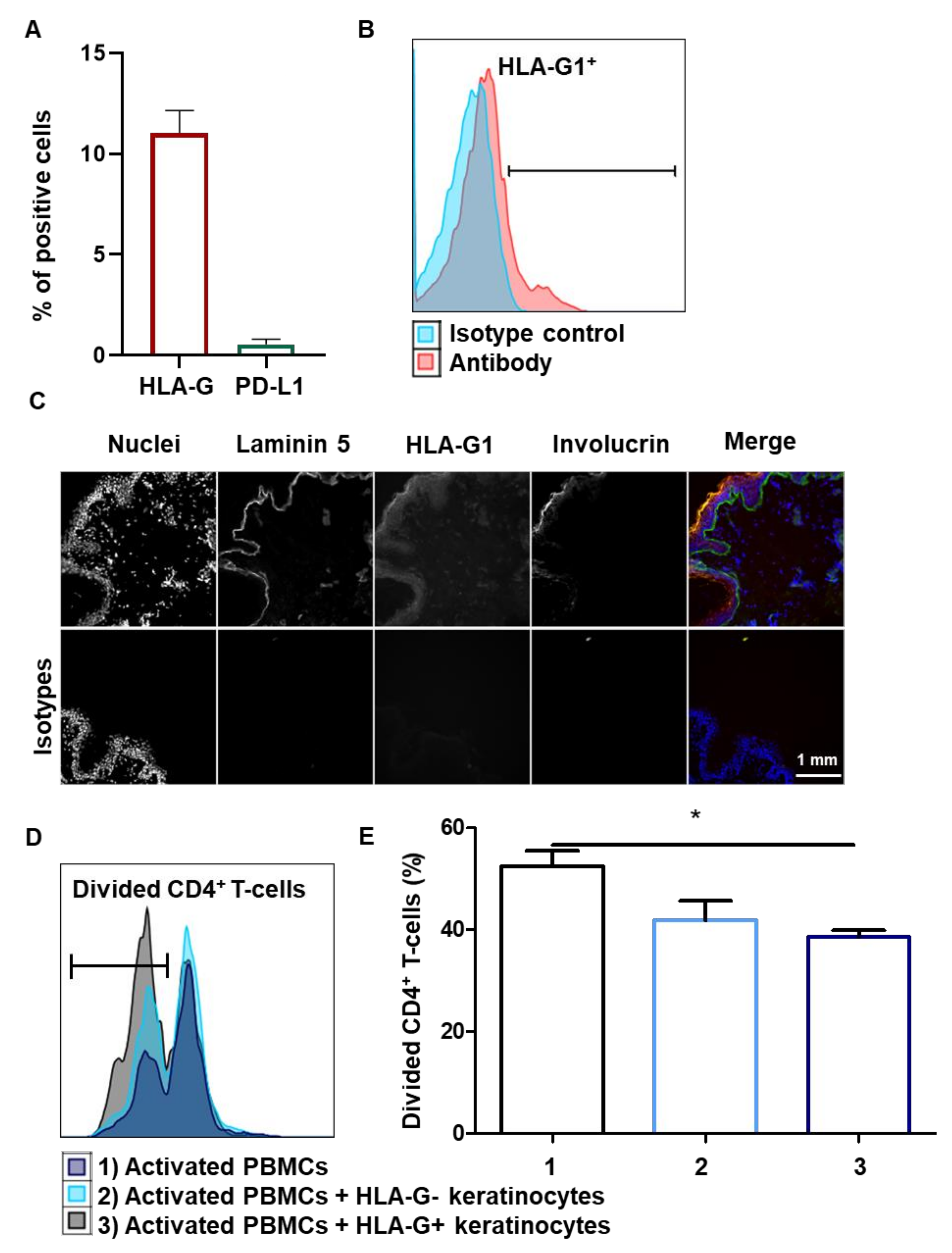
3.1. Amplified Keratinocytes Are Hypoimmunogenic and Limit CD4+ T-Cell Proliferation
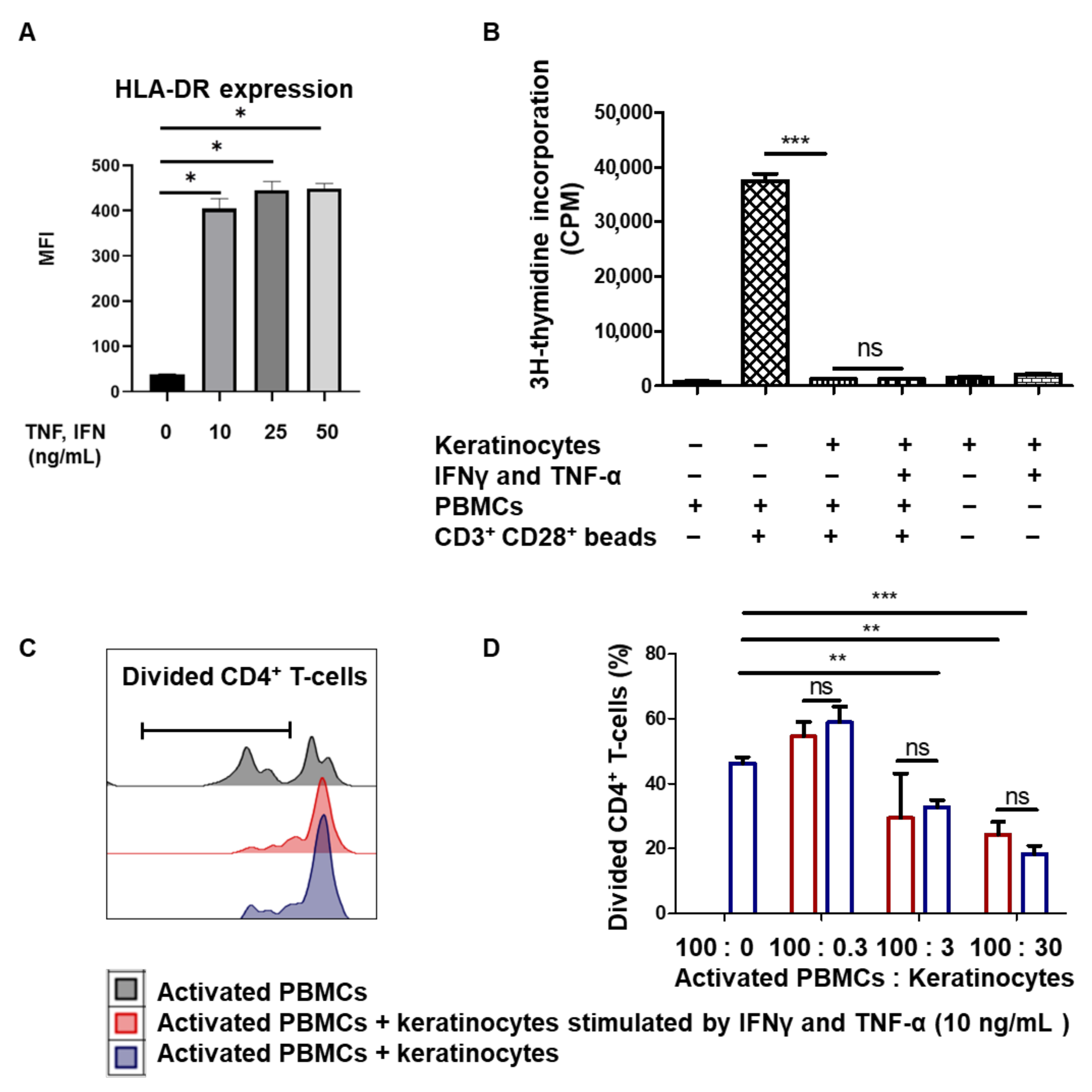
3.2. Amplified Keratinocytes Limit CD4+ T-Cell Proliferation Whether in Inflammatory Conditions or Not
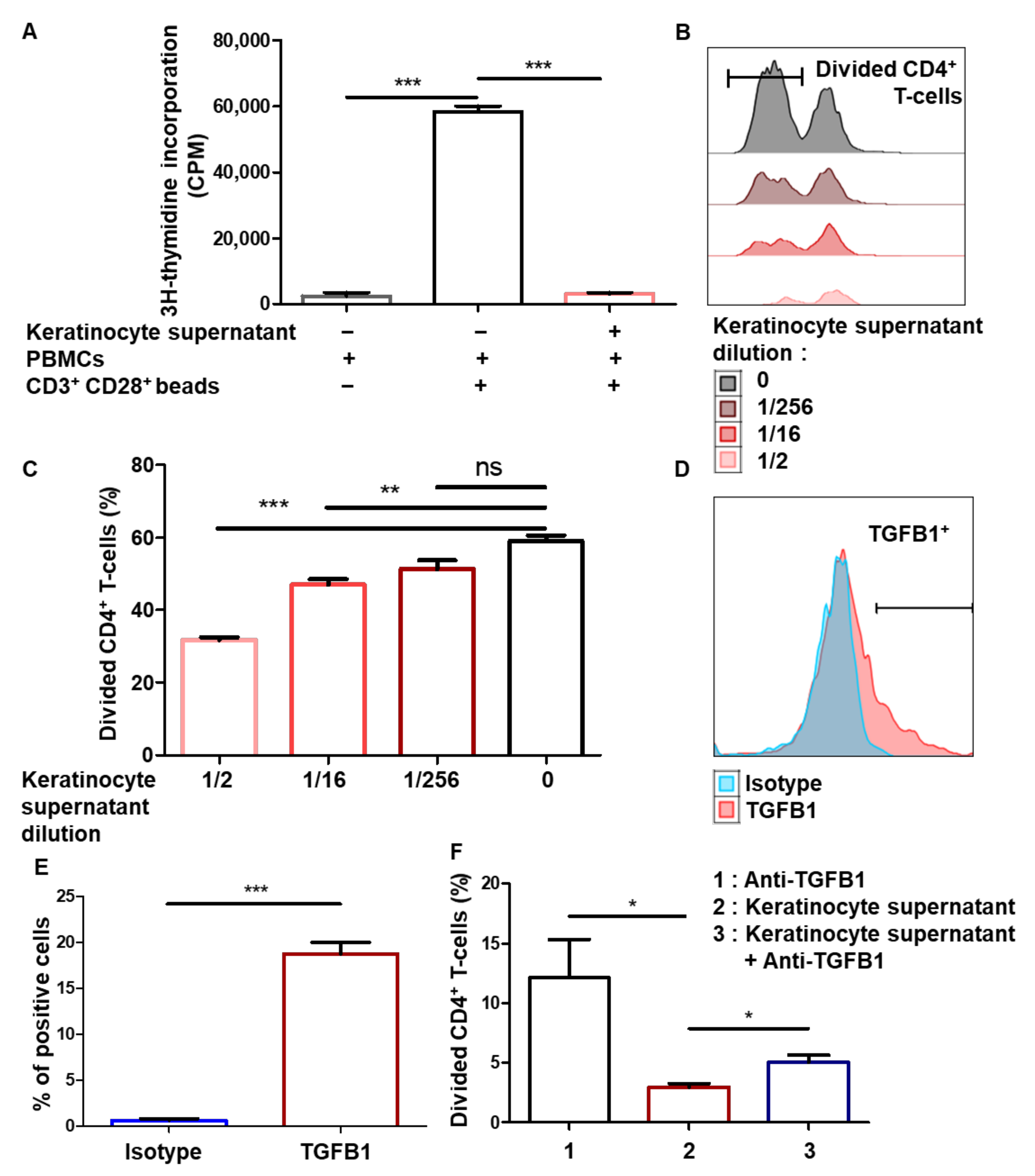
3.3. Immunomodulation Mediated by Amplified Keratinocytes Involves Soluble Factors
3.4. Immunomodulation by Amplified Keratinocytes Involves the PD-L1 Immune Checkpoint Protein
3.5. Tissue keratinocytes Express HLA-G1, Which Contributes to Their Immunomodulatory Properties
3.6. Inducible Expression of HLA-G1 in Amplified Keratinocytes Increases Their Immunomodulatory Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HLA-G | human leukocyte antigen G |
| MHC | major histocompatibility complex |
| PD-L1 | programmed death-ligand 1 |
| IFN-γ | interferon-γ |
| TGFB1 | transforming growth factor-β1 |
| TNF-α | tumor necrosis factor-α |
| EGF | epidermal growth factor |
| NK | natural killer |
| CPM | count per minute |
| TRE | tetracycline response element |
| Tet | tetracycline |
References
- Gallico, G.G.; O’connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent Coverage of Large Burn Wounds with Autologous Cultured Human Epithelium. N. Engl. J. Med. 1984, 311, 448–451. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the Entire Human Epidermis Using Transgenic Stem Cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Alexaline, M.M.; Trouillas, M.; Nivet, M.; Bourreau, E.; Leclerc, T.; Duhamel, P.; Martin, M.T.; Doucet, C.; Fortunel, N.O.; Lataillade, J.-J. Bioengineering a Human Plasma-Based Epidermal Substitute with Efficient Grafting Capacity and High Content in Clonogenic Cells. Stem Cells Transl. Med. 2015, 4, 643–654. [Google Scholar] [CrossRef]
- Agrawal, N.A.; Zavlin, D.; Louis, M.R.; Reece, E.M. Stem Cells and Plastic Surgery. Semin. Plast. Surg. 2019, 33, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of Cultured Human Epidermal Cells into Multiple Epithelia Suitable for Grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- Cirodde, A.; Leclerc, T.; Jault, P.; Duhamel, P.; Lataillade, J.-J.; Bargues, L. Cultured Epithelial Autografts in Massive Burns: A Single-Center Retrospective Study with 63 Patients. Burns 2011, 37, 964–972. [Google Scholar] [CrossRef]
- Sood, R.; Roggy, D.; Zieger, M.; Balledux, J.; Chaudhari, S.; Koumanis, D.J.; Mir, H.S.; Cohen, A.; Knipe, C.; Gabehart, K.; et al. Cultured Epithelial Autografts for Coverage of Large Burn Wounds in Eighty-Eight Patients: The Indiana University Experience. J. Burn Care Res. 2010, 31, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Quinby, W.C.; Bondoc, C.C.; Cosimi, A.B.; Russell, P.S.; Szyfelbein, S.K. Immunosuppression and Temporary Skin Transplantation in the Treatment of Massive Third Degree Burns. Ann. Surg. 1975, 182, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Wendt, J.R.; Ulich, T.; Rao, P.N. Long-Term Survival of Human Skin Allografts in Patients with Immunosuppression. Plast. Reconstr. Surg. 2004, 113, 1347–1354. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.-G.; Xu, Y.; et al. An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Bluestone, J.A. Control of Peripheral T-Cell Tolerance and Autoimmunity via the CTLA-4 and PD-1 Pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; Gonçalves, R.M.; Menier, C.; Dausset, J.; Carosella, E.D. Direct Evidence to Support the Role of HLA-G in Protecting the Fetus from Maternal Uterine Natural Killer Cytolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 11520–11525. [Google Scholar] [CrossRef] [PubMed]
- Ponte, M.; Cantoni, C.; Biassoni, R.; Tradori-Cappai, A.; Bentivoglio, G.; Vitale, C.; Bertone, S.; Moretta, A.; Moretta, L.; Mingari, M.C. Inhibitory Receptors Sensing HLA-G1 Molecules in Pregnancy: Decidua-Associated Natural Killer Cells Express LIR-1 and CD94/NKG2A and Acquire P49, an HLA-G1-Specific Receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 5674–5679. [Google Scholar] [CrossRef]
- Carosella, E.D.; Rouas-Freiss, N.; Tronik-Le Roux, D.; Moreau, P.; LeMaoult, J. HLA-G: An Immune Checkpoint Molecule. Adv. Immunol. 2015, 127, 33–144. [Google Scholar] [CrossRef]
- Carosella, E.D.; Moreau, P.; Lemaoult, J.; Rouas-Freiss, N. HLA-G: From Biology to Clinical Benefits. Trends Immunol. 2008, 29, 125–132. [Google Scholar] [CrossRef]
- Lila, N.; Carpentier, A.; Amrein, C.; Khalil-Daher, I.; Dausset, J.; Carosella, E.D. Implication of HLA-G Molecule in Heart-Graft Acceptance. Lancet 2000, 355, 2138. [Google Scholar] [CrossRef]
- Créput, C.; Durrbach, A.; Menier, C.; Guettier, C.; Samuel, D.; Dausset, J.; Charpentier, B.; Carosella, E.D.; Rouas-Freiss, N. Human Leukocyte Antigen-G (HLA-G) Expression in Biliary Epithelial Cells Is Associated with Allograft Acceptance in Liver-Kidney Transplantation. J. Hepatol. 2003, 39, 587–594. [Google Scholar] [CrossRef]
- Naji, A.; Le Rond, S.; Durrbach, A.; Krawice-Radanne, I.; Creput, C.; Daouya, M.; Caumartin, J.; LeMaoult, J.; Carosella, E.D.; Rouas-Freiss, N. CD3+CD4low and CD3+CD8low Are Induced by HLA-G: Novel Human Peripheral Blood Suppressor T-Cell Subsets Involved in Transplant Acceptance. Blood 2007, 110, 3936–3948. [Google Scholar] [CrossRef] [PubMed]
- Deschaseaux, F.; Delgado, D.; Pistoia, V.; Giuliani, M.; Morandi, F.; Durrbach, A. HLA-G in Organ Transplantation: Towards Clinical Applications. Cell. Mol. Life Sci. 2011, 68, 397–404. [Google Scholar] [CrossRef]
- Brugière, O.; Thabut, G.; Krawice-Radanne, I.; Rizzo, R.; Dauriat, G.; Danel, C.; Suberbielle, C.; Mal, H.; Stern, M.; Schilte, C.; et al. Role of HLA-G as a Predictive Marker of Low Risk of Chronic Rejection in Lung Transplant Recipients: A Clinical Prospective Study. Am. J. Transplant. 2015, 15, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Horuzsko, A.; Lenfant, F.; Munn, D.H.; Mellor, A.L. Maturation of Antigen-Presenting Cells Is Compromised in HLA-G Transgenic Mice. Int. Immunol. 2001, 13, 385–394. [Google Scholar] [CrossRef][Green Version]
- LeMaoult, J.; Daouya, M.; Wu, J.; Loustau, M.; Horuzsko, A.; Carosella, E.D. Synthetic HLA-G Proteins for Therapeutic Use in Transplantation. FASEB J. 2013, 27, 3643–3651. [Google Scholar] [CrossRef]
- Naji, A.; Menier, C.; Morandi, F.; Agaugue, S.; Maki, G.; Ferretti, E.; Bruel, S.; Pistoia, V.; Carosella, E.D.; Rouas-Freiss, N. Binding of HLA-G to ITIM-Bearing Ig-like Transcript 2 Receptor Suppresses B Cell Responses. J. Immunol. 2014, 192, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Lefebvre, A.T.; Ajith, A.; Portik-Dobos, V.; Horuzsko, D.D.; Mulloy, L.L.; Horuzsko, A. Mouse Models for Studies of HLA-G Functions in Basic Science and Pre-Clinical Research. Hum. Immunol. 2016, 77, 711–719. [Google Scholar] [CrossRef]
- Cai, Y.-J.; Huang, L.; Leung, T.-Y.; Burd, A. A Study of the Immune Properties of Human Umbilical Cord Lining Epithelial Cells. Cytotherapy 2014, 16, 631–639. [Google Scholar] [CrossRef]
- Zhao, L.; Teklemariam, T.; Hantash, B.M. Heterelogous Expression of Mutated HLA-G Decreases Immunogenicity of Human Embryonic Stem Cells and Their Epidermal Derivatives. Stem Cell Res. 2014, 13, 342–354. [Google Scholar] [CrossRef]
- Fortunel, N.O.; Chadli, L.; Coutier, J.; Lemaître, G.; Auvré, F.; Domingues, S.; Bouissou-Cadio, E.; Vaigot, P.; Cavallero, S.; Deleuze, J.-F.; et al. KLF4 Inhibition Promotes the Expansion of Keratinocyte Precursors from Adult Human Skin and of Embryonic-Stem-Cell-Derived Keratinocytes. Nat. Biomed. Eng. 2019, 3, 985–997. [Google Scholar] [CrossRef]
- Fortunel, N.O.; Cadio, E.; Vaigot, P.; Chadli, L.; Moratille, S.; Bouet, S.; Roméo, P.-H.; Martin, M.T. Exploration of the Functional Hierarchy of the Basal Layer of Human Epidermis at the Single-Cell Level Using Parallel Clonal Microcultures of Keratinocytes. Exp. Dermatol. 2010, 19, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic Cells Dictate Responses to PD-L1 Blockade Cancer Immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef] [PubMed]
- Van Caam, A.; Aarts, J.; van Ee, T.; Vitters, E.; Koenders, M.; van de Loo, F.; van Lent, P.; van den Hoogen, F.; Thurlings, R.; Vonk, M.C.; et al. TGFβ-Mediated Expression of TGFβ-Activating Integrins in SSc Monocytes: Disturbed Activation of Latent TGFβ? Arthritis Res. Ther. 2020, 22, 42. [Google Scholar] [CrossRef]
- Delisle, J.-S.; Giroux, M.; Boucher, G.; Landry, J.-R.; Hardy, M.-P.; Lemieux, S.; Jones, R.G.; Wilhelm, B.T.; Perreault, C. The TGF-β-Smad3 Pathway Inhibits CD28-Dependent Cell Growth and Proliferation of CD4 T Cells. Genes Immun. 2013, 14, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dupin, C.; Lhuillier, E.; Létuvé, S.; Pretolani, M.; Thabut, G.; Mal, H.; Carosella, E.; Schilte, C.; Mordant, P.; Castier, Y.; et al. Inhibition of T Cell Alloreactivity by Bronchial Epithelium Is Impaired in Lung Transplant Recipients, Through Pathways Involving TGF-β, IL-10 and HLA-G. Transplantation 2017, 101, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Sivan, V.; Vozenin-Brotons, M.-C.; Tricaud, Y.; Lefaix, J.-L.; Cosset, J.-M.; Dubray, B.; Martin, M.T. Altered Proliferation and Differentiation of Human Epidermis in Cases of Skin Fibrosis after Radiotherapy. Int. J. Radiat. Oncol. 2002, 53, 385–393. [Google Scholar] [CrossRef]
- Du, W.-J.; Reppel, L.; Leger, L.; Schenowitz, C.; Huselstein, C.; Bensoussan, D.; Carosella, E.D.; Han, Z.-C.; Rouas-Freiss, N. Mesenchymal Stem Cells Derived from Human Bone Marrow and Adipose Tissue Maintain Their Immunosuppressive Properties After Chondrogenic Differentiation: Role of HLA-G. Stem Cells Dev. 2016, 25, 1454–1469. [Google Scholar] [CrossRef]
- McIntire, R.H.; Morales, P.J.; Petroff, M.G.; Colonna, M.; Hunt, J.S. Recombinant HLA-G5 and -G6 Drive U937 Myelomonocytic Cell Production of TGF-Beta1. J. Leukoc. Biol. 2004, 76, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Song, B.; Liu, F.; Sun, D.; Wang, K.; Qu, H. TGF-β Induces HLA-G Expression through Inhibiting MiR-152 in Gastric Cancer Cells. J. Biomed. Sci. 2015, 22, 107. [Google Scholar] [CrossRef]
- Urosevic, M. HLA-G in the Skin—Friend or Foe? Semin. Cancer Biol. 2007, 17, 480–484. [Google Scholar] [CrossRef]
- Ulbrecht, M.; Rehberger, B.; Strobel, I.; Messer, G.; Kind, P.; Degitz, K.; Bieber, T.; Weiss, E.H. HLA-G: Expression in Human Keratinocytes in Vitro and in Human Skin in Vivo. Eur. J. Immunol. 1994, 24, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ritprajak, P.; Hashiguchi, M.; Tsushima, F.; Chalermsarp, N.; Azuma, M. Keratinocyte-Associated B7-H1 Directly Regulates Cutaneous Effector CD8 + T Cell Responses. J. Immunol. 2010, 184, 4918–4925. [Google Scholar] [CrossRef]
- Miao, X.; Xu, R.; Fan, B.; Chen, J.; Li, X.; Mao, W.; Hua, S.; Li, B. PD-L1 Reverses Depigmentation in Pmel-1 Vitiligo Mice by Increasing the Abundance of Tregs in the Skin. Sci. Rep. 2018, 8, 1605. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 Expression in the Tumor Microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Aractingi, S.; Briand, N.; Le Danff, C.; Viguier, M.; Bachelez, H.; Michel, L.; Dubertret, L.; Carosella, E.D. HLA-G and NK Receptor Are Expressed in Psoriatic Skin: A Possible Pathway for Regulating Infiltrating T Cells? Am. J. Pathol. 2001, 159, 71–77. [Google Scholar] [CrossRef]
- Khosrotehrani, K.; Danff, C.L.; Reynaud-Mendel, B.; Dubertret, L.; Carosella, E.D.; Aractingi, S. HLA-G Expression in Atopic Dermatitis. J. Investig. Dermatol. 2001, 117, 750–752. [Google Scholar] [CrossRef]
- Gazit, E.; Slomov, Y.; Goldberg, I.; Brenner, S.; Loewenthal, R. HLA-G Is Associated with Pemphigus Vulgaris in Jewish Patients. Hum. Immunol. 2004, 65, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E.; Loewenthal, R. The Immunogenetics of Pemphigus Vulgaris. Autoimmun. Rev. 2005, 4, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-C.; Lee, Y.H. Association of HLA-G Polymorphisms with Systemic Lupus Erythematosus and Correlation between Soluble HLA-G Levels and the Disease: A Meta-Analysis. Z. Rheumatol. 2021, 80, 96–102. [Google Scholar] [CrossRef]
- Aractingi, S.; Kanitakis, J.; Euvrard, S.; Danff, C.L.; Carosella, E.D. Selective Expression of HLA-G in Malignant and Premalignant Skin Specimens in Kidney Transplant Recipients. Int. J. Cancer 2003, 106, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Urosevic, M.; Kamarashev, J.; Burg, G.; Dummer, R. Primary Cutaneous CD8+ and CD56+ T-Cell Lymphomas Express HLA-G and Killer-Cell Inhibitory Ligand, ILT2. Blood 2004, 103, 1796–1798. [Google Scholar] [CrossRef]
- Urosevic, M.; Kempf, W.; Zagrodnik, B.; Panizzon, R.; Burg, G.; Dummer, R. HLA-G Expression in Basal Cell Carcinomas of the Skin Recurring after Radiotherapy. Clin. Exp. Dermatol. 2005, 30, 422–425. [Google Scholar] [CrossRef]
- Geertsen, R.C.; Hofbauer, G.F.L.; Yue, F.-Y.; Manolio, S.; Burg, G.; Dummer, R. Higher Frequency of Selective Losses of HLA-A and -B Allospecificities in Metastasis Than in Primary Melanoma Lesions. J. Investig. Dermatol. 1998, 111, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, S.; Marincola, F.M. Loss of HLA Class I Antigens by Melanoma Cells: Molecular Mechanisms, Functional Significance and Clinical Relevance. Immunol. Today 1995, 16, 487–494. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Kärre, K. In Search of the “Missing Self”: MHC Molecules and NK Cell Recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Paul, P.; Rouas-Freiss, N.; Khalil-Daher, I.; Moreau, P.; Riteau, B.; Le Gal, F.A.; Avril, M.F.; Dausset, J.; Guillet, J.G.; Carosella, E.D. HLA-G Expression in Melanoma: A Way for Tumor Cells to Escape from Immunosurveillance. Proc. Natl. Acad. Sci. USA 1998, 95, 4510–4515. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Cabestré, F.A.; Le Gal, F.A.; Khalil-Daher, I.; Le Danff, C.; Schmid, M.; Mercier, S.; Avril, M.F.; Dausset, J.; Guillet, J.G.; et al. Heterogeneity of HLA-G Gene Transcription and Protein Expression in Malignant Melanoma Biopsies. Cancer Res. 1999, 59, 1954–1960. [Google Scholar]
- Dumont, C.; Jacquier, A.; Verine, J.; Noel, F.; Goujon, A.; Wu, C.-L.; Hung, T.-M.; Desgrandchamps, F.; Culine, S.; Carosella, E.D.; et al. CD8+PD-1-ILT2+ T Cells Are an Intratumoral Cytotoxic Population Selectively Inhibited by the Immune Checkpoint HLA-G. Cancer Immunol. Res. 2019, 27, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Dumont, C.; Carosella, E.D.; Rouas-Freiss, N.; LeMaoult, J. Cytometry-Based Analysis of HLA-G Functions According to ILT2 Expression. Hum. Immunol. 2020, 81, 168–177. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mestrallet, G.; Auvré, F.; Schenowitz, C.; Carosella, E.D.; LeMaoult, J.; Martin, M.T.; Rouas-Freiss, N.; Fortunel, N.O. Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints. Cells 2021, 10, 1438. https://doi.org/10.3390/cells10061438
Mestrallet G, Auvré F, Schenowitz C, Carosella ED, LeMaoult J, Martin MT, Rouas-Freiss N, Fortunel NO. Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints. Cells. 2021; 10(6):1438. https://doi.org/10.3390/cells10061438
Chicago/Turabian StyleMestrallet, Guillaume, Frédéric Auvré, Chantal Schenowitz, Edgardo D. Carosella, Joel LeMaoult, Michèle T. Martin, Nathalie Rouas-Freiss, and Nicolas O. Fortunel. 2021. "Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints" Cells 10, no. 6: 1438. https://doi.org/10.3390/cells10061438
APA StyleMestrallet, G., Auvré, F., Schenowitz, C., Carosella, E. D., LeMaoult, J., Martin, M. T., Rouas-Freiss, N., & Fortunel, N. O. (2021). Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints. Cells, 10(6), 1438. https://doi.org/10.3390/cells10061438