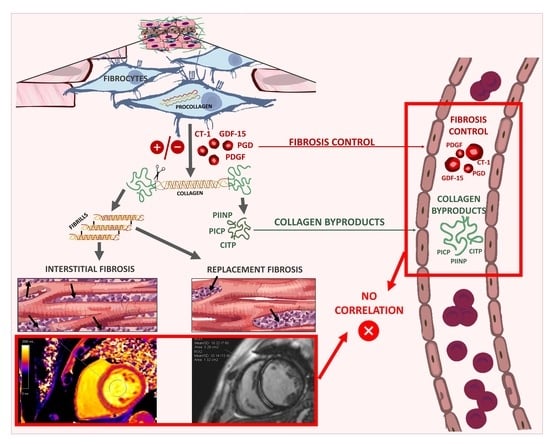
Lack of Relationship between Fibrosis-Related Biomarkers and Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis in Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac Magnetic Resonance
2.2.1. Assessment of Replacement Fibrosis
2.2.2. Assessment of Interstitial Fibrosis
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparisons of Biomarkers between DCM Patients, Stratified According to LGE and ECV
3.3. Associations between LGE and Biomarkers
3.4. Associations between ECV and Biomarkers
4. Discussion
4.1. Rationale of Circulating Markers of Fibrosis
4.2. Invasive vs. Non-Invasive Assessments of Cardiac Fibrosis
4.3. Relationship between Circulating Fibrosis-Related Molecules and EMB-Assessed Cardiac Fibrosis
4.4. Relationship between Circulating Fibrosis-Related Molecules and CMR-Assessed Cardiac Fibrosis
4.5. Relationship between Cardiac-Specific Biomarkers and Replacement and Interstitial Fibrosis
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Li, A.-H.; Liu, P.P.; Villarreal, F.J.; Garcia, R.A. Dynamic Changes in Myocardial Matrix and Relevance to Disease. Circ. Res. 2014, 114, 916–927. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Dziewięcka, E.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Holcman, K.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Kaciczak, M.; Podolec, P.; Kostkiewicz, M.; et al. Relationships between Pulmonary Hypertension Risk, Clinical Profiles, and Outcomes in Dilated Cardiomyopathy. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Cojan-Minzat, B.O.; Zlibut, A.; Muresan, I.D.; Cionca, C.; Horvat, D.; Kiss, E.; Revnic, R.; Florea, M.; Ciortea, R.; Agoston-Coldea, L. Left Ventricular Geometry and Replacement Fibrosis Detected by cMRI Are Associated with Major Adverse Cardiovascular Events in Nonischemic Dilated Cardiomyopathy. J. Clin. Med. 2020, 9, 1997. [Google Scholar] [CrossRef] [PubMed]
- Rubiś, P.; Wiśniowska-Śmialek, S.; Wypasek, E.; Biernacka-Fijalkowska, B.; Rudnicka-Sosin, L.; Dziewiecka, E.; Faltyn, P.; Khachatryan, L.; Karabinowska, A.; Kozanecki, A.; et al. Fibrosis of extracellular matrix is related to the duration of the disease but is unrelated to the dynamics of collagen metabolism in dilated cardiomyopathy. Inflamm. Res. 2016, 65, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.H.; Ismail, N.A.; Dweck, M.R.; et al. Association of Fibrosis With Mortality and Sudden Cardiac Death in Patients With Nonischemic Dilated Cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left Ventricular Remodeling in Heart Failure: Current Concepts in Clinical Significance and Assessment. Jacc: Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 1–24. [Google Scholar] [CrossRef]
- López, B.; González, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; José, G.S.; Querejeta, R.; Díez, J. Circulating Biomarkers of Myocardial Fibrosis. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kuhl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2007, 29, 270–276. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Rubiś, P. The diagnostic work-up of genetic and inflammatory dilated cardiomyopathy. E-J. Cardiol. Pract. 2015, 13, 19. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Matilla, L.; Arrieta, V.; Jover, E.; Garcia-Peña, A.; Martinez-Martinez, E.; Sadaba, R.; Alvarez, V.; Navarro, A.; Fernandez-Celis, A.; Gainza, A.; et al. Soluble St2 induces cardiac fibroblast activation and collagen synthesis via Neuropilin-1. Cells 2020, 9, 1667. [Google Scholar] [CrossRef] [PubMed]
- Meagher, P.; Lee, X.; Lee, J.; Visram, A.; Friedberg, M.; Connelly, K. Cardiac fibrosis: Key role of integrins in cardiac homeostasis and remodeling. Cells 2021, 10, 770. [Google Scholar] [CrossRef]
- Delaunay, M.; Osman, H.; Kaiser, S.; Diviani, D. The role of cyclic AMP signaling in cardiac fibrosis. Cells 2019, 9, 69. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef]
- Heymans, S.; González, A.; Pizard, A.; Papageorgiou, A.P.; López-Andrés, N.; Jaisser, F.; Thum, T.; Zannad, F.; Díez, J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur. J. Heart Fail. 2015, 17, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cao, Y.; Song, J.; Dong, N.; Kong, X.; Wang, J.; Yuan, Y.; Zhu, X.; Yan, X.; Greiser, A.; et al. Association between myocardial extracellular volume and strain analysis through cardiovascular magnetic resonance with histological myocardial fibrosis in patients awaiting heart transplantation. J. Cardiovasc. Magn. Reson. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial interstitial fibrosis in heart failure. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, R.; López, B.; González, A.; Sánchez, E.; Larman, M.; Ubago, J.L.M.; Díez, J. Increased collagen type i synthesis in patients with heart failure of hypertensive origin. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef]
- Izawa, H.; Murohara, T.; Nagata, K.; Isobe, S.; Asano, H.; Amano, T.; Ichihara, S.; Kato, T.; Ohshima, S.; Murase, Y.; et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy. Circulation 2005, 112, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qiao, S.; Song, Y.; Liu, Y.; Tang, Y.; Deng, L.; Yuan, J.; Hu, F.; Yang, W. Procollagen type I carboxy-terminal propeptide (PICP) and MMP-2 are potential biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2019, 43, 107150. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; López, B.; Querejeta, R.; Echegaray, K.; José, G.S.; Moreno, M.U.; Beaumont, F.J.; González, A.; Díez, J. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J. Hypertens. 2017, 35, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Rubiś, P.; Holcman, K.; Dziewięcka, E.; Wiśniowska-Śmialek, S.; Karabinowska, A.; Szymonowicz, M.; Khachatryan, L.; Wypasek, E.; Garlitski, A.; Gackowski, A.; et al. Relationships between circulating galectin-3, extracellular matrix fibrosis and outcomes in dilated cardiomyopathy. Adv. Clin. Exp. Med. 2021, 30, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Piek, A.; Schouten, E.M.; Van De Kolk, C.W.; Mueller, C.; Mebazaa, A.; Voors, A.A.; De Boer, R.A.; Silljé, H.H. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics 2018, 8, 4155–4169. [Google Scholar] [CrossRef] [PubMed]
- Rubiś, P.; Totoń-Żurańska, J.; Wiśniowska-Śmiałek, S.; Holcman, K.; Kołton-Wróż, M.; Wołkow, P.; Wypasek, E.; Natorska, J.; Rudnicka-Sosin, L.; Pawlak, A.; et al. Relations between circulating microRNAs (miR-21, miR-26, miR-29, miR-30 and miR-133a), extracellular matrix fibrosis and serum markers of fibrosis in dilated cardiomyopathy. Int. J. Cardiol. 2017, 231, 201–206. [Google Scholar] [CrossRef]
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Solal, A.C.; Padeletti, L.; et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100. [Google Scholar] [CrossRef]
- Münch, J.; Avanesov, M.; Bannas, P.; Säring, D.; Krämer, E.; Mearini, G.; Carrier, L.; Suling, A.; Lund, G.; Patten, M. Serum matrix metalloproteinases as quantitative biomarkers for myocardial fibrosis and sudden cardiac death risk stratification in patients with hypertrophic cardiomyopathy. J. Card. Fail. 2016, 22, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Lepojärvi, E.S.; Piira, O.-P.; Pääkkö, E.; Lammentausta, E.; Risteli, J.; Miettinen, J.A.; Perkiömäki, J.S.; Huikuri, H.V.; Junttila, M.J. Serum PINP, PIIINP, galectin-3, and ST2 as surrogates of myocardial fibrosis and echocardiographic left venticular diastolic filling properties. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.V.; Gurshchenkov, A.V.; Mitrofanova, L.B.; Ryzhkov, A.V.; Kazakova, E.E.; Badaev, K.D.; Gordeev, M.L.; Moiseeva, O.M. Clinical significance of different assesment methods of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Kardiologiia 2020, 60, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Redon, J.; Martínez, F.; Solaz, E.; Calaforra, O.; Andrés, M.S.; Lopez, B.; Díez, J.; Oberbauer, R.; Adlbrecht, C.; et al. Cardiac magnetic resonance-derived fibrosis, strain and molecular biomarkers of fibrosis in hypertensive heart disease. J. Hypertens. 2020, 38, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Foussier, C.; Barral, P.; Jerosh-Herold, M.; Gariboldi, V.; Rapacchi, S.; Gallon, A.; Bartoli, A.; Bentatou, Z.; Guye, M.; Bernard, M.; et al. Quantification of diffuse myocardial fibrosis using CMR extracellular volume fraction and serum biomarkers of collagen turnover with histologic quantification as standard of reference. Diagn. Interv. Imaging 2021, 102, 163–169. [Google Scholar] [CrossRef]
- Quick, S.; Waessnig, N.K.; Kandler, N.; Poitz, D.M.; Schoen, S.; Ibrahim, K.; Strasser, R.H.; Speiser, U. Soluble ST2 and myocardial fibrosis in 3T cardiac magnetic resonance. Scand. Cardiovasc. J. 2015, 49, 361–366. [Google Scholar]
- Ellims, A.H.; Taylor, A.J.; Mariani, J.A.; Ling, L.-H.; Iles, L.M.; Maeder, M.T.; Kaye, D.M. Evaluating the utility of circulating biomarkers of collagen synthesis in hypertrophic cardiomyopathy. Circ. Heart Fail. 2014, 7, 271–278. [Google Scholar] [CrossRef]
- Fang, L.; Ellims, A.H.; Moore, X.-L.; White, D.A.; Taylor, A.J.; Chin-Dusting, J.; Dart, A.M. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J. Transl. Med. 2015, 13, 1–12. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sakai, C.; Harimoto, K.; Yamano, M.; Miki, S.; Kamitani, T. Usefulness of high-sensitivity cardiac troponin T and brain natriuretic peptide as biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2013, 112, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Carolyn, Y.; Abbasi, S.A.; Neilan, T.T.G.; Shah, R.; Chen, Y.; Heydari, B.B.; Cirino, A.; Alison, L.; Lakdawala, N.; et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ. Cardiovasc. Imaging 2013, 6, 415–422. [Google Scholar] [CrossRef]
- Costello, B.T.; Stub, D.; Hare, J.; Ellims, A.H.; Wang, X.; Smith, K.; Bernard, S.; Nehme, Z.; Stephenson, M.; Bray, J.E.; et al. Comparison of magnetic resonance analysis of myocardial scarring with biomarker release following s-t elevation myocardial infarction. Hear. Lung Circ. 2019, 28, 397–405. [Google Scholar] [CrossRef]
- Karaahmet, T.; Tigen, K.; Dundar, C.; Pala, S.; Guler, A.; Kilicgedik, A.; Cevik, C.; Mahmutyazicioglu, K.; Isiklar, I.; Basaran, Y. The effect of cardiac fibrosis on left ventricular remodeling, diastolic function, and n-terminal pro-b-type natriuretic peptide levels in patients with nonischemic dilated cardiomyopathy. Echocardiography 2010, 27, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Son, J.W.; Chung, H.; Park, C.H.; Kim, Y.-J.; Chang, H.-J.; Hong, G.-R.; Kim, T.H.; Ha, J.-W.; Choi, B.W.; et al. Relationship between myocardial extracellular space expansion estimated with post-contrast t1 mapping mri and left ventricular remodeling and neurohormonal activation in patients with dilated cardiomyopathy. Korean J. Radiol. 2015, 16, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Tachi, M.; Amano, Y.; Kobayashi, Y.; Mizuno, K.; Kumita, S. Evaluation of nonscarred myocardial T1 value using contrast-enhanced look-locker cardiac MRI and its relationship to cardiac function in dilated cardiomyopathy: Comparison of 1.5 and 3.0 Tesla MRI. J. Magn. Reson. Imaging 2013, 38, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Child, N.; Suna, G.; Dabir, D.; Yap, M.-L.; Rogers, T.; Kathirgamanathan, M.; Arroyo-Ucar, E.; Hinojar, R.; Mahmoud, I.; Young, C.; et al. Comparison of MOLLI, shMOLLLI, and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur. Heart J. Cardiovasc. Imaging 2017, 19, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, Ł.; Petryka, J.; Spiewak, M.; Miłosz-Wieczorek, B.; Werys, K.; Małek, Ł.; Polanska-Skrzypczyk, M.; Ojrzynska, N.; Kubik, A.; Marczak, M.; et al. Biventricular mechanics in prediction of severe myocardial fibrosis in patients with dilated cardiomyopathy: CMR study. Eur. J. Radiol. 2017, 91, 71–81. [Google Scholar] [CrossRef]
- Yi, J.-E.; Park, J.; Lee, H.-J.; Shin, D.G.; Kim, Y.; Kim, M.; Kwon, K.; Pyun, W.B.; Kim, Y.J.; Joung, B. Prognostic implications of late gadolinium enhancement at the right ventricular insertion point in patients with non-ischemic dilated cardiomyopathy: A multicenter retrospective cohort study. PLoS ONE 2018, 13, e0208100. [Google Scholar] [CrossRef]
| Without LGE (n = 56) | With LGE (n = 44) | p-Value | ECV ≤ Median (n = 50) | ECV > Median (n = 50) | p-Value | |
|---|---|---|---|---|---|---|
| Age (year) | 44.86 ± 11.28 | 45.42 ± 12.29 | 0.76 | 41.87 ± 11.13 | 50.06 ± 11.06 | 0.0015 |
| Male (n, %) | 49 (87.5%) | 38 (86.4%) | 0.87 | 40 (80%) | 45 (90%) | 0.13 |
| BMI (kg/m2) | 28.21 ± 5.58 | 28.88 ± 5.97 | 0.54 | 29.08 ± 5.75 | 28.2 ± 6.13 | 0.50 |
| HF symptoms time (month) | 18.69 ± 24.73 | 12.84 ± 21.7 | 0.32 | 13.75 ± 19.15 | 16.07 ± 27.26 | 0.41 |
| Diabetes mellitus (n, %) | 7 (12.5%) | 7 (15.9%) | 0.63 | 7 (14%) | 8 (16%) | 0.76 |
| Hypercholesterolemia (n, %) | 31 (55.4%) | 30 (68.2%) | 0.19 | 31 (62%) | 28 (56%) | 0.66 |
| Hypertension (n, %) | 5 (8.9%) | 15 (34.1%) | 0.002 | 9 (18%) | 11 (22%) | 0.60 |
| COPD (n, %) | 1 (1.8%) | 5 (11.4%) | 0.045 | 1 (2%) | 6 (12%) | 0.09 |
| Atrial fibrillation (n, %) | 12 (21.4%) | 14 (31.8%) | 0.24 | 7 (14%) | 22 (44%) | 0.02 |
| NYHA class | 1.73 ± 0.6 | 1.88 ± 0.63 | 0.26 | 1.72 ± 0.56 | 1.97 ± 0.65 | 0.10 |
| SBP (mmHg) | 118.9 ± 17.3 | 121.8 ± 21.8 | 0.46 | 121.2 ± 16.8 | 118.6 ± 22.1 | 0.53 |
| DBP (mmHg) | 75.16 ± 12.35 | 80.3 ± 14.25 | 0.03 | 76.68 ± 11.89 | 78.52 ± 14.24 | 0.51 |
| Heart rate (bpm) | 70.54 ± 12.69 | 72.72 ± 14.71 | 0.74 | 69.81 ± 15.11 | 74.58 ± 12.98 | 0.12 |
| QRS (ms) | 95.64 ± 24.17 | 106.14 ± 29.9 | 0.02 | 100.23 ± 28.07 | 102.05 ± 28.58 | 0.63 |
| Ventricular arrhythmia (n, %) | 17 (31.5%) | 14 (32.6%) | 0.91 | 7 (14%) | 26 (52%) | <0.001 |
| 6MWT—distance (m) | 451.6 ± 90.4 | 438.7 ± 94.5 | 0.49 | 464.6 ± 89.2 | 416.1 ± 92.5 | 0.01 |
| LVEDd/BSA (mm/m2) | 32.03 ± 4.51 | 30.99 ± 5.16 | 0.16 | 31.26 ± 4.35 | 31.85 ± 5.6 | 0.58 |
| IVS (mm) | 9.65 ± 1.92 | 10.52 ± 2.47 | 0.05 | 9.66 ± 1.84 | 10.43 ± 2.58 | 0.11 |
| LVEF (%) | 30.88 ± 10.14 | 28.06 ± 9.98 | 0.17 | 31.22 ± 9.89 | 27.47 ± 11.15 | 0.10 |
| RVd (mm/m2) | 39.3 ± 6.4 | 39.34 ± 7.21 | 0.90 | 38.48 ± 6.58 | 40.52 ± 7.25 | 0.17 |
| LAA (cm2) | 26.24 ± 6.79 | 29.98 ± 9.62 | 0.05 | 25.81 ± 8.2 | 30.98 ± 8.01 | <0.001 |
| RAA (cm2) | 20.16 ± 6.79 | 21.69 ± 6.24 | 0.10 | 19.07 ± 5.26 | 23.4 ± 7.44 | 0.001 |
| E/e’ | 9.17 ± 5.69 | 12.23 ± 5.77 | 0.003 | 9.96 ± 5.9 | 11.54 ± 6.33 | 0.25 |
| MR ≥ moderate (n, %) | 17 (30.4%) | 17 (38.6%) | 0.39 | 9 (18%) | 27 (54%) | <0.001 |
| TR ≥ moderate (n, %) | 7 (12.5%) | 4 (9.1%) | 0.59 | 2 (4%) | 9 (18%) | 0.04 |
| TRV (m/s) | 2.58 ± 1.39 | 2.32 ± 1.23 | 0.37 | 2.32 ± 1.23 | 2.74 ± 1.44 | 0.14 |
| LV mass (g) | 165.4 ± 44.2 | 203.5 ± 53.2 | <0.001 | 172.7 ± 48.3 | 193.2 ± 55.3 | 0.13 |
| % LGE (%) | 0 | 4.55 ± 5.02 | <0.001 | 1.88 ± 1.59 | 5.85 ± 5.67 | 0.01 |
| T1 native (ms) | 1207 ± 188 | 1285.2 ± 64.2 | 0.009 | 1255.5 ± 104.9 | 1274.7 ± 145.1 | <0.001 |
| T1 post contrast (ms) | 470.7 ± 53.6 | 471.3 ± 44.1 | 0.95 | 485.3 ± 49.4 | 458.2 ± 42.4 | 0.007 |
| ECV (%) | 28.0 ± 5.4 | 29.8 ± 4.2 | 0.01 | 25.3 ± 1.8 | 32.4 ± 4.5 | <0.001 |
| Hct (%) | 42.26 ± 3.73 | 44.43 ± 5 | 0.01 | 43.49 ± 4.57 | 42.91 ± 4.6 | 0.55 |
| WBC (M/uL) | 6.96 ± 2.05 | 8.52 ± 1.99 | <0.001 | 7.25 ± 2.09 | 8.1 ± 2.27 | 0.07 |
| Creatinine (umol/L) | 90.34 ± 41.34 | 92.7 ± 21.83 | 0.13 | 92.91 ± 46.57 | 90.77 ± 21 | 0.64 |
| Uric acid (umol/L) | 400.4 ± 112.2 | 450.4 ± 114.6 | 0.04 | 386.11 ± 102.29 | 455.51 ± 126.41 | 0.01 |
| Cholesterol LDL (mmol/L) | 3.14 ± 0.95 | 3 ± 0.85 | 0.45 | 3.29 ± 0.94 | 2.82 ± 0.81 | 0.01 |
| BB (n, %) | 56 (100%) | 44 (100%) | 1.00 | 50 (100%) | 50 (100%) | 1.00 |
| ARNI/ACEi (n, %) | 56 (100%) | 43 (97.7%) | 0.26 | 50 (100%) | 49 (98%) | 0.31 |
| MRA (n, %) | 55 (98.2%) | 41 (93.2%) | 0.20 | 48 (96.0%) | 48 (96.0%) | 1.00 |
| Diuretic dosage (mg/day) | 35.12 ± 73.31 | 46.5 ± 42.48 | 0.03 | 28.95 ± 34.98 | 58.69 ± 82.12 | 0.01 |
| Without LGE (n = 56) | With LGE (n = 44) | p-Value | ECV ≤ Median (n = 50) | ECV > Median (n = 50) | p-Value | |
|---|---|---|---|---|---|---|
| NT-proBNP (pg/mL) | 345 (109.5–1047) | 972 (432–1818) | <0.001 | 389 (81- 793.5) | 1371 (750–2652) | <0.001 |
| hsTnT (ng/mL) | 0.007 (0.005–0.014) | 0.013 (0.009–0.02) | <0.001 | 0.009 (0.007–0.018) | 0.013 (0.007–0.02) | <0.001 |
| CT-1 (pg/mL) | 42.3 (6.1–169.2) | 81.9 (4.9–225.1) | 0.81 | 60.3 (6.1–189) | 37.1 (4.9–225.1) | 0.69 |
| PDGF-BB (pg/mL) | 254.7 (139.3–334.3) | 215.4 (135–362) | 0.36 | 221.6 (133–334) | 216.8 (139–331) | 0.95 |
| GDF-15 (pg/mL) | 26.5 (18.3–47.1) | 28.3 (20.3–60.9) | 0.67 | 24.9 (18.1–40.8) | 33.7 (23.3–60.3) | 0.08 |
| PICP (ng/mL) | 95.5 (65.7–173.4) | 75.8 (68.4–204) | 0.72 | 97.7 (71.7–306) | 77.1 (65.7–146) | 0.09 |
| PIIINP (ng/L) | 161.2 (127.9–350.5) | 163.9 (135–445) | 0.79 | 165.5 (129–571) | 157 (127.9–309) | 0.26 |
| CTIP (ng/mL) | 0.32 (0.22–0.38) | 0.28 (0.22–0.34) | 0.91 | 0.28 (0.21–0.35) | 0.29 (0.23–0.35) | 0.41 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| QRS (ms) | 1.015 (0.999–1.031) | 0.06 | ||
| LAA (cm2) | 1.058 (1.005–1.115) | 0.03 | 0.973 (0.91–1.04) | 0.42 |
| Hb (g/dL) | 1.322 (1.003–1.742) | 0.04 | 1.112 (0.799–1.548) | 0.52 |
| WBC (M/uL) | 1.451 (1.168–1.801) | <0.001 | 1.288 (0.997–1.664) | 0.05 |
| Uric acid (umol/L) | 1.004 (0.999–1.008) | 0.05 | ||
| hsTnT (ng/mL) | 3 × 1011 (6 × 10−3–4 × 1033) | 0.10 | ||
| log10 (NT-proBNP) | 3.343 (1.619–6.901) | 0.001 | 2.979 (1.216–7.296) | 0.02 |
| LV mass (g) | 1.016 (1.007–1.026) | <0.001 | 1.016 (1.004–1.029) | 0.009 |
| loop diuretics (mg/d) | 1.003 (0.996–1.010) | 0.38 | ||
| QRS (ms) | 1.015 (0.999–1.031) | 0.06 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Standard Coefficient | p-Value | Standard Coefficient | p-Value | |
| Age (years) | 0.13 ± 0.04 | 0.002 | 0.03 ± 0.04 | 0.45 |
| NYHA class | 2.37 ± 0.81 | 0.005 | 0.47 ± 0.87 | 0.59 |
| Atrial fibrillation | −1.47 ± 0.56 | 0.01 | −1.09 ± 0.55 | 0.05 |
| Ventricular arrhythmias | −1.57 ± 0.53 | 0.004 | −0.73 ± 0.53 | 0.17 |
| COPD | −1.03 ± 1.03 | 0.32 | ||
| IVS (mm) | 0.43 ± 0.23 | 0.07 | ||
| LAA (cm2) | 0.09 ± 0.06 | 0.13 | ||
| Uric acid (umol/L) | 0.011 ± 0.004 | 0.02 | 0.004 ± 0.004 | 0.31 |
| Cholesterol LDL (mmol/L) | −1.56 ± 0.57 | 0.008 | −0.97 ± 0.63 | 0.13 |
| hsTnT (ng/mL) | 11.92 ± 4.31 | 0.007 | 8.31 ± 3.70 | 0.03 |
| log10 (NT-proBNP) | 3.04 ± 0.67 | <0.001 | 1.78 ± 0.80 | 0.03 |
| hsCRP (mg/dL) | 0.09 ± 0.06 | 0.13 | ||
| Loop diuretics (mg/day) | 0.02 ± 0.01 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiś, P.; Dziewięcka, E.; Szymańska, M.; Banyś, R.; Urbańczyk-Zawadzka, M.; Krupiński, M.; Mielnik, M.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Podolec, P.; et al. Lack of Relationship between Fibrosis-Related Biomarkers and Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis in Dilated Cardiomyopathy. Cells 2021, 10, 1295. https://doi.org/10.3390/cells10061295
Rubiś P, Dziewięcka E, Szymańska M, Banyś R, Urbańczyk-Zawadzka M, Krupiński M, Mielnik M, Wiśniowska-Śmiałek S, Karabinowska A, Podolec P, et al. Lack of Relationship between Fibrosis-Related Biomarkers and Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis in Dilated Cardiomyopathy. Cells. 2021; 10(6):1295. https://doi.org/10.3390/cells10061295
Chicago/Turabian StyleRubiś, Paweł, Ewa Dziewięcka, Magdalena Szymańska, Robert Banyś, Małgorzata Urbańczyk-Zawadzka, Maciej Krupiński, Małgorzata Mielnik, Sylwia Wiśniowska-Śmiałek, Aleksandra Karabinowska, Piotr Podolec, and et al. 2021. "Lack of Relationship between Fibrosis-Related Biomarkers and Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis in Dilated Cardiomyopathy" Cells 10, no. 6: 1295. https://doi.org/10.3390/cells10061295
APA StyleRubiś, P., Dziewięcka, E., Szymańska, M., Banyś, R., Urbańczyk-Zawadzka, M., Krupiński, M., Mielnik, M., Wiśniowska-Śmiałek, S., Karabinowska, A., Podolec, P., Winiarczyk, M., Gliniak, M., Kaciczak, M., Robak, J., Karapetyan, A., & Wypasek, E. (2021). Lack of Relationship between Fibrosis-Related Biomarkers and Cardiac Magnetic Resonance-Assessed Replacement and Interstitial Fibrosis in Dilated Cardiomyopathy. Cells, 10(6), 1295. https://doi.org/10.3390/cells10061295