Antiretroviral Drugs Impact Autophagy with Toxic Outcomes
Abstract
1. Introduction
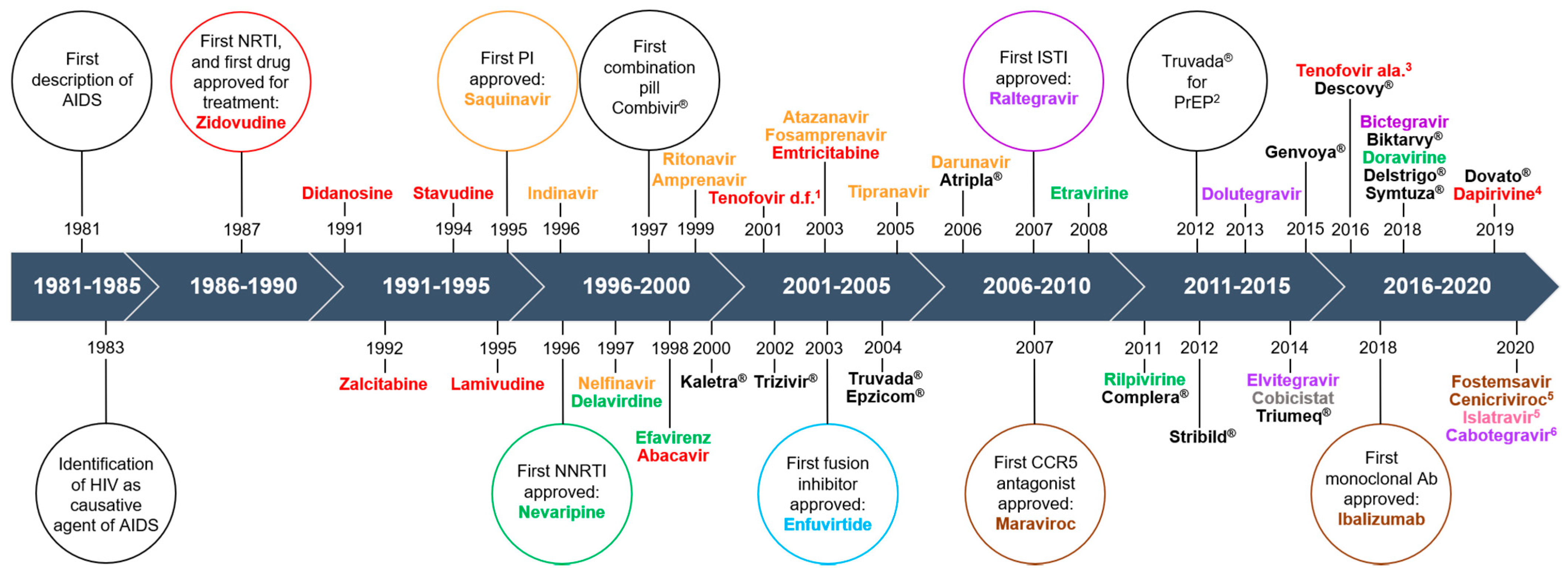
1.1. Antiretroviral Therapy
| Antiretroviral Drug | Cell Type/Animal Model | Disease Process | Cell Toxicity/Effect | Autophagy Effect 1,* | Ref. |
|---|---|---|---|---|---|
| Rev. Transc. Inhibitors | |||||
| Efavirenz | SH-SY5Y, primary rat neurons | Neurotoxicity, HAND | Mitochondrial, apoptosis | Induced autophagy/mitophagy | [6] |
| Hep3B, Hela | Hepatoxicity | Mitochondrial | *Dose-dependent auto/mitophagy inhibition | [7] | |
| Hep3B, primary rat neurons | Hepato-, neurotoxicity | ROS, mitochondrial, ER stress, cell death | Increased LC3-II | [8] | |
| primary human keratinocytes | Cutaneous reactions | Terminal differentiation, cell death | Decreased phospho-mTOR, increased LC3-lI | [9] | |
| EA.hy926, HUVEC | Cardiac, endothelial toxicity | ER stress, decreased meshwork, viability | Increased number of APG | [10] | |
| hCMEC/D3, human BMVEC, Tg HIV mice | Neurotoxicity, HAND | ER stress | * Inhibited autophagy | [11] | |
| U251-MG | Neurotoxicity, HAND | Mitochondrial | Inhibited autophagy/mitophagy | [12] | |
| Zidovudine | C2C12 | Myopathy | ROS, mitochondrial, cell viability | * Inhibited autophagy | [13] |
| 293T, 3T3-F442A | Lipoatrophy | ROS, mitochondrial, cell death | * Inhibited autophagy | [14] | |
| HepG2, HUH7 | Hepatotoxicity | ROS, mitochondrial, apoptosis | * Inhibited autophagy | [15] | |
| male Wister rats, hepatocytes | Hepatocarcinogenesis | Mitochondrial | Initiation inhibition, maturation inhibition | [16] | |
| Primary Sprag.-Dawl. rat oocytes | Low fertility | Decreased maturity/cleavage, apoptosis | Increased autophagy | [17] | |
| Primary human PBMC | Immunologic recovery | ROS, mitochondrial, apoptosis | * No change in autophagy activity factor | [18] | |
| HUVEC, human aortic endothelial cells | Cardiac, endothelial toxicity | Mitochondrial | * Increased LC3-II, mito.: lysosome co-local. | [19] | |
| Stavudine | 293T, 3T3-F442A | Lipodystrophy | ROS, mitochondrial, apoptosis | * Inhibited autophagy | [14] |
| HepG2, HUH7 | Hepatoxicity | ROS, mitochondrial, cell death | * Inhibited autophagy | [15] | |
| Lamivudine | Primary Sprag.-Dawl. rat oocytes | Low fertility | Decreased maturity/cleavage, apoptosis | Increased autophagy | [17] |
| HUVEC, human aortic endothelial cells | Cardiac, endothelial toxicity | ROS, mitochondrial, apoptosis | * Increased LC3-II, mito.:lysosome co-local. | [19] | |
| Protease Inhibitors | |||||
| Lopinavir/Ritonavir | 3T3-L1, human SGBS adipocytes | Lipodystrophy | ER stress, inhibited differentiation, apoptosis | * Inhibited autophagy | [20] |
| Primary mouse hepatocytes | Hepatoxicity | ER stress, dec. ROS response, cell death | Increased LC3-II | [21] | |
| Human JEG3, 3A-subE cells | Placenta health | ER stress | * Increased number of APG | [22] | |
| Atazanavir | Human JEG3 | Placenta health | ER stress | Increased number of APG | [22] |
| SE872 | Lipodystrophy | Decreased lipid stores, differentiation | Increased autophagy/mitophagy | [23] | |
| Saquinavir | Chub-S7 | Lipodystrophy | ROS, mitochondrial, apoptosis | * Increased autophagy genes mRNA, APG | [24] |
| Combinations | |||||
| TDF + FTC + DTG2 | Primary Sprag.-Dawl. rat microglia | HAND | Increased mRNA for inflammatory markers | Inhibited autophagy, lysosome dysfunction | [25] |
| TDF + FTC + DTG | Primary Sprag.-Dawl. rat microglia | HAND | ROS | Inhibited autophagy, lysosome dysfunction | [26] |
| TEN + FTC + RAL 3 | Primary human astrocytes | HAND | Effects aside from autophagy not assessed | * Inhibited autophagy | [27] |
| ZDV + SQV + NVP + Intlnh 4 | Primary Sprag.-Dawl. rat neurons | HAND | Decreased neuron health markers, ATP | Increased autophagy | [28] |
| FTC + RTV + ATV 5 | HIV infected primary human astrocytes | HAND | Increased viral and cytokine production | Increased p62 | [29] |
| 2 or 3-drug regimens 6 | Primary Human PBMC | Immune senescence | Increased pro- & anti-apoptotic gene mRNA | * Decreased BECN1, increased LC3 mRNA | [30] |
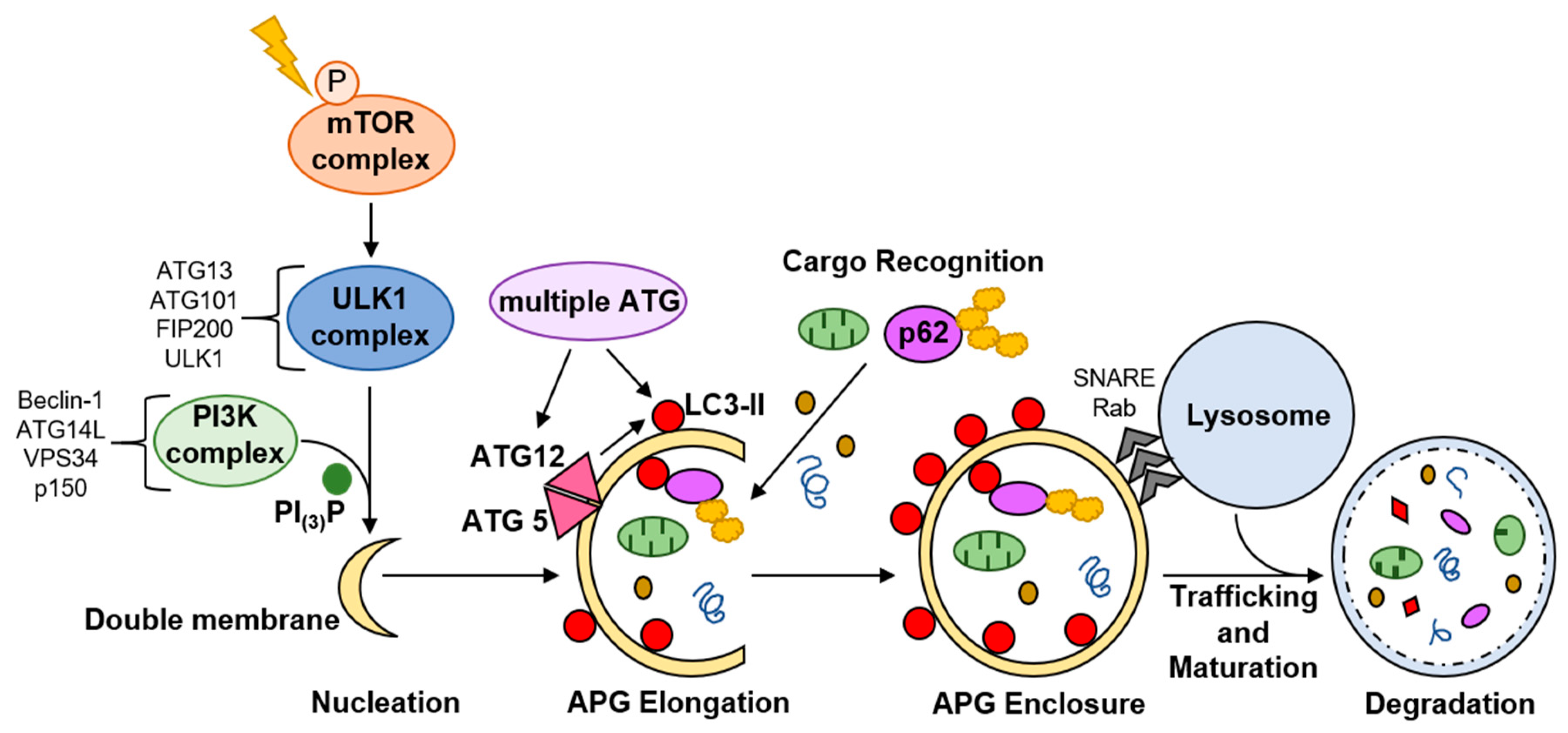
1.2. Autophagy
2. Reverse Transcriptase Inhibitors
2.1. Efavirenz
2.2. Zidovudine and Stavudine
2.3. Lamivudine
3. Protease Inhibitors
3.1. Lopinavir/Ritonavir
3.2. Atazanavir
3.3. Saquinavir
4. Combination Antiretroviral Drugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 3 March 2021).
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Goh, O.; Kroon, E.; Colby, D.; Sacdalan, C.; Pinyakorn, S.; Prueksakaew, P.; Reiss, P.; Ananworanich, J.; Valcour, V.; et al. Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res. Ther. 2020, 17, 1. [Google Scholar] [CrossRef]
- Fettiplace, A.; Stainsby, C.; Winston, A.; Givens, N.; Puccini, S.; Vannappagari, V.; Hsu, R.; Fusco, J.; Quercia, R.; Aboud, M.; et al. Psychiatric Symptoms in Patients Receiving Dolutegravir. J. Acquir. Immune Defic. Syndr. 2017, 74, 423–431. [Google Scholar] [CrossRef]
- Menard, A.; Montagnac, C.; Solas, C.; Meddeb, L.; Dhiver, C.; Tomei, C.; Ravaux, I.; Tissot-Dupont, H.; Mokhtari, S.; Colson, P.; et al. Neuropsychiatric adverse effects on dolutegravir: An emerging concern in Europe. AIDS 2017, 31, 1201–1203. [Google Scholar] [CrossRef]
- Purnell, P.R.; Fox, H.S. Efavirenz induces neuronal autophagy and mitochondrial alterations. J. Pharmacol. Exp. Ther. 2014, 351, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Gomez-Sucerquia, L.J.; Gortat, A.; Blas-Garcia, A.; Esplugues, J.V. Compromising mitochondrial function with the antiretroviral drug efavirenz induces cell survival-promoting autophagy. Hepatology 2011, 54, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Blas-Garcia, A.; Polo, M.; Alegre, F.; Funes, H.A.; Martinez, E.; Apostolova, N.; Esplugues, J.V. Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: A comparison with efavirenz. J. Antimicrob. Chemother. 2014, 69, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Oh, J.E.; Yi, J.K.; Kim, R.H.; Shin, K.H.; Mitsuyasu, R.; Park, N.H.; Kang, M.K. Efavirenz induces autophagy and aberrant differentiation in normal human keratinocytes. Int. J. Mol. Med. 2013, 31, 1305–1312. [Google Scholar] [CrossRef]
- Weiss, M.; Kost, B.; Renner-Muller, I.; Wolf, E.; Mylonas, I.; Bruning, A. Efavirenz Causes Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy in Endothelial Cells. Cardiovasc. Toxicol. 2016, 16, 90–99. [Google Scholar] [CrossRef]
- Bertrand, L.; Toborek, M. Dysregulation of Endoplasmic Reticulum Stress and Autophagic Responses by the Antiretroviral Drug Efavirenz. Mol. Pharmacol. 2015, 88, 304–315. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Gruevska, A.; Victor, V.M.; Gonzalez-Polo, R.A.; Yakhine-Diop, S.M.S.; Fuentes, J.M.; Esplugues, J.V.; Blas-Garcia, A.; Apostolova, N. Mitophagy in human astrocytes treated with the antiretroviral drug Efavirenz: Lack of evidence or evidence of the lack. Antivir. Res. 2019, 168, 36–50. [Google Scholar] [CrossRef]
- Lin, H.; Stankov, M.V.; Hegermann, J.; Budida, R.; Panayotova-Dimitrova, D.; Schmidt, R.E.; Behrens, G.M.N. Zidovudine-Mediated Autophagy Inhibition Enhances Mitochondrial Toxicity in Muscle Cells. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Stankov, M.V.; Panayotova-Dimitrova, D.; Leverkus, M.; Schmidt, R.E.; Behrens, G.M. Thymidine analogues suppress autophagy and adipogenesis in cultured adipocytes. Antimicrob. Agents Chemother. 2013, 57, 543–551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stankov, M.V.; Panayotova-Dimitrova, D.; Leverkus, M.; Vondran, F.W.; Bauerfeind, R.; Binz, A.; Behrens, G.M. Autophagy inhibition due to thymidine analogues as novel mechanism leading to hepatocyte dysfunction and lipid accumulation. AIDS 2012, 26, 1995–2006. [Google Scholar] [CrossRef]
- Santos-Llamas, A.; Monte, M.J.; Marin, J.J.G.; Perez, M.J. Dysregulation of autophagy in rat liver with mitochondrial DNA depletion induced by the nucleoside analogue zidovudine. Arch. Toxicol. 2018, 92, 2109–2118. [Google Scholar] [CrossRef]
- Tang, L.; Yang, S.; Wang, H.; Gu, H.; Xia, X.; Feng, Y.; Yang, Z.; Zhao, S.; Su, C.; Su, Z.; et al. Nucleoside reverse transcriptase inhibitor-induced rat oocyte dysfunction and low fertility mediated by autophagy. Oncotarget 2018, 9, 3895–3907. [Google Scholar] [CrossRef]
- Wallace, Z.R.; Sanderson, S.; Simon, A.K.; Dorrell, L. Exposure to zidovudine adversely affects mitochondrial turnover in primary T cells. Antivir. Res. 2016, 133, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.Y.; Hebert, V.Y.; Hayes, D.M.; Robinson, C.N.; Glover, M.; Dugas, T.R. Nucleoside reverse transcriptase inhibitors induce a mitophagy-associated endothelial cytotoxicity that is reversed by coenzyme Q10 cotreatment. Toxicol. Sci. 2013, 134, 323–334. [Google Scholar] [CrossRef]
- Zha, B.S.; Wan, X.; Zhang, X.; Zha, W.; Zhou, J.; Wabitsch, M.; Wang, G.; Lyall, V.; Hylemon, P.B.; Zhou, H. HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLoS ONE 2013, 8, e59514. [Google Scholar] [CrossRef]
- Hu, J.; Han, H.; Lau, M.Y.; Lee, H.; MacVeigh-Aloni, M.; Ji, C. Effects of combined alcohol and anti-HIV drugs on cellular stress responses in primary hepatocytes and hepatic stellate and kupffer cells. Alcohol. Clin. Exp. Res. 2015, 39, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A.; Kimmich, T.; Brem, G.J.; Buchholtz, M.L.; Mylonas, I.; Kost, B.; Weizsacker, K.; Gingelmaier, A. Analysis of endoplasmic reticulum stress in placentas of HIV-infected women treated with protease inhibitors. Reprod Toxicol 2014, 50, 122–128. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Pinti, M.; Nasi, M.; Riccio, M.; Carnevale, G.; Cavallini, G.M.; Sala de Oyanguren, F.J.; O’Connor, J.E.; Mussini, C.; et al. The protease inhibitor atazanavir triggers autophagy and mitophagy in human preadipocytes. AIDS 2012, 26, 2017–2026. [Google Scholar] [CrossRef]
- Polus, A.; Bociaga-Jasik, M.; Czech, U.; Goralska, J.; Cialowicz, U.; Chojnacka, M.; Polus, M.; Jurowski, K.; Dembinska-Kiec, A. The human immunodeficiency virus (HIV1) protease inhibitor sanquinavir activates autophagy and removes lipids deposited in lipid droplets. J. Physiol. Pharmacol. 2017, 68, 283–293. [Google Scholar]
- Tripathi, A.; Thangaraj, A.; Chivero, E.T.; Periyasamy, P.; Callen, S.; Burkovetskaya, M.E.; Guo, M.L.; Buch, S. Antiretroviral-Mediated Microglial Activation Involves Dysregulated Autophagy and Lysosomal Dysfunction. Cells 2019, 8. [Google Scholar] [CrossRef]
- Tripathi, A.; Thangaraj, A.; Chivero, E.T.; Periyasamy, P.; Burkovetskaya, M.E.; Niu, F.; Guo, M.L.; Buch, S. N-Acetylcysteine Reverses Antiretroviral-Mediated Microglial Activation by Attenuating Autophagy-Lysosomal Dysfunction. Front. Neurol. 2020, 11, 840. [Google Scholar] [CrossRef]
- Cheney, L.; Guzik, H.; Macaluso, F.P.; Macian, F.; Cuervo, A.M.; Berman, J.W. HIV Nef and Antiretroviral Therapy Have an Inhibitory Effect on Autophagy in Human Astrocytes that May Contribute to HIV-Associated Neurocognitive Disorders. Cells 2020, 9. [Google Scholar] [CrossRef]
- Sanchez, A.B.; Varano, G.P.; de Rozieres, C.M.; Maung, R.; Catalan, I.C.; Dowling, C.C.; Sejbuk, N.E.; Hoefer, M.M.; Kaul, M. Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob. Agents Chemother. 2016, 60, 168–179. [Google Scholar] [CrossRef]
- Rodriguez, M.; Lapierre, J.; Ojha, C.R.; Pawitwar, S.; Karuppan, M.K.M.; Kashanchi, F.; El-Hage, N. Morphine counteracts the antiviral effect of antiretroviral drugs and causes upregulation of p62/SQSTM1 and histone-modifying enzymes in HIV-infected astrocytes. J. Neurovirol. 2019. [Google Scholar] [CrossRef]
- Serrano, A.; El Haddad, S.; Moal, F.; Prazuck, T.; Legac, E.; Robin, C.; Brule, F.; Charpentier, S.; Normand, T.; Legrand, A.; et al. Dysregulation of apoptosis and autophagy gene expression in peripheral blood mononuclear cells of efficiently treated HIV-infected patients. AIDS 2018, 32, 1579–1587. [Google Scholar] [CrossRef]
- Bento, C.F.; Renna, M.; Ghislat, G.; Puri, C.; Ashkenazi, A.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem. 2016, 85, 685–713. [Google Scholar] [CrossRef]
- World Health Organization. Update of Recommendations on First-And Second-Line Antiretroviral Regimens; WHO/CDS/HIV/19.15; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Best, B.M.; Koopmans, P.P.; Letendre, S.L.; Capparelli, E.V.; Rossi, S.S.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Mbeo, G.; McCutchan, J.A.; et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J. Antimicrob. Chemother. 2011, 66, 354–357. [Google Scholar] [CrossRef]
- Tashima, K.T.; Caliendo, A.M.; Ahmad, M.; Gormley, J.M.; Fiske, W.D.; Brennan, J.M.; Flanigan, T.P. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J. Infect. Dis. 1999, 180, 862–864. [Google Scholar] [CrossRef]
- Staszewski, S.; Morales-Ramirez, J.; Tashima, K.T.; Rachlis, A.; Skiest, D.; Stanford, J.; Stryker, R.; Johnson, P.; Labriola, D.F.; Farina, D.; et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N. Engl. J. Med. 1999, 341, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, C.; Telenti, A.; Decosterd, L.A.; Greub, G.; Biollaz, J.; Buclin, T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 2001, 15, 71–75. [Google Scholar] [CrossRef]
- Burger, D.; van der Heiden, I.; la Porte, C.; van der Ende, M.; Groeneveld, P.; Richter, C.; Koopmans, P.; Kroon, F.; Sprenger, H.; Lindemans, J.; et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: The effect of gender, race, and CYP2B6 polymorphism. Br. J. Clin. Pharmacol. 2006, 61, 148–154. [Google Scholar] [CrossRef]
- Carr, D.F.; la Porte, C.J.; Pirmohamed, M.; Owen, A.; Cortes, C.P. Haplotype structure of CYP2B6 and association with plasma efavirenz concentrations in a Chilean HIV cohort. J. Antimicrob. Chemother. 2010, 65, 1889–1893. [Google Scholar] [CrossRef]
- Lopez, S.; Coll, O.; Durban, M.; Hernandez, S.; Vidal, R.; Suy, A.; Moren, C.; Casademont, J.; Cardellach, F.; Mataro, D.; et al. Mitochondrial DNA depletion in oocytes of HIV-infected antiretroviral-treated infertile women. Antivir. Ther. 2008, 13, 833–838. [Google Scholar]
- Bostan, A.; Demeestere, I.; Vanderwinden, J.M.; Devreker, F.; Englert, Y. Nucleoside analog stavudine depletes mitochondrial DNA with no organelle loss in mouse oocytes. Curr. HIV Res. 2010, 8, 127–133. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 10, 1–382. [Google Scholar] [CrossRef]
- World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection; WHO/CDS/HIV/18.51; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- KALETRA North Chicago, IL: AbbVie Inc. Available online: https://www.rxabbvie.com/pdf/kaletratabpi.pdf (accessed on 8 April 2021).
- Chougrani, I.; Luton, D.; Matheron, S.; Mandelbrot, L.; Azria, E. Safety of protease inhibitors in HIV-infected pregnant women. HIV AIDS 2013, 5, 253–262. [Google Scholar] [CrossRef][Green Version]
- Snijdewind, I.J.M.; Smit, C.; Godfried, M.H.; Bakker, R.; Nellen, J.; Jaddoe, V.W.V.; van Leeuwen, E.; Reiss, P.; Steegers, E.A.P.; van der Ende, M.E. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS ONE 2018, 13, e0191389. [Google Scholar] [CrossRef] [PubMed]
- Saleska, J.L.; Turner, A.N.; Maierhofer, C.; Clark, J.; Kwiek, J.J. Use of Antiretroviral Therapy During Pregnancy and Adverse Birth Outcomes Among Women Living With HIV-1 in Low- and Middle-Income Countries: A Systematic Review. J. Acquir. Immune Defic. Syndr. 2018, 79, 1–9. [Google Scholar] [CrossRef]
- Floridia, M.; Dalzero, S.; Giacomet, V.; Tamburrini, E.; Masuelli, G.; Savasi, V.; Spinillo, A.; Tassis, B.; Franceschetti, L.; Degli Antoni, A.M.; et al. Pregnancy and neonatal outcomes in women with HIV-1 exposed to integrase inhibitors, protease inhibitors and non-nucleoside reverse transcriptase inhibitors: An observational study. Infection 2020, 48, 249–258. [Google Scholar] [CrossRef]
- Dos Reis, H.L.B.; Boldrini, N.A.T.; Rangel, A.F.R.; Barros, V.F.; Mercon de Vargas, P.R.; Miranda, A.E. Placental growth disorders and perinatal adverse outcomes in Brazilian HIV-infected pregnant women. PLoS ONE 2020, 15, e0231938. [Google Scholar] [CrossRef] [PubMed]
- Theron, G.; Brummel, S.; Fairlie, L.; Pinilla, M.; McCarthy, K.; Owor, M.; Chinula, L.; Makanani, B.; Violari, A.; Moodley, D.; et al. Pregnancy outcomes of women conceiving on antiretroviral therapy (ART) compared to those commenced on ART during pregnancy. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Yampolsky, M.; Shlakhter, O.; Deng, D.; Kala, S.; Walmsley, S.L.; Murphy, K.E.; Yudin, M.H.; MacGillivray, J.; Loutfy, M.; Dunk, C.; et al. Exploring the impact of HIV infection and antiretroviral therapy on placenta morphology. Placenta 2021, 104, 102–109. [Google Scholar] [CrossRef]
- Mohammadi, H.; Papp, E.; Cahill, L.; Rennie, M.; Banko, N.; Pinnaduwage, L.; Lee, J.; Kibschull, M.; Dunk, C.; Sled, J.G.; et al. HIV antiretroviral exposure in pregnancy induces detrimental placenta vascular changes that are rescued by progesterone supplementation. Sci. Rep. 2018, 8, 6552. [Google Scholar] [CrossRef] [PubMed]
- Fraichard, C.; Bonnet-Serrano, F.; Laguillier-Morizot, C.; Hebert-Schuster, M.; Lai-Kuen, R.; Sibiude, J.; Fournier, T.; Cohen, M.; Guibourdenche, J. Protease Inhibitor Anti-HIV, Lopinavir, Impairs Placental Endocrine Function. Int. J. Mol. Sci. 2021, 22, 683. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.; Arlier, S.; Guzeloglu-Kayisli, O.; Tabak, M.S.; Ekiz, T.; Semerci, N.; Larsen, K.; Schatz, F.; Lockwood, C.J.; Kayisli, U.A. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int. J. Mol. Sci. 2017, 18, 792. [Google Scholar] [CrossRef]
- Conradie, F.; Zorrilla, C.; Josipovic, D.; Botes, M.; Osiyemi, O.; Vandeloise, E.; Eley, T.; Child, M.; Bertz, R.; Hu, W.; et al. Safety and exposure of once-daily ritonavir-boosted atazanavir in HIV-infected pregnant women. HIV Med. 2011, 12, 570–579. [Google Scholar] [CrossRef]
- Reyetaz Montreal, Canada: Bristol-Myers Squibb. Available online: https://packageinserts.bms.com/pi/pi_reyataz.pdf (accessed on 8 April 2021).
- Croom, K.F.; Dhillon, S.; Keam, S.J. Atazanavir: A review of its use in the management of HIV-1 infection. Drugs 2009, 69, 1107–1140. [Google Scholar] [CrossRef]
- Crutchley, R.D.; Ma, Q.; Sulaiman, A.; Hochreitter, J.; Morse, G.D. Within-patient atazanavir trough concentration monitoring in HIV-1-infected patients. J. Pharm. Pract. 2011, 24, 216–222. [Google Scholar] [CrossRef]
- Le Tiec, C.; Barrail, A.; Goujard, C.; Taburet, A.M. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharm. 2005, 44, 1035–1050. [Google Scholar] [CrossRef]
- Bociaga-Jasik, M.; Polus, A.; Goralska, J.; Czech, U.; Gruca, A.; Sliwa, A.; Garlicki, A.; Mach, T.; Dembinska-Kiec, A. Metabolic effects of the HIV protease inhibitor--saquinavir in differentiating human preadipocytes. Pharmacol. Rep. 2013, 65, 937–950. [Google Scholar] [CrossRef]
- Merry, C.; Barry, M.G.; Mulcahy, F.; Ryan, M.; Heavey, J.; Tjia, J.F.; Gibbons, S.E.; Breckenridge, A.M.; Back, D.J. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 1997, 11, F29–F33. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.D.; Hartigan, P.M.; Simberkoff, M.S.; Day, P.L.; Diamond, G.R.; Dickinson, G.M.; Drusano, G.L.; Egorin, M.J.; George, W.L.; Gordin, F.M.; et al. A controlled trial of early versus late treatment with zidovudine in symptomatic human immunodeficiency virus infection. Results of the Veterans Affairs Cooperative Study. N. Engl. J. Med. 1992, 326, 437–443. [Google Scholar] [CrossRef]
- Aboulker, J.P.; Swart, A.M. Preliminary analysis of the Concorde trial. Concorde Coordinating Committee. Lancet 1993, 341, 889–890. [Google Scholar] [CrossRef]
- Johnson, V.A.; Hirsch, M.S. New developments in combination chemotherapy of anti-human immunodeficiency virus drugs. Ann. N. Y. Acad. Sci. 1990, 616, 318–327. [Google Scholar] [CrossRef]
- Calcagno, A.; Di Perri, G.; Bonora, S. Pharmacokinetics and pharmacodynamics of antiretrovirals in the central nervous system. Clin. Pharm. 2014, 53, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Price, R.W.; Gisslen, M. Antiretroviral drug treatment of CNS HIV-1 infection. J. Antimicrob. Chemother. 2012, 67, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M.; Nath, A. Where does HIV hide? A focus on the central nervous system. Curr. Opin. HIV AIDS 2013, 8, 165–169. [Google Scholar] [CrossRef]
- Di Malta, C.; Cinque, L.; Settembre, C. Transcriptional Regulation of Autophagy: Mechanisms and Diseases. Front. Cell Dev. Biol. 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [PubMed]
- High, K.P.; Brennan-Ing, M.; Clifford, D.B.; Cohen, M.H.; Currier, J.; Deeks, S.G.; Deren, S.; Effros, R.B.; Gebo, K.; Goronzy, J.J.; et al. HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J. Acquir. Immune Defic. Syndr. 2012, 60 (Suppl. 1), 18. [Google Scholar] [CrossRef]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, D.; Wit, F.W.; Burkle, A.; Oehlke, S.; Kootstra, N.A.; Winston, A.; Franceschi, C.; Garagnani, P.; Pirazzini, C.; Libert, C.; et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 2019, 33, 259–268. [Google Scholar] [CrossRef]
- Bellisai, C.; Sciamanna, I.; Rovella, P.; Giovannini, D.; Baranzini, M.; Pugliese, G.M.; Zeya Ansari, M.S.; Milite, C.; Sinibaldi-Vallebona, P.; Cirilli, R.; et al. Reverse transcriptase inhibitors promote the remodelling of nuclear architecture and induce autophagy in prostate cancer cells. Cancer Lett. 2020, 478, 133–145. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2020, 1–13. [Google Scholar] [CrossRef]
- Patties, I.; Kortmann, R.D.; Menzel, F.; Glasow, A. Enhanced inhibition of clonogenic survival of human medulloblastoma cells by multimodal treatment with ionizing irradiation, epigenetic modifiers, and differentiation-inducing drugs. J. Exp. Clin. Cancer Res. 2016, 35, 94. [Google Scholar] [CrossRef]
- Liu, W.; Song, X.L.; Zhao, S.C.; He, M.; Wang, H.; Chen, Z.; Xiang, W.; Yi, G.; Qi, S.; Liu, Y. Antitumor Activity and Mechanism of a Reverse Transcriptase Inhibitor, Dapivirine, in Glioblastoma. J. Cancer 2018, 9, 117–128. [Google Scholar] [CrossRef]
- Kushchayeva, Y.; Jensen, K.; Recupero, A.; Costello, J.; Patel, A.; Klubo-Gwiezdzinska, J.; Boyle, L.; Burman, K.; Vasko, V. The HIV protease inhibitor nelfinavir down-regulates RET signaling and induces apoptosis in medullary thyroid cancer cells. J. Clin. Endocrinol. Metab. 2014, 99, E734–E745. [Google Scholar] [CrossRef]
- Gills, J.J.; Lopiccolo, J.; Tsurutani, J.; Shoemaker, R.H.; Best, C.J.; Abu-Asab, M.S.; Borojerdi, J.; Warfel, N.A.; Gardner, E.R.; Danish, M.; et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin. Cancer Res. 2007, 13, 5183–5194. [Google Scholar] [CrossRef]
- Johnson, C.E.; Hunt, D.K.; Wiltshire, M.; Herbert, T.P.; Sampson, J.R.; Errington, R.J.; Davies, D.M.; Tee, A.R. Endoplasmic reticulum stress and cell death in mTORC1-overactive cells is induced by nelfinavir and enhanced by chloroquine. Mol. Oncol. 2015, 9, 675–688. [Google Scholar] [CrossRef]
- La Rosa, F.; Saresella, M.; Marventano, I.; Piancone, F.; Ripamonti, E.; Al-Daghri, N.; Bazzini, C.; Zoia, C.P.; Conti, E.; Ferrarese, C.; et al. Stavudine Reduces NLRP3 Inflammasome Activation and Modulates Amyloid-beta Autophagy. J. Alzheimers Dis. 2019, 72, 401–412. [Google Scholar] [CrossRef]
- Gulick, R.M.; Flexner, C. Long-Acting HIV Drugs for Treatment and Prevention. Annu Rev. Med. 2019, 70, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Gnanadhas, D.P.; Dash, P.K.; Sillman, B.; Bade, A.N.; Lin, Z.; Palandri, D.L.; Gautam, N.; Alnouti, Y.; Gelbard, H.A.; McMillan, J.; et al. Autophagy facilitates macrophage depots of sustained-release nanoformulated antiretroviral drugs. J. Clin. Investig. 2017, 127, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Gnanadhas, D.P.; Dash, P.K.; Machhi, J.; Lin, Z.; McMillan, J.; Edagwa, B.; Gelbard, H.; Gendelman, H.E.; Gorantla, S. Modulating cellular autophagy for controlled antiretroviral drug release. Nanomedicine 2018, 13, 2139–2154. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheney, L.; Barbaro, J.M.; Berman, J.W. Antiretroviral Drugs Impact Autophagy with Toxic Outcomes. Cells 2021, 10, 909. https://doi.org/10.3390/cells10040909
Cheney L, Barbaro JM, Berman JW. Antiretroviral Drugs Impact Autophagy with Toxic Outcomes. Cells. 2021; 10(4):909. https://doi.org/10.3390/cells10040909
Chicago/Turabian StyleCheney, Laura, John M. Barbaro, and Joan W. Berman. 2021. "Antiretroviral Drugs Impact Autophagy with Toxic Outcomes" Cells 10, no. 4: 909. https://doi.org/10.3390/cells10040909
APA StyleCheney, L., Barbaro, J. M., & Berman, J. W. (2021). Antiretroviral Drugs Impact Autophagy with Toxic Outcomes. Cells, 10(4), 909. https://doi.org/10.3390/cells10040909





