Carboxylated Poly-l-Lysine as a Macromolecular Cryoprotective Agent Enables the Development of Defined and Xeno-Free Human Sperm Cryopreservation Reagents
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Cryopreservation Reagents
2.3. Study Design
2.4. Sperm Preparation, Cryopreservation, and Thawing
2.5. Sperm Viability Test (SVT)
2.6. SDF Analysis Using the TUNEL Assay
2.7. Quantitative Analysis of Sperm Motility with a Longevity Curve
2.8. Assessment of the Capacitation Status
2.9. Assessment of the Acrosome Reaction
2.10. Statistical Analysis
3. Results
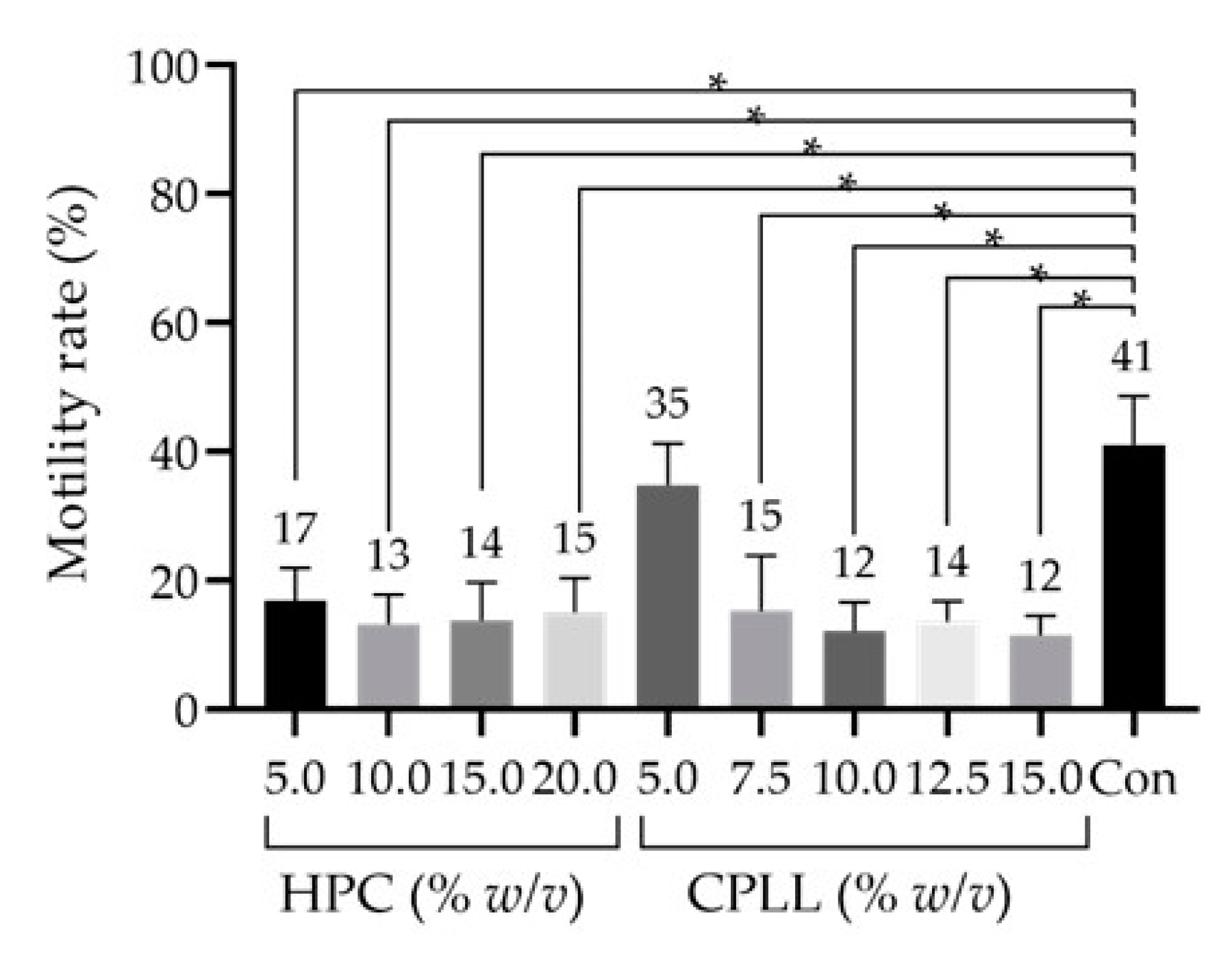
3.1. Effect of Macro-Mw CPAs
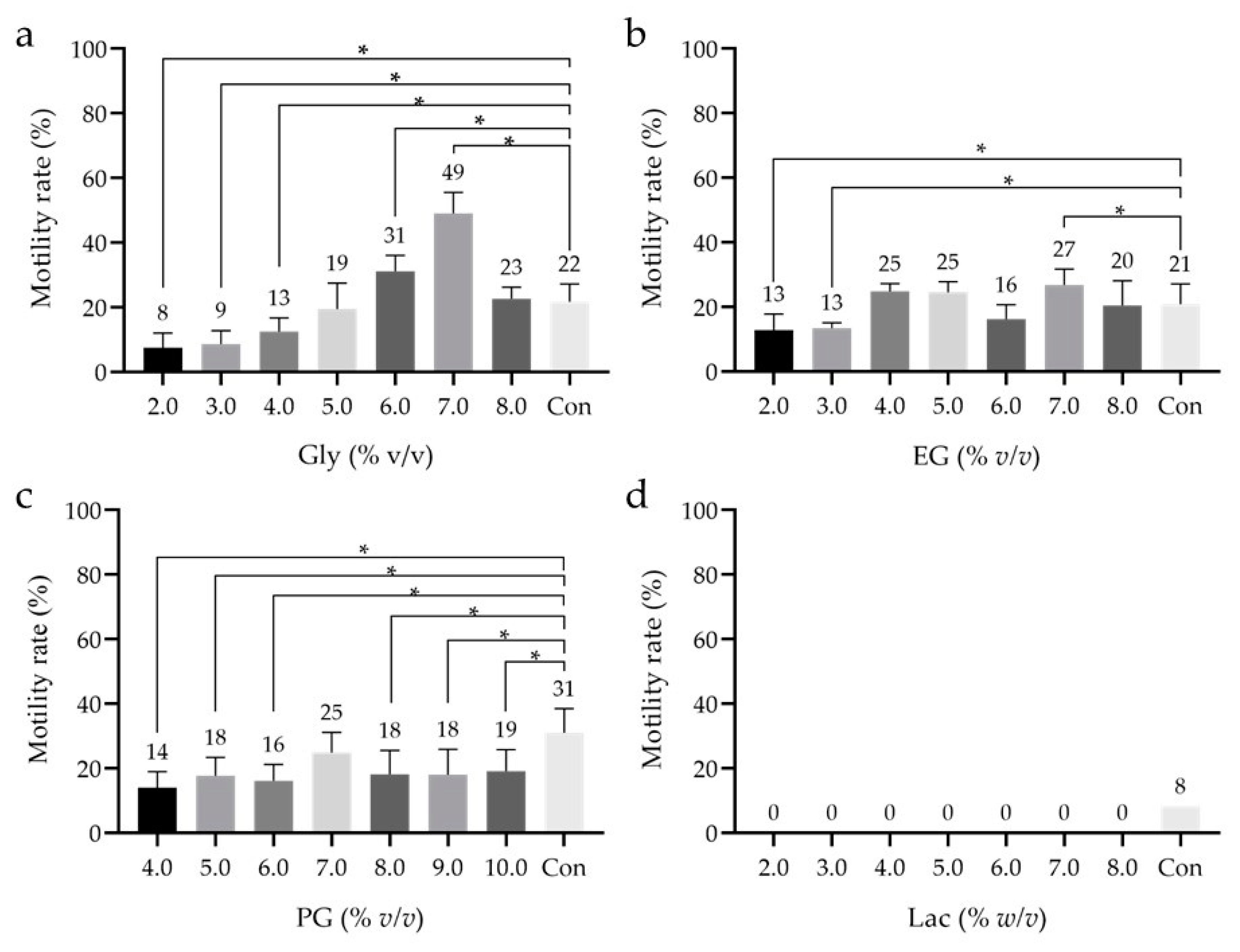
3.2. Effect of Low-Mw CPAs
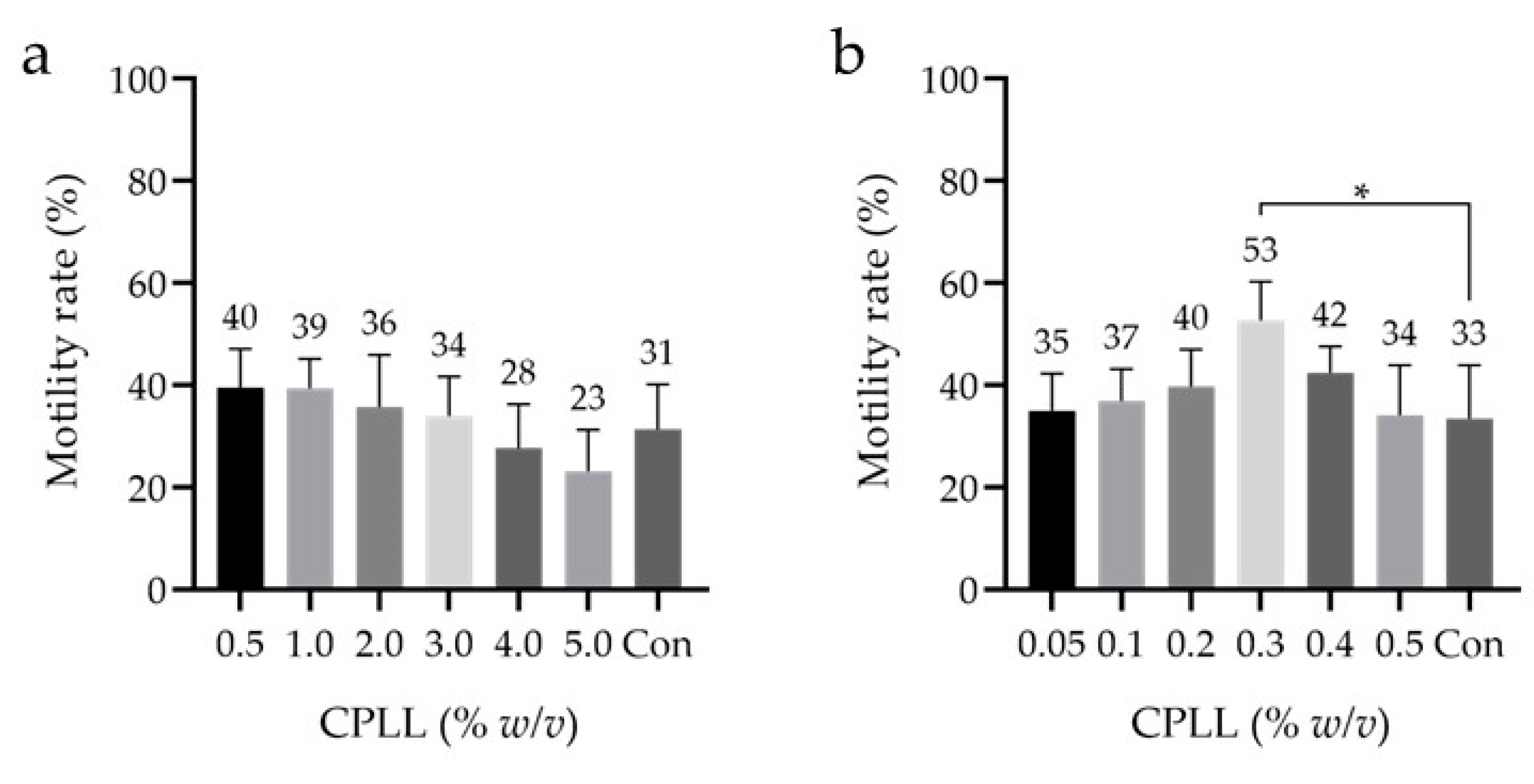
3.3. Detailed Effect of CPLL
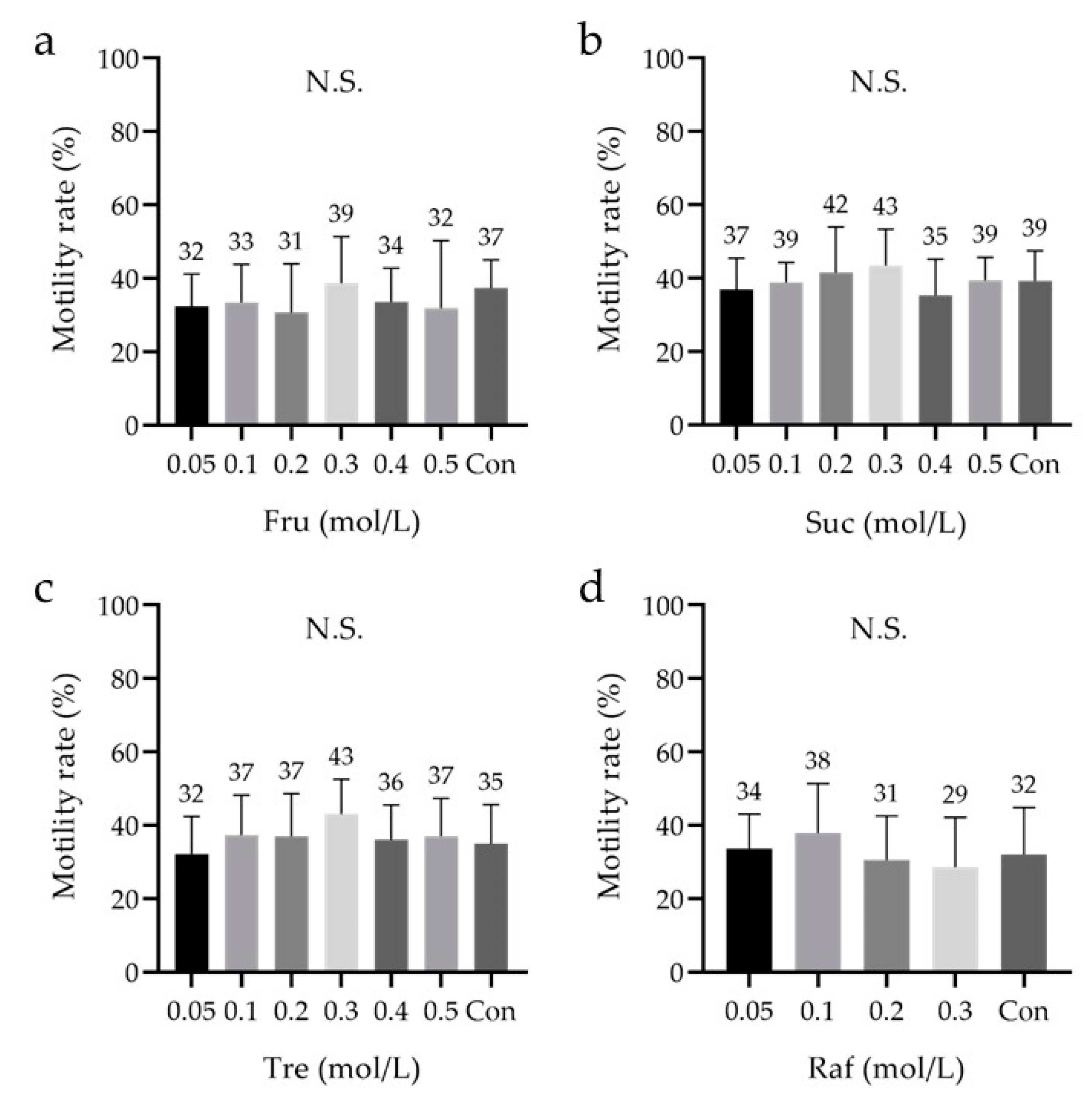
3.4. Effect of Saccharides
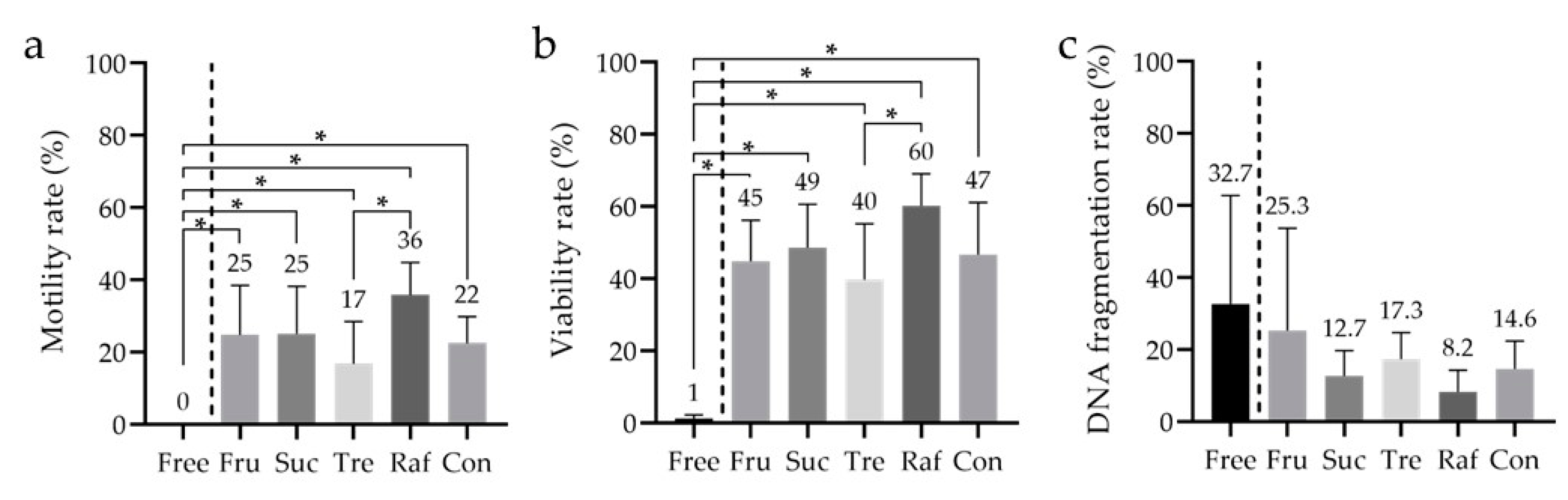
3.5. Analysis of the Sperm Viability Test (SVT) and DNA Fragmentation
3.6. Quantitative Analysis of Sperm Motility with a Longevity Curve, Capacitation Status, and Acrosome Reaction
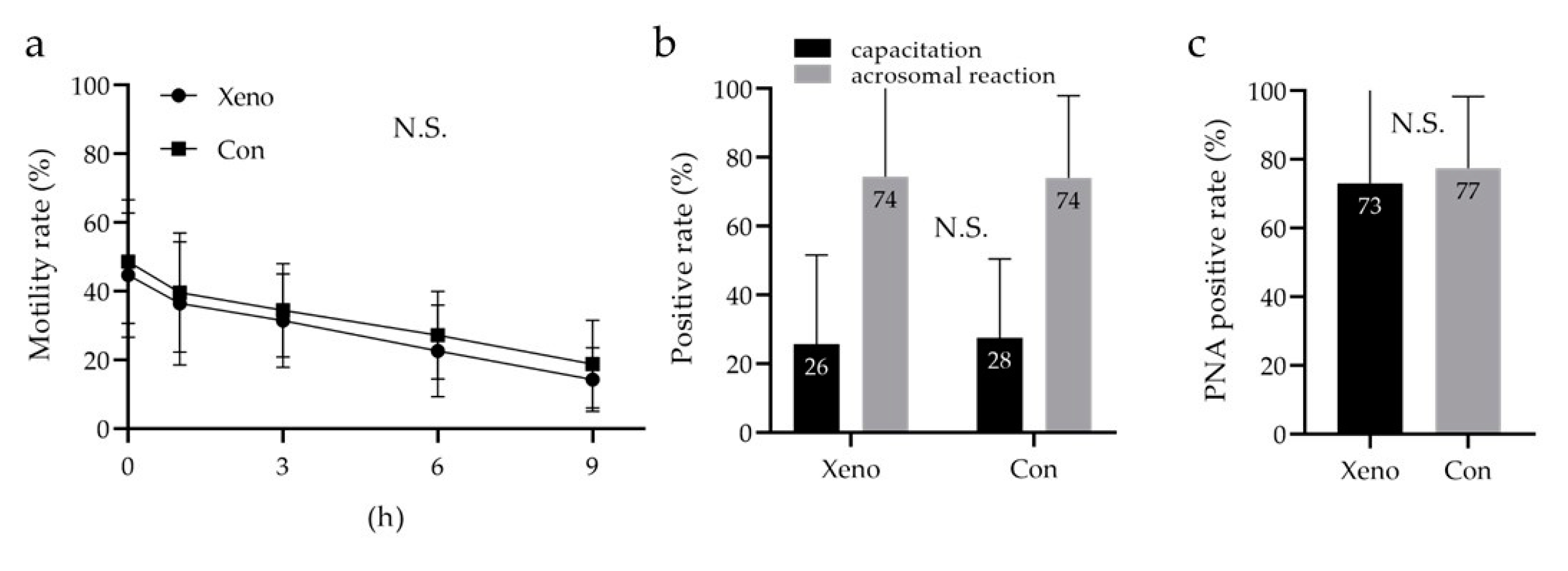
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V. Sperm storage for cancer patients in the UK: A review of current practice. Hum. Reprod. 2011, 26, 2935–2943. [Google Scholar] [CrossRef]
- Pukazhenthi, B.; Comizzoli, P.; Travis, A.J.; Wildt, D.E. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod. Fertil. Dev. 2006, 18, 77–90. [Google Scholar] [CrossRef]
- Marschall, S.; Hrabe de Angelis, M. Cryopreservation of mouse spermatozoa: Double your mouse space. Trends Genet. 1999, 15, 128–131. [Google Scholar] [CrossRef]
- Van Steirteghem, A.; Nagy, P.; Joris, H.; Janssenswillen, C.; Staessen, C.; Verheyen, G.; Camus, M.; Tournaye, H.; Devroey, P. Results of intracytoplasmic sperm injection with ejaculated, fresh and frozen-thawed epididymal and testicular spermatozoa. Hum. Reprod. 1998, 13 (Suppl. 1), 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H. Cryopreservation of semen from domestic livestock. Methods Mol. Biol. 2015, 1257, 277–287. [Google Scholar] [CrossRef]
- Byrd, W.; Bradshaw, K.; Carr, B.; Edman, C.; Odom, J.; Ackerman, G. A prospective randomized study of pregnancy rates following intrauterine and intracervical insemination using frozen donor sperm. Fertil. Steril. 1990, 53, 521–527. [Google Scholar] [CrossRef]
- Hamberger, L.; Lundin, K.; Sjogren, A.; Soderlund, B. Indications for intracytoplasmic sperm injection. Hum. Reprod. 1998, 13 (Suppl. 1), 128–133. [Google Scholar] [CrossRef] [PubMed]
- Sermon, K. Novel technologies emerging for preimplantation genetic diagnosis and preimplantation genetic testing for aneuploidy. Expert Rev. Mol. Diagn. 2017, 17, 71–82. [Google Scholar] [CrossRef]
- Walls, M.L.; Hart, R.J. In vitro maturation. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 53, 60–72. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Shapiro, D.; Chang, C.C. Vitrification of the human embryo: A more efficient and safer in vitro fertilization treatment. Fertil. Steril. 2020, 113, 241–247. [Google Scholar] [CrossRef]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv. Urol. 2012, 2012, 854837. [Google Scholar] [CrossRef]
- Pedro, P.B.; Zhu, S.E.; Makino, N.; Sakurai, T.; Edashige, K.; Kasai, M. Effects of hypotonic stress on the survival of mouse oocytes and embryos at various stages. Cryobiology 1997, 35, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Polge, C. Fertilizing capacity of bull spermatozoa after freezing at 79 degrees C. Nature 1952, 169, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Macias Garcia, B.; Ortega Ferrusola, C.; Aparicio, I.M.; Miro-Moran, A.; Morillo Rodriguez, A.; Gallardo Bolanos, J.M.; Gonzalez Fernandez, L.; Balao da Silva, C.M.; Rodriguez Martinez, H.; Tapia, J.A.; et al. Toxicity of glycerol for the stallion spermatozoa: Effects on membrane integrity and cytoskeleton, lipid peroxidation and mitochondrial membrane potential. Theriogenology 2012, 77, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Shimada, M. The Development of a Sperm Cryopreservation and Thawing Method based on Species-Specific and Common Sperm Biology. J. Mamm. Ova Res. 2014, 31, 96–101. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Edashige, K.; Asano, A.; An, T.Z.; Kasai, M. Restoration of resistance to osmotic swelling of vitrified mouse embryos by short-term culture. Cryobiology 1999, 38, 273–280. [Google Scholar] [CrossRef]
- Graham, E.F.; Crabo, B.G.; Brown, K.I. Effect of some zwitter ion buffers on the freezing and storage of spermatozoa. I. Bull. J. Dairy Sci. 1972, 55, 372–378. [Google Scholar] [CrossRef]
- Hallak, J.; Sharma, R.K.; Wellstead, C.; Agarwal, A. Cryopreservation of human spermatozoa: Comparison of TEST-yolk buffer and glycerol. Int. J. Fertil. Womens Med. 2000, 45, 38–42. [Google Scholar]
- Larson, J.M.; McKinney, K.A.; Mixon, B.A.; Burry, K.A.; Wolf, D.P. An intrauterine insemination-ready cryopreservation method compared with sperm recovery after conventional freezing and post-thaw processing. Fertil. Steril. 1997, 68, 143–148. [Google Scholar] [CrossRef]
- Mori, C.; Yabuuchi, A.; Ezoe, K.; Murata, N.; Takayama, Y.; Okimura, T.; Uchiyama, K.; Takakura, K.; Abe, H.; Wada, K.; et al. Hydroxypropyl cellulose as an option for supplementation of cryoprotectant solutions for embryo vitrification in human assisted reproductive technologies. Reprod. Biomed. Online 2015, 30, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Coello, A.; Campos, P.; Remohí, J.; Meseguer, M.; Cobo, A. A combination of hydroxypropyl cellulose and trehalose as supplementation for vitrification of human oocytes: A retrospective cohort study. J. Assist. Reprod. Genet. 2016, 33, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Hebles, M.; Migueles, B.; Dorado, M.; Aguilera, L.; Gonzalez, M.; Piqueras, P.; Lucas, A.; Montero, L.; Sanchez-Martin, P.; et al. Hydroxypropyl cellulose supplementation in vitrification solutions: A prospective study with donor oocytes. J. Assist. Reprod. Genet. 2017, 34, 417–422. [Google Scholar] [CrossRef]
- Matsumura, K.; Bae, J.Y.; Hyon, S.H. Polyampholytes as cryoprotective agents for mammalian cell cryopreservation. Cell Transplant. 2010, 19, 691–699. [Google Scholar] [CrossRef]
- Maehara, M.; Sato, M.; Watanabe, M.; Matsunari, H.; Kokubo, M.; Kanai, T.; Sato, M.; Matsumura, K.; Hyon, S.H.; Yokoyama, M.; et al. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 2013, 13, 58. [Google Scholar] [CrossRef]
- Jin, O.S.; Lee, J.H.; Shin, Y.C.; Lee, E.J.; Lee, J.J.; Matsumura, K.; Hyon, S.H.; Han, D.W. Cryoprotection of fibroblasts by carboxylated poly-l-lysine upon repeated freeze/thaw cycles. Cryo Lett. 2013, 34, 396–403. [Google Scholar]
- Watanabe, H.; Kohaya, N.; Kamoshita, M.; Fujiwara, K.; Matsumura, K.; Hyon, S.H.; Ito, J.; Kashiwazaki, N. Efficient production of live offspring from mouse oocytes vitrified with a novel cryoprotective agent, carboxylated epsilon-poly-l-lysine. PLoS ONE 2013, 8, e83613. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hyon, S.H. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials 2009, 30, 4842–4849. [Google Scholar] [CrossRef]
- Stubbs, C.; Bailey, T.L.; Murray, K.; Gibson, M.I. Polyampholytes as Emerging Macromolecular Cryoprotectants. Biomacromolecules 2020, 21, 7–17. [Google Scholar] [CrossRef]
- Matsumura, K.; Hayashi, F.; Nagashima, T.; Hyon, S.H. Long-term cryopreservation of human mesenchymal stem cells using carboxylated poly-l-lysine without the addition of proteins or dimethyl sulfoxide. J. Biomater. Sci. Polym. Ed. 2013, 24, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Bae, J.Y.; Kim, H.H.; Hyon, S.H. Effective vitrification of human induced pluripotent stem cells using carboxylated epsilon-poly-l-lysine. Cryobiology 2011, 63, 76–83. [Google Scholar] [CrossRef]
- Fujikawa, T.; Ando, T.; Gen, Y.; Hyon, S.H.; Kubota, C. Cryopreservation of bovine somatic cells using antifreeze polyamino-acid (carboxylated poly-l-lysine). Cryobiology 2017, 76, 140–145. [Google Scholar] [CrossRef]
- Bailey, T.L.; Stubbs, C.; Murray, K.; Tomás, R.M.F.; Otten, L.; Gibson, M.I. Synthetically Scalable Poly(ampholyte) Which Dramatically Enhances Cellular Cryopreservation. Biomacromolecules 2019, 20, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Johnson, M.A.; Fisher, R.; Burke, N.A.D.; Stöver, H.D.H. Synthetic Polyampholytes as Macromolecular Cryoprotective Agents. Langmuir 2019, 35, 1807–1817. [Google Scholar] [CrossRef]
- Tsuji, Y.; Iwasaki, T.; Ogata, H.; Matsumoto, Y.; Kokeguchi, S.; Matsumura, K.; Hyon, S.H.; Shiotani, M. The Beneficial Effect of Carboxylated Poly-l-Lysine on Cryosurvival of Vitrified Early Stage Embryos. Cryo Lett. 2017, 38, 1–6. [Google Scholar]
- Kamoshita, M.; Kato, T.; Fujiwara, K.; Namiki, T.; Matsumura, K.; Hyon, S.H.; Ito, J.; Kashiwazaki, N. Successful vitrification of pronuclear-stage pig embryos with a novel cryoprotective agent, carboxylated epsilon-poly-l-lysine. PLoS ONE 2017, 12, e0176711. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Imamura, S.; Tokumaru, M.; Ando, T.; Gen, Y.; Hyon, S.H.; Kubota, C. Cryoprotective effect of antifreeze polyamino-acid (Carboxylated Poly-l-Lysine) on bovine sperm: A technical note. Cryobiology 2018, 82, 159–162. [Google Scholar] [CrossRef]
- Tariq, A.; Ahmad, M.; Iqbal, S.; Riaz, M.I.; Tahir, M.Z.; Ghafoor, A.; Riaz, A. Effect of carboxylated poly l-Lysine as a cryoprotectant on post-thaw quality and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull semen. Theriogenology 2020, 144, 8–15. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Barratt, C.L.; Mortimer, D.; Jouannet, P. ‘How to count sperm properly’: Checklist for acceptability of studies based on human semen analysis. Hum. Reprod. 2016, 31, 227–232. [Google Scholar] [CrossRef]
- Sharma, R.; Ahmad, G.; Esteves, S.C.; Agarwal, A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: Protocol, reference values, and quality control. J. Assist. Reprod. Genet. 2016, 33, 291–300. [Google Scholar] [CrossRef]
- Kong, M.; Diaz, E.S.; Morales, P. Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol. Reprod. 2009, 80, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Szasz, F.; Sirivaidyapong, S.; Cheng, F.P.; Voorhout, W.F.; Marks, A.; Colenbrander, B.; Solti, L.; Gadella, B.M. Detection of calcium ionophore induced membrane changes in dog sperm as a simple method to predict the cryopreservability of dog semen. Mol. Reprod. Dev. 2000, 55, 289–298. [Google Scholar] [CrossRef]
- Zavos, P.M.; Sofikitis, N.; Toda, T.; Miyagawa, I. Improvements in qualitative characteristics of cryopreserved human spermatozoa following recovery via the SpermPrep II filtration method. Tohoku J. Exp. Med. 1991, 165, 283–290. [Google Scholar] [CrossRef][Green Version]
- Lovelock, J.E.; Bishop, M.W. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 1959, 183, 1394–1395. [Google Scholar] [CrossRef]
- Zavos, P.M.; Correa, J.R.; Sofikitis, N.; Kofinas, G.D.; Zarmakoupis, P.N. A method of short-term cryostorage and selection of viable sperm for use in the various assisted reproductive techniques. Tohoku J. Exp. Med. 1995, 176, 75–81. [Google Scholar] [CrossRef][Green Version]
- Benet, J.; Navarro, J.; Genesca, A.; Egozcue, J.; Templado, C. Chromosome abnormalities in human spermatozoa after albumin or TEST-Yolk capacitation. Hum. Reprod. 1991, 6, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, F.; Paffoni, A.; Papasso Brambilla, E.; Bonetti, S.; Brevini, T.A.; Ragni, G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: Comparative analysis between human and animal models. Fertil. Steril. 2006, 85 (Suppl. 1), 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Glander, H.J.; Colditz, U. [Comparison between cryoprotective potentials of glycerol, dimethylsulphoxide, dimethylformamide, and ethylene glycol in cryopreservation of human semen (author’s transl)]. Zentralbl. Gynakol. 1981, 103, 840–848. [Google Scholar]
- Lee, Y.A.; Kim, Y.H.; Kim, B.J.; Jung, M.S.; Auh, J.H.; Seo, J.T.; Park, Y.S.; Lee, S.H.; Ryu, B.Y. Cryopreservation of mouse spermatogonial stem cells in dimethylsulfoxide and polyethylene glycol. Biol. Reprod. 2013, 89, 109. [Google Scholar] [CrossRef]
- Okuda, Y.; Seita, Y.; Hisamatsu, S.; Sonoki, S.; Shino, M.; Masaoka, T.; Inomata, T.; Kamijo, S.; Kashiwazaki, N. Fertility of spermatozoa cryopreserved with 2% acetamide or glycerol through artificial insemination in the Japanese white rabbit. Exp. Anim. 2007, 56, 29–34. [Google Scholar] [CrossRef][Green Version]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Hanada, A.; Nagase, H. Cryoprotective effects of some amides on rabbit spermatozoa. J. Reprod. Fertil. 1980, 60, 247–252. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B.; Wu, J.; Phan, J.; Rasch, C.; Chang, A.; Zendejas, E. Cryopreservation of organs by vitrification: Perspectives and recent advances. Cryobiology 2004, 48, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tanrikut, C.; Wright, D.L.; Lee, G.Y.; Toner, M.; Biggers, J.D.; Toth, T.L. Cryopreservation of human spermatozoa with minimal non-permeable cryoprotectant. Cryobiology 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Yildiz, C.; Ottaviani, P.; Law, N.; Ayearst, R.; Liu, L.; McKerlie, C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction 2007, 133, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Critser, J.K.; Huse-Benda, A.R.; Aaker, D.V.; Arneson, B.W.; Ball, G.D. Cryopreservation of human spermatozoa. III. The effect of cryoprotectants on motility. Fertil. Steril. 1988, 50, 314–320. [Google Scholar] [CrossRef]
- Gandini, L.; Lombardo, F.; Lenzi, A.; Spano, M.; Dondero, F. Cryopreservation and sperm DNA integrity. Cell Tissue Bank 2006, 7, 91–98. [Google Scholar] [CrossRef]
- Ozkavukcu, S.; Erdemli, E.; Isik, A.; Oztuna, D.; Karahuseyinoglu, S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J. Assist. Reprod. Genet. 2008, 25, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef]
- Meamar, M.; Zribi, N.; Cambi, M.; Tamburrino, L.; Marchiani, S.; Filimberti, E.; Fino, M.G.; Biggeri, A.; Menezo, Y.; Forti, G.; et al. Sperm DNA fragmentation induced by cryopreservation: New insights and effect of a natural extract from Opuntia ficus-indica. Fertil. Steril. 2012, 98, 326–333. [Google Scholar] [CrossRef]
- Lusignan, M.F.; Li, X.; Herrero, B.; Delbes, G.; Chan, P.T.K. Effects of different cryopreservation methods on DNA integrity and sperm chromatin quality in men. Andrology 2018, 6, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Meyers, S.A.; Ball, B.A. Capacitation-like changes in equine spermatozoa following cryopreservation. Theriogenology 2006, 65, 1531–1550. [Google Scholar] [CrossRef] [PubMed]
| Reageants | Concentrations | Units | n | Evaluation Item—Statistical Analysis | ||
|---|---|---|---|---|---|---|
| Figure 1 | low-Mw CPAs | Glyc | 4 | % v/v | 6 | Motility—Dunnett’s test |
| macro-Mw CPAs | HPC | 5, 10, 15, 20 | % w/v | |||
| CPLL | 5, 7.5, 10, 12.5, 15 | |||||
| saccharides | — | — | — | |||
| Figure 2 | low-Mw CPAs | Glyc | 2, 3, 4, 5, 6, 7, 8 | % v/v | 9 | Motility—Dunnett’s test |
| EG | 2, 3, 4, 5, 6, 7, 8 | 9 | ||||
| PG | 4, 5, 6, 7, 8, 9, 10 | 9 | ||||
| Lac | 2, 3, 4, 5, 6, 7, 8, | % w/v | 1 | |||
| macro-Mw CPAs | CPLL | 5 | % w/v | / | ||
| saccharides | — | — | — | / | ||
| Figure 3 | low-Mw CPAs | Glyc | 7 | % v/v | / | Motility—Dunnett’s test |
| macro-Mw CPAs | CPLL | 0.05, 0.1, 0.2, 0.3, 0.4,0.5, 1, 2, 3, 4, 5 | % w/v | 18 | ||
| saccharides | — | — | — | / | ||
| Figure 4 | low-Mw CPAs | Glyc | 7 | % v/v | / | Motility—Dunnett’s test |
| macro-Mw CPAs | CPLL | 5 | % w/v | / | ||
| saccharides | Fru | 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 | M | 9 | ||
| Suc | 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 | 9 | ||||
| Tre | 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 | 9 | ||||
| Raf | 0.05, 0.1, 0.2, 0.3 | 9 | ||||
| Figure 5 | low-Mw CPAs | Glyc | 7 | % v/v | 8 | Motility—Tukey’s multiple comparison test Viability—Tukey’s multiple comparison test SDF—Tukey’s multiple comparison test |
| macro-Mw CPAs | CPLL | 5 | % w/v | |||
| saccharides | Fru | 0,3 | M | |||
| Suc | 0,3 | |||||
| Tre | 0,3 | |||||
| Raf | 0,1 | |||||
| Figure 6 | low-Mw CPAs | Glyc | 7 | % v/v | 9 | Quantitative analysis of sperm motility with a longevity curve—Two way–repeated measures analysis Capacitation status—Mann–Whitney U test Acrosomal reaction—Mann–Whitney U test |
| macro-Mw CPAs | CPLL | 5 | % w/v | |||
| saccharides | Raf | 0,1 | M | |||
| 105 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, H.; Nishioka, M.; Maezawa, T.; Kitano, Y.; Terada-Yoshikawa, K.; Tachibana, R.; Kato, M.; Hyon, S.-h.; Gen, Y.; Tanaka, K.; et al. Carboxylated Poly-l-Lysine as a Macromolecular Cryoprotective Agent Enables the Development of Defined and Xeno-Free Human Sperm Cryopreservation Reagents. Cells 2021, 10, 1435. https://doi.org/10.3390/cells10061435
Takeuchi H, Nishioka M, Maezawa T, Kitano Y, Terada-Yoshikawa K, Tachibana R, Kato M, Hyon S-h, Gen Y, Tanaka K, et al. Carboxylated Poly-l-Lysine as a Macromolecular Cryoprotective Agent Enables the Development of Defined and Xeno-Free Human Sperm Cryopreservation Reagents. Cells. 2021; 10(6):1435. https://doi.org/10.3390/cells10061435
Chicago/Turabian StyleTakeuchi, Hiroki, Mikiko Nishioka, Tadashi Maezawa, Yuko Kitano, Kento Terada-Yoshikawa, Ryota Tachibana, Manabu Kato, Suong-hyu Hyon, Yuki Gen, Kayo Tanaka, and et al. 2021. "Carboxylated Poly-l-Lysine as a Macromolecular Cryoprotective Agent Enables the Development of Defined and Xeno-Free Human Sperm Cryopreservation Reagents" Cells 10, no. 6: 1435. https://doi.org/10.3390/cells10061435
APA StyleTakeuchi, H., Nishioka, M., Maezawa, T., Kitano, Y., Terada-Yoshikawa, K., Tachibana, R., Kato, M., Hyon, S.-h., Gen, Y., Tanaka, K., Toriyabe, K., Nii, M., Kondo, E., & Ikeda, T. (2021). Carboxylated Poly-l-Lysine as a Macromolecular Cryoprotective Agent Enables the Development of Defined and Xeno-Free Human Sperm Cryopreservation Reagents. Cells, 10(6), 1435. https://doi.org/10.3390/cells10061435







