Cancer Salt Nostalgia
Abstract
:1. Introduction
2. Salt Induced Tumorigenesis
3. Salt: A Double-Edged Sword in Tumorigenesis
4. Application of Sodium MRI as a Prognostic and/or Diagnostic Marker
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leslie, T.K.; James, A.D.; Zaccagna, F.; Grist, J.; Deen, S.; Kennerley, A.; Riemer, F.; Kaggie, J.D.; Gallagher, F.A.; Gilbert, F.J.; et al. Sodium homeostasis in the tumour microenvironment. Biochim. Biophys. Acta Bioenergy 2019, 1872, 188304. [Google Scholar] [CrossRef] [PubMed]
- Poku, L.O.; Phil, M.; Cheng, Y.; Wang, K.; Sun, X. 23 Na-MRI as a Noninvasive Biomarker for Cancer Diagnosis and Prognosis. J. Magn. Reson. Imaging 2021, 53, 995–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boada, F.E.; Qian, Y.; Nemoto, E.; Jovin, T.; Jungreis, C.; Jones, S.C.; Weimer, J.; Lee, V. Sodium MRI and the Assessment of Irreversible Tissue Damage During Hyper-Acute Stroke. Transl. Stroke Res. 2012, 3, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Schepkin, V.D.; Ross, B.D.; Chenevert, T.L.; Rehemtulla, A.; Sharma, S.; Kumar, M.; Stojanovska, J. Sodium magnetic resonance imaging of chemotherapeutic response in a rat glioma. Magn. Reson. Med. 2005, 53, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.; Riemer, F.; McLean, M.A.; Kaggie, J.; Robb, F.; Tropp, J.S.; Warren, A.; Brattm, O.; Shah, N.; Gnanapragasam, V.J.; et al. Quantifi-cation of Total and Intracellular Sodium Concentration in Primary Prostate Cancer and Adjacent Normal Prostate Tissue with Magnetic Resonance Imaging. Invest. Radiol. 2018, 53, 450–456. [Google Scholar] [CrossRef]
- Ouwerkerk, R.; Jacobs, M.A.; Macura, K.J.; Wolff, A.; Stearns, V.; Mezban, S.D.; Khouri, N.F.; Bluemke, D.; Bottomley, P.A. Elevated tissue sodium concentration in malignant breast lesions detected with non-invasive 23Na MRI. Breast Cancer Res. Treat. 2007, 106, 151–160. [Google Scholar] [CrossRef]
- Zaric, O.; Pinker, K.; Zbyn, S.; Strasser, B.; Robinson, S.; Minarikova, L.; Gruber, S.; Farr, A.; Singer, C.; Helbich, T.H.; et al. Quantitative Sodium MR Imaging at 7 T: Initial Results and Comparison with Diffusion-weighted Imaging in Patients with Breast Tumors. Radiology 2016, 280, 39–48. [Google Scholar] [CrossRef]
- Rahbar, H.; Partridge, S.C. Multiparametric MR Imaging of Breast Cancer. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Roumelioti, M.-E.; Glew, R.H.; Khitan, Z.J.; Rondon-Berrios, H.; Argyropoulos, C.P.; Malhotra, D.; Raj, D.S.; Agaba, I.E.; Rohrscheib, M.; Murata, G.H.; et al. Fluid balance concepts in medicine: Principles and practice. World J. Nephrol. 2018, 7, 1–28. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. mAbs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Lang, F.; Stournaras, C. Ion channels in cancer: Future perspectives and clinical potential. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130108. [Google Scholar] [CrossRef] [Green Version]
- McGrail, D.; McAndrews, K.M.; Brandenburg, C.P.; Ravikumar, N.; Kieu, Q.M.N.; Dawson, M.R. Osmotic Regulation Is Required for Cancer Cell Survival under Solid Stress. Biophys. J. 2015, 109, 1334–1337. [Google Scholar] [CrossRef] [Green Version]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [Green Version]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutriens 2019, 11, 1970. [Google Scholar] [CrossRef] [Green Version]
- Messerli, F.H.; Hofstetter, L.; Bangalore, S. Salt and heart disease: A second round of bad science? Lancet 2018, 392, 456–458. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Provenzano, M.; De Stefano, T.; Vita, C.; Chiodini, P.; Minutolo, R.; De Nicola, L.; Conte, G. Dietary Salt Re-striction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Tait, B.D.; Hudson, F.; Cantwell, L.; Brewin, G.; Holdsworth, R.; Bennett, G.; Jose, M. Review article: Luminex technology for HLA antibody detection in organ transplantation. Nephrology 2009, 14, 247–254. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Svetkey, L.P.; Chen, Y.T.; McKeown, S.P.; Preis, L.; Wilson, A.F. Preliminary evidence of linkage of salt sensitivity in black Americans at the beta 2-adrenergic receptor locus. Hypertension 1997, 29, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, S.K.; Inserra, P.; Solkoff, D.; Watson, R.R. The Role of Cytokines and Chemokines on Tumor Progression: A Review. Cancer Detect. Prev. 1999, 23, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M. Modulation of MMP-2 and MMP-9 by cytokines, mitogens and inhibitors in lung cancer and malignant mesothelioma cell lines. Oncol. Rep. 2009, 22, 1283–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vindrieux, D.; Escobar, P.; Lazennec, G. Emerging roles of chemokines in prostate cancer. Endocr. Relat. Cancer 2009, 16, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Tiriveedhi, V.; Takenaka, M.; Ramachandran, S.; Gelman, A.E.; Subramanian, V.; Patterson, G.A.; Mohanakumar, T. T Regulatory Cells Play a Significant Role in Modulating MHC Class I Antibody-Induced Obliterative Airway Disease. Arab. Archaeol. Epigr. 2012, 12, 2663–2674. [Google Scholar] [CrossRef] [Green Version]
- Chulpanova, D.S.; Kitaeva, K.V.; Green, A.R.; Rizvanov, A.A.; Solovyeva, V.V. Molecular Aspects and Future Perspectives of Cyto-kine-Based Anti-cancer Immunotherapy. Front. Cell Dev. Biol. 2020, 8, 402. [Google Scholar] [CrossRef]
- Kirabo, A. A new paradigm of sodium regulation in inflammation and hypertension. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R706–R710. [Google Scholar] [CrossRef]
- Afsar, B.; Kuwabara, M.; Ortiz, A.; Yerlikaya, A.; Siriopol, D.; Covic, A.; Rodríguez-Iturbe, B.; Johnson, R.J.; Kanbay, M. Salt Intake and Immunity. Hypertens 2018, 72, 19–23. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene–Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef]
- Dyer, A.; Stamler, J.; Berkson, D.; Lindberg, H.; Stevens, E. High Blood-Pressure: A Risk Factor for Cancer Mortality? Lancet 1975, 305, 1051–1056. [Google Scholar] [CrossRef]
- Stocks, T.; Van Hemelrijck, M.; Manjer, J.; Bjørge, T.; Ulmer, H.; Hallmans, G.; Lindkvist, B.; Selmer, R.; Nagel, G.; Tretli, S.; et al. Blood Pressure and Risk of Cancer Incidence and Mortality in the Metabolic Syndrome and Cancer Project. Hypertens 2012, 59, 802–810. [Google Scholar] [CrossRef]
- Largent, A.J.; McEligot, A.J.; Ziogas, A.; Reid, C.; Hess, J.; Leighton, N.; Peel, D.; Anton-Culver, H. Hypertension, diuretics and breast cancer risk. J. Hum. Hypertens. 2006, 20, 727–732. [Google Scholar] [CrossRef]
- Grossman, E.; Messerli, F.H.; Boyko, V.; Goldbourt, U. Is there an association between hypertension and cancer mortality? Am. J. Med. 2002, 112, 479–486. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K.; Voelkel, N.F. Endothelial cells and pulmonary arterial hypertension: Apoptosis, proliferation, interaction and transdifferentiation. Respir. Res. 2009, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Sanada, H.; Jones, J.E.; Jose, P.A. Genetics of Salt-Sensitive Hypertension. Curr. Hypertens. Rep. 2011, 13, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Cone, C.D. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J. Theor. Biol. 1971, 30, 151–181. [Google Scholar] [CrossRef]
- Nagy, I.Z.; Lustyik, G.; Nagy, V.Z.; Zarándi, B.; Bertoni-Freddari, C. Intracellular Na+:K+ ratios in human cancer cells as revealed by energy dispersive x-ray microanalysis. J. Cell Biol. 1981, 90, 769–777. [Google Scholar] [CrossRef]
- Sparks, R.L.; Pool, T.B.; Smith, N.K.; Cameron, I.L. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983, 43, 73–77. [Google Scholar]
- Brown, M.J.; Williams, B.; Morant, S.V.; Webb, D.J.; Caulfield, M.J.; Cruickshank, J.K.; Ford, I.; McInnes, G.; Sever, P.; Salsbury, J.; et al. Effect of amiloride, or amiloride plus hydrochlorothiazide, versus hydrochlorothiazide on glucose tolerance and blood pressure (PATHWAY-3): A parallel-group, double-blind randomised phase 4 trial. Lancet Diabetes Endocrinol. 2016, 4, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Cameron, I.L.; Hunter, E.K. Effect of cancer cachexia and amiloride treatment on the intracellular sodium content in tissue cells. Cancer Res. 1983, 43, 1074–1078. [Google Scholar]
- Cameron, I.L.; Smith, N.K.; Pool, T.B.; Sparks, R.L. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980, 40, 1493–1500. [Google Scholar]
- Guzel, R.M.; Ogmen, K.; Ilieva, K.M.; Fraser, S.P.; Djamgoz, M.B.A. Colorectal cancer invasiveness in vitro: Predominant contribution of neonatal Nav1.5 under normoxia and hypoxia. J. Cell. Physiol. 2019, 234, 6582–6593. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Dai, Y.; Qian, C.; Dong, Y.; Chen, Z.; Meng, L.; Jiang, Z.; Huang, T.; Hu, J.; et al. Voltage-gated sodium channel Nav1.5 promotes proliferation, migration and invasion of oral squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2019, 51, 562–570. [Google Scholar] [CrossRef]
- Campbell, T.M.; Main, M.J.; Fitzgerald, E.M. Functional expression of the voltage-gated Na+-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J. Cell Sci. 2013, 126, 4939–4949. [Google Scholar] [CrossRef] [Green Version]
- Gradek, F.; Lopez-Charcas, O.; Chadet, S.; Poisson, L.; Ouldamer, L.; Goupille, C.; Jourdan, M.-L.; Chevalier, S.; Moussata, D.; Besson, P.; et al. Sodium Channel Nav1.5 Controls Epithelial-to-Mesenchymal Transition and Invasiveness in Breast Cancer Cells Through its Regulation by the Salt-Inducible Kinase. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Díaz, D.; Delgadillo, D.M.; Hernández-Gallegos, E.; Ramírez-Domínguez, M.E.; Hinojosa, L.M.; Ortiz, C.S.; Berumen, J.; Camacho, J.; Gomora, J.C. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J. Cell. Physiol. 2006, 210, 469–478. [Google Scholar] [CrossRef]
- Gumushan Aktas, H.; Akgun, T. Naringenin inhibits prostate cancer metastasis by blocking voltage-gated sodium channels. Biomed. Pharm. 2018, 106, 770–775. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, N.; Wang, B.; Wang, L.; Zhou, C.; Yan, Y.; He, J.; Ren, Y. Significant prognostic values of aquaporin mRNA expression in breast cancer. Cancer Manag. Res. 2019, 11, 1503–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, S.; Chen, H.; Liu, M.; Gan, L.; Li, C.; Zhang, W.; Lv, L.; Mei, Z. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/beta-catenin pathway. Aging 2020, 12, 1527–1544. [Google Scholar] [CrossRef]
- Kusayama, M.; Wada, K.; Nagata, M.; Ishimoto, S.; Takahashi, H.; Yoneda, M.; Nakajima, A.; Okura, M.; Kogo, M.; Kamisaki, Y. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011, 102, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Chetry, M.; Huang, K.; Peng, Y.; Wang, J.; Wang, J.; Zhou, Y.; Shen, Y.; Xue, Y.; Ji, K. Significance of aquaporins’ expression in the prognosis of gastric cancer. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, B.; Liskova, V.; Babula, P.; Krizanova, O. Role of Sodium/Calcium Exchangers in Tumors. Biomolecules 2020, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Estevez, G.N.N.; Tersariol, I.L.D.S. Na+/Ca2+ exchangers: Unexploited opportunities for cancer therapy? Biochem. Pharmacol. 2019, 163, 357–361. [Google Scholar] [CrossRef]
- Amara, S.; Ivy, M.T.; Myles, E.L.; Tiriveedhi, V. Sodium channel gammaENaC mediates IL-17 synergized high salt induced in-flammatory stress in breast cancer cells. Cell Immunol. 2016, 302, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bondarava, M.; Li, T.; Endl, E.; Wehner, F. Alpha-ENaC is a functional element of the hypertonicity-induced cation channel in HepG2 cells and it mediates proliferation. Pflugers Arch. 2009, 458, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Rooj, A.K.; McNicholas, C.M.; Bartoszewski, R.; Bebok, Z.; Benos, D.J.; Fuller, C.M. Glioma-specific cation conductance regulates mi-gration and cell cycle progression. J. Biol. Chem. 2012, 287, 4053–4065. [Google Scholar] [CrossRef] [Green Version]
- Berdiev, B.K.; Xia, J.; McLean, L.A.; Markert, J.M.; Gillespie, G.Y.; Mapstone, T.B.; Naren, A.P.; Jovov, B.; Bubien, J.K.; Ji, H.-L.; et al. Acid-sensing Ion Channels in Malignant Gliomas. J. Biol. Chem. 2003, 278, 15023–15034. [Google Scholar] [CrossRef] [Green Version]
- Babaer, D.; Amara, S.; Ivy, M.; Zhao, Y.; Lammers, P.E.; Titze, J.M.; Tiriveedhi, V. High salt induces P-glycoprotein mediated treatment resistance in breast cancer cells through store operated calcium influx. Oncotarget 2018, 9, 25193–25205. [Google Scholar] [CrossRef] [Green Version]
- Tran, Q.; Lee, H.; Kim, C.; Kong, G.; Gong, N.; Kwon, S.H.; Park, J.; Kim, S.-H.; Park, J. Revisiting the Warburg Effect: Diet-Based Strategies for Cancer Prevention. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Gladden, L.B.; Nijsten, M.W.; Jones, K.B. Lactate and cancer: Revisiting the warburg effect in an era of lactate shut-tling. Front. Nutr. 2014, 1, 27. [Google Scholar]
- Amara, S.; Zheng, M.; Tiriveedhi, V. Oleanolic Acid Inhibits High Salt-Induced Exaggeration of Warburg-like Metabolism in Breast Cancer Cells. Cell Biophys. 2016, 74, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Amara, S.; Alotaibi, D.; Tiriveedhi, V. NFAT5/STAT3 interaction mediates synergism of high salt with IL-17 towards induction of VEGF-A expression in breast cancer cells. Oncol. Lett. 2016, 12, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Amara, S.; Majors, C.; Roy, B.; Hill, S.; Rose, K.L.; Myles, E.L.; Tiriveedhi, V. Critical role of SIK3 in mediating high salt and IL-17 synergy leading to breast cancer cell proliferation. PLoS ONE 2017, 12, e0180097. [Google Scholar] [CrossRef] [Green Version]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phas-es-elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 1511–1567. [Google Scholar] [CrossRef]
- Vesely, M.D.; Schreiber, R.D. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 2013, 1284, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Willebrand, R.; Hamad, I.; Van Zeebroeck, L.; Kiss, M.; Bruderek, K.; Geuzens, A.; Swinnen, D.; Côrte-Real, B.F.; Markó, L.; Lebegge, E.; et al. High Salt Inhibits Tumor Growth by Enhancing Anti-tumor Immunity. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- He, W.; Xu, J.; Mu, R.; Li, Q.; Lv, D.L.; Huang, Z.; Zhang, J.; Wang, C.; Dong, L. High-salt diet inhibits tumour growth in mice via reg-ulating myeloid-derived suppressor cell differentiation. Nat. Commun. 2020, 11, 1732. [Google Scholar] [CrossRef]
- Wu, C.; Yosef, N.; Thalhamer, T.; Zhu, C.; Xiao, S.; Kishi, Y.; Regev, A.; Kuchroo, V.K. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK. Nat. Cell Biol. 2013, 496, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives auto-immune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Istomine, R.; Alvarez, F.; Al-Aubodah, T.-A.; Shi, X.Q.; Takano, T.; Thornton, A.M.; Shevach, E.M.; Zhang, J.; Piccirillo, C.A. Salt Sensing by Serum/Glucocorticoid-Regulated Kinase 1 Promotes Th17-like Inflammatory Adaptation of Foxp3+ Regulatory T Cells. Cell Rep. 2020, 30, 1515–1529.e4. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zha, S.; Shen, X.; Zhao, Y.; Li, L.; Yang, L.; Lei, M.; Liu, W. NFAT5 mediates hypertonic stress-induced atherosclerosis via ac-tivating NLRP3 inflammasome in endothelium. Cell Commun. Signal. 2019, 17, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, H.Y.; Singh, A.; Shukla, P.; Anupam, R.; Mondal, R.K.; Mishra, P.K.; Srivastava, R.K. High dietary salt intake correlates with modulated Th17-Treg cell balance resulting in enhanced bone loss and impaired bone-microarchitecture in male mice. Sci. Rep. 2018, 8, 2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, A.L.; Kitz, A.; Wu, C.; Lowther, D.E.; Rodriguez, D.M.; Vudattu, N.; Deng, S.; Herold, K.C.; Kuchroo, V.K.; Kleinewietfeld, M.; et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Investig. 2015, 125, 4212–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
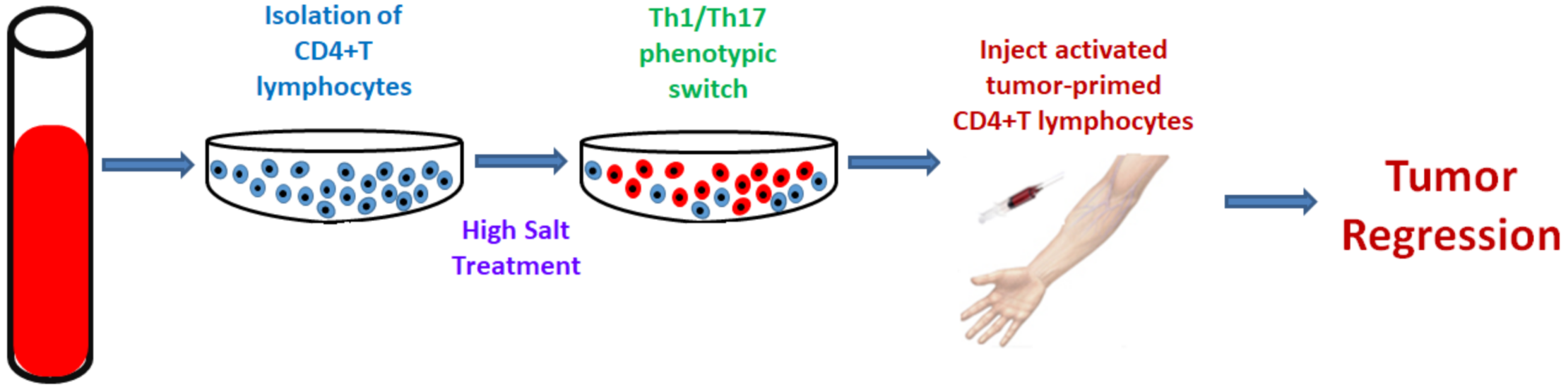
- Tiriveedhi, V.; Ivy, M.; Myles, E.; Zent, R.; Rathmell, J.; Titze, J. Ex Vivo High Salt Activated Tumor-Primed CD4+T Lymphocytes Exert a Potent Anti-Cancer Response. Cancers 2021, 13, 1690. [Google Scholar] [CrossRef]
- Amara, S.; Whalen, M.; Tiriveedhi, V. High salt induces anti-inflammatory MPhi2-like phenotype in peripheral macrophages. Biochem. Biophys. Rep. 2016, 7, 1–9. [Google Scholar]
- Fischereder, M.; Michalke, B.; Schmoeckel, E.; Habicht, A.; Kunisch, R.; Pavelic, I.; Szabados, B.; Schönermarck, U.; Nelson, P.J.; Stangl, M. Sodium storage in human tissues is mediated by glycosaminoglycan expression. Am. J. Physiol. Physiol. 2017, 313, F319–F325. [Google Scholar] [CrossRef]
- Boer, P.; Braam, B.; Fransen, R.; Boer, W.H.; Koomans, H.A. Reliable atomic absorption analysis of sodium and potassium in rat renal tubular fluid. Kidney Int. 1994, 45, 1211–1214. [Google Scholar] [CrossRef] [Green Version]
- Stankey, J.; Akbulut, C.; Romero, J.; Govindasamy-Lucey, S. Evaluation of X-ray fluorescence spectroscopy as a method for the rapid and direct determination of sodium in cheese. J. Dairy Sci. 2015, 98, 5040–5051. [Google Scholar] [CrossRef] [Green Version]
- Da-Col, J.A.; Bueno, M.I.M.S.; Melquiades, F.L. Fast and Direct Na and K Determination in Table, Marine, and Low-Sodium Salts by X-ray Fluorescence and Chemometrics. J. Agric. Food Chem. 2015, 63, 2406–2412. [Google Scholar] [CrossRef]
- Refardt, J.; Sailer, C.O.; Chifu, I.; Winzeler, B.; Schnyder, I.; Fassnacht, M.; Fenske, W.K.; Christ-Crain, M. The challenges of sodium measurements: Indirect versus direct ion-selective method. Eur. J. Endocrinol. 2019, 181, 193–199. [Google Scholar] [CrossRef]
- Madelin, G.; Lee, J.-S.; Regatte, R.R.; Jerschow, A. Sodium MRI: Methods and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 79, 14–47. [Google Scholar] [CrossRef] [Green Version]
- Parish, T.B.; Fieno, D.S.; Fitzgerald, S.W.; Judd, R.M. Theoretical basis for sodium and potassium MRI of the human heart at 1.5 T. Magn. Reson. Med. 1997, 38, 653–661. [Google Scholar] [CrossRef]
- Riemer, F.; Solanky, B.S.; Stehning, C.; Clemence, M.; Wheeler-Kingshott, C.A.; Golay, X. Sodium ((23)Na) ultra-short echo time im-aging in the human brain using a 3D-Cones trajectory. MAGMA 2014, 27, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Gurney, P.T.; Hargreaves, B.A.; Nishimura, D.G. Design and analysis of a practical 3D cones trajectory. Magn. Reson. Med. 2006, 55, 575–582. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef] [Green Version]
- Dahl, L.K. Effects of chronic excess salt feeding. Induction of self-sustaining hypertension in rats. J. Exp. Med. 1961, 114, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Strazzullo, P.; D’Elia, L.; Kandala, N.B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allu, A.S.; Tiriveedhi, V. Cancer Salt Nostalgia. Cells 2021, 10, 1285. https://doi.org/10.3390/cells10061285
Allu AS, Tiriveedhi V. Cancer Salt Nostalgia. Cells. 2021; 10(6):1285. https://doi.org/10.3390/cells10061285
Chicago/Turabian StyleAllu, Aashish S., and Venkataswarup Tiriveedhi. 2021. "Cancer Salt Nostalgia" Cells 10, no. 6: 1285. https://doi.org/10.3390/cells10061285
APA StyleAllu, A. S., & Tiriveedhi, V. (2021). Cancer Salt Nostalgia. Cells, 10(6), 1285. https://doi.org/10.3390/cells10061285