Stability of Imprinting and Differentiation Capacity in Naïve Human Cells Induced by Chemical Inhibition of CDK8 and CDK19
Abstract
1. Introduction
2. Materials and Methods
2.1. Human PSC Resources
2.2. Human PSC Culture Conditions
2.3. Derivation of Primordial Germ Cells (PGCs)
2.4. Derivation of Trophoblast Stem Cells (TSCs)
2.5. Teratoma Formation Assay
2.6. RNA Isolation and qRT-PCR
2.7. RNA-Seq Transcriptomic Analyses
2.8. Differential Gene Expression Comparison of Published Studies
2.9. DNA Methylation
2.10. Exome Sequencing
2.11. Imprinting Score
2.12. Statistical Analysis
3. Results
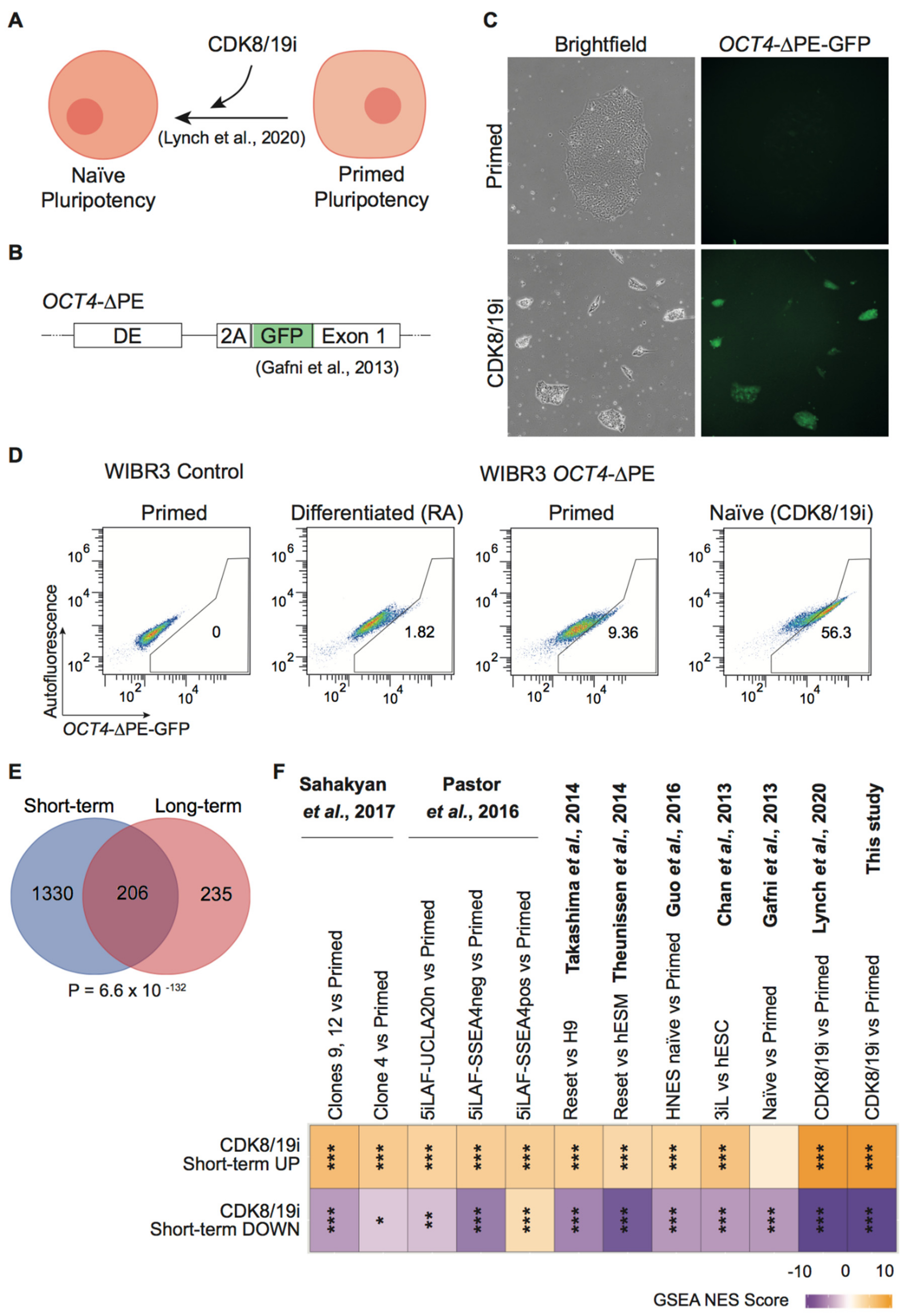
3.1. CDK8/19i Stabilizes Naïve Human PSCs over Long-Term Passaging
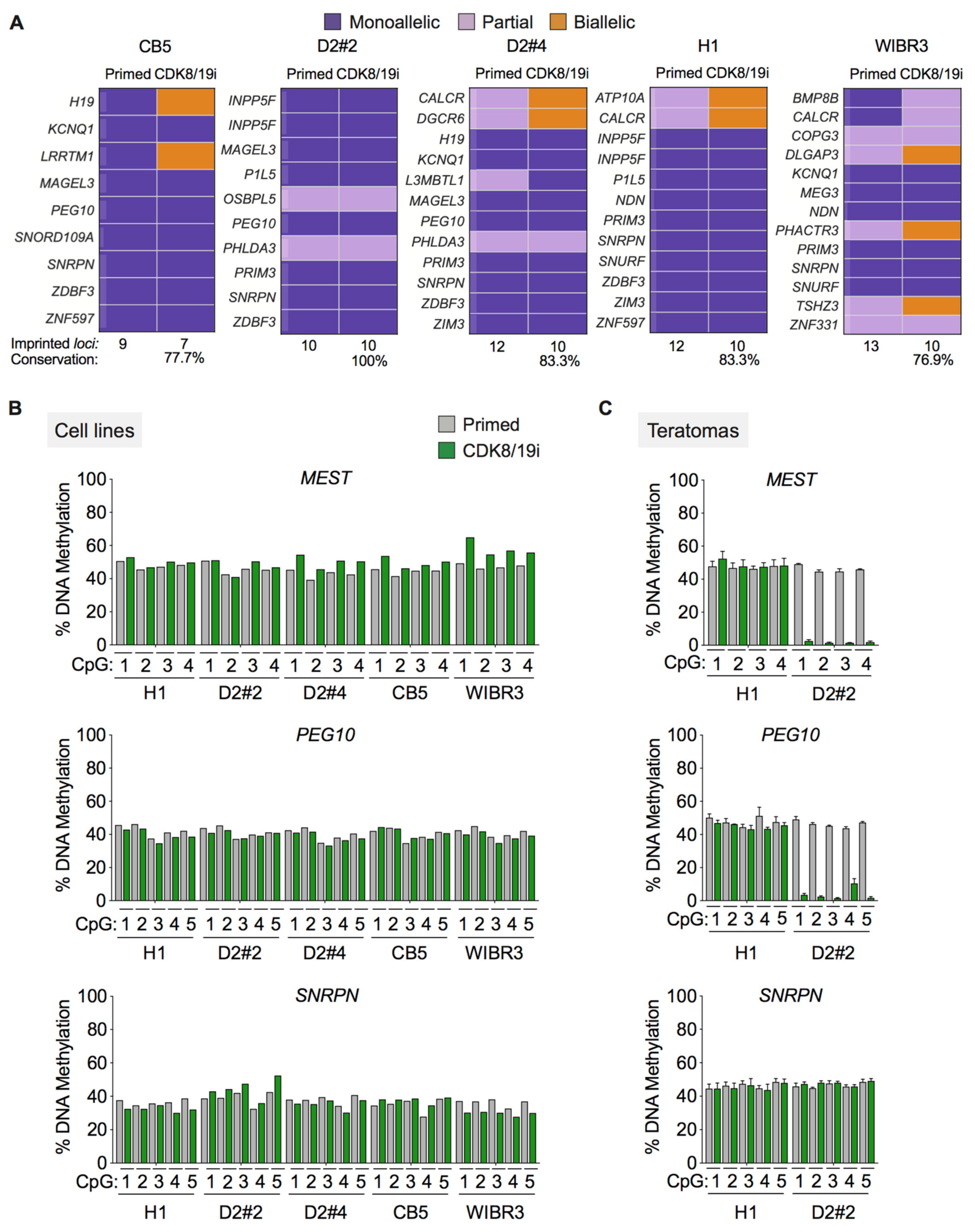
3.2. Long-Term Preservation of Genomic Imprints in CDK8/19i-Naïve Human PSCs
3.3. Differentiation Potential of CDK8/19i-Naïve Human PSCs into Primordial Germ Cells
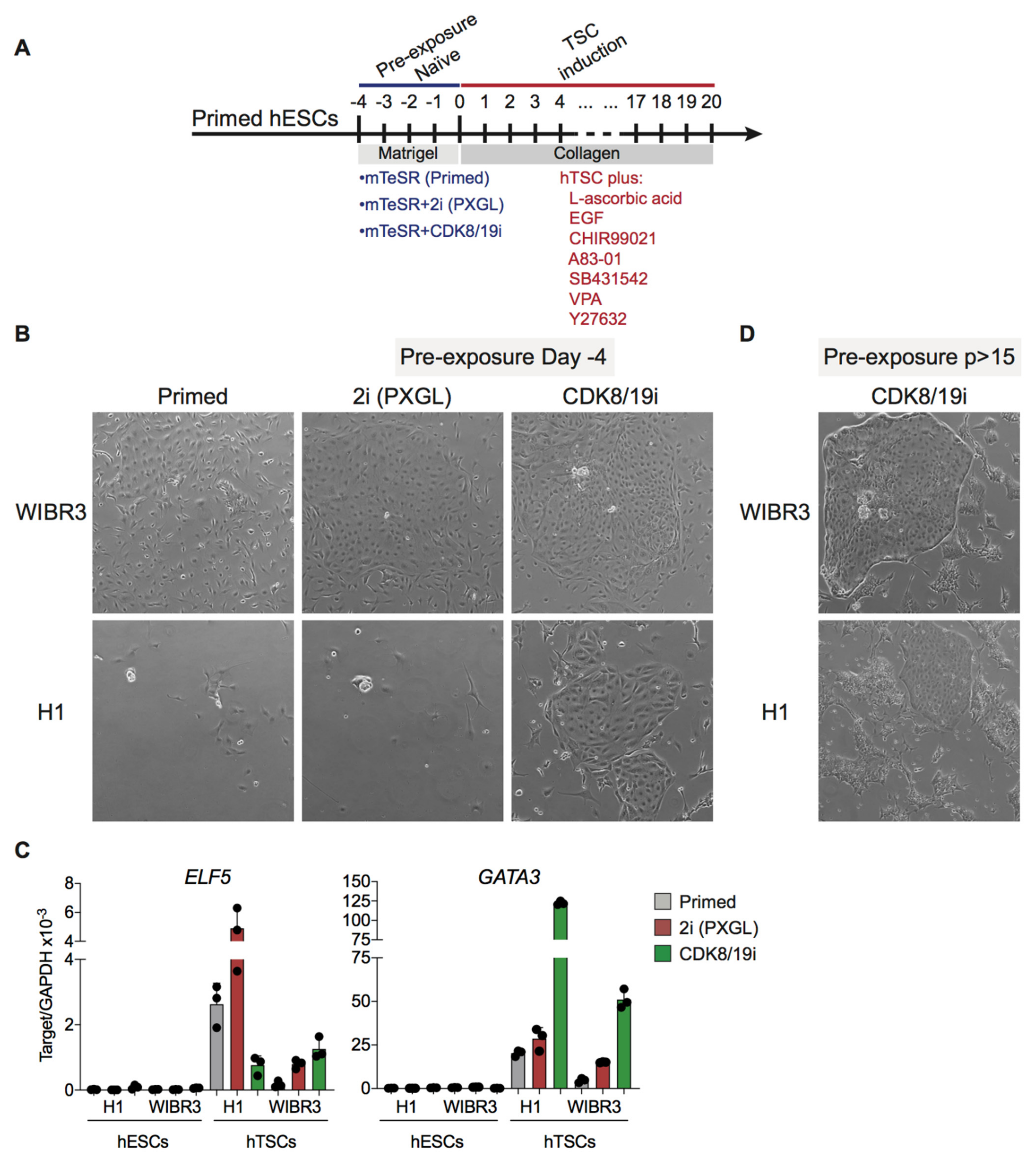
3.4. Differentiation Potential of CDK8/19i-Naïve Human PSCs into Trophoblast Stem Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boroviak, T.; Loos, R.; Bertone, P.; Smith, A.; Nichols, J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 2014, 16, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.; Kalkan, T.; Menafra, R.; Denissov, S.; Jones, K.; Hofemeister, H.; Nichols, J.; Kranz, A.; Stewart, A.F.; Smith, A.; et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 2012, 149, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef]
- Hanna, J.; Cheng, A.W.; Saha, K.; Kim, J.; Lengner, C.J.; Soldner, F.; Cassady, J.P.; Muffat, J.; Carey, B.W.; Jaenisch, R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA 2010, 107, 9222–9227. [Google Scholar] [CrossRef]
- Chan, Y.-S.; Göke, J.; Ng, J.-H.; Lu, X.; Uy-Gonzales, K.A.; Tan, C.-P.; Tng, W.-Q.; Hong, Z.-Z.; Lim, Y.-S.; Ng, H.-H. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 2013, 13, 663–675. [Google Scholar] [CrossRef]
- Gafni, O.; Weinberger, L.; Mansour, A.A.; Manor, Y.S.; Chomsky, E.; Ben-Yosef, D.; Kalma, Y.; Viukov, S.; Maza, I.; Zviran, A.; et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. [Google Scholar] [CrossRef]
- Guo, G.; von Meyenn, F.; Rostovskaya, M.; Clarke, J.; Dietmann, S.; Baker, D.; Sahakyan, A.; Myers, S.; Bertone, P.; Reik, W.; et al. Epigenetic resetting of human pluripotency. Development 2017, 144, 2748–2763. [Google Scholar] [CrossRef]
- Takashima, Y.; Guo, G.; Loos, R.; Nichols, J.; Ficz, G.; Krueger, F.; Oxley, D.; Santos, F.; Clarke, J.; Mansfield, W.; et al. Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell 2014, 158, 1254–1269. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Powell, B.E.; Wang, H.; Mitalipova, M.; Faddah, D.A.; Reddy, J.; Fan, Z.P.; Maetzel, D.; Ganz, K.; Shi, L.; et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 2014, 15, 471–487. [Google Scholar] [CrossRef]
- Ware, C.B.; Nelson, A.M.; Mecham, B.; Hesson, J.; Zhou, W.; Jonlin, E.C.; Jimenez-Caliani, A.J.; Deng, X.; Cavanaugh, C.; Cook, S.; et al. Derivation of naïve human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4484–4489. [Google Scholar] [CrossRef]
- Liu, X.; Nefzger, C.M.; Rossello, F.J.; Chen, J.; Knaupp, A.S.; Firas, J.; Ford, E.; Pflueger, J.; Paynter, J.M.; Chy, H.S.; et al. Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nat. Methods 2017, 14, 1055–1062. [Google Scholar] [CrossRef]
- Avior, Y.; Eggan, K.; Benvenisty, N. Cancer-related mutations identified in primed and naive human pluripotent stem cells. Cell Stem Cell 2019, 25, 456–461. [Google Scholar] [CrossRef]
- Bar, S.; Schachter, M.; Eldar-Geva, T.; Benvenisty, N. Large-scale analysis of loss of imprinting in human pluripotent stem cells. Cell Rep. 2017, 19, 957–968. [Google Scholar] [CrossRef]
- Pastor, W.A.; Chen, D.; Liu, W.; Kim, R.; Sahakyan, A.; Lukianchikov, A.; Plath, K.; Jacobsen, S.E.; Clark, A.T. Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. Cell Stem Cell 2016, 18, 323–329. [Google Scholar] [CrossRef]
- Theunissen, T.W.; Friedli, M.; He, Y.; Planet, E.; O’Neil, R.C.; Markoulaki, S.; Pontis, J.; Wang, H.; Iouranova, A.; Imbeault, M.; et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Von Meyenn, F.; Iurlaro, M.; Habibi, E.; Liu, N.Q.; Salehzadeh-Yazdi, A.; Santos, F.; Petrini, E.; Milagre, I.; Yu, M.; Xie, Z.; et al. Impairment of DNA methylation maintenance is the main cause of global demethylation in naive embryonic stem cells. Mol. Cell 2016, 62, 1–14. [Google Scholar]
- Li, J.; Wang, R.; Hu, X.; Gao, Y.; Wang, Z.; Li, J.; Wong, J. Activated MEK/ERK pathway drives widespread and coordinated overexpression of UHRF1 and DNMT1 in cancer cells. Sci. Rep. 2019, 9, 907. [Google Scholar] [CrossRef]
- Di Stefano, B.; Ueda, M.; Sabri, S.; Brumbaugh, J.; Huebner, A.J.; Sahakyan, A.; Clement, K.; Clowers, K.J.; Erickson, A.R.; Shioda, K.; et al. Reduced MEK inhibition preserves genomic stability in naive human embryonic stem cells. Nat. Methods 2018, 15, 732–740. [Google Scholar] [CrossRef]
- Lynch, C.J.; Bernad, R.; Martínez-Val, A.; Shahbazi, M.N.; Nóbrega-Pereira, S.; Calvo, I.; Blanco-Aparicio, C.; Tarantino, C.; Garreta, E.; Richart-Ginés, L.; et al. Global hyperactivation of enhancers stabilizes human and mouse naive pluripotency through inhibition of CDK8/19 Mediator kinases. Nat. Cell Biol. 2020, 22, 1223–1238. [Google Scholar] [CrossRef]
- Martinez-Val, A.; Lynch, C.J.; Calvo, I.; Ximénez-Embún, P.; Garcia, F.; Zarzuela, E.; Serrano, M.; Munoz, J. Dissection of two routes to naïve pluripotency using different kinase inhibitors. Nat. Commun. 2021, 12, 1863. [Google Scholar] [CrossRef]
- Kojima, Y.; Sasaki, K.; Yokobayashi, S.; Sakai, Y.; Nakamura, T.; Yabuta, Y.; Nakaki, F.; Nagaoka, S.; Woltjen, K.; Hotta, A.; et al. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Dong, C.; Beltcheva, M.; Gontarz, P.; Zhang, B.; Popli, P.; Fischer, L.A.; Khan, S.A.; Park, K.M.; Yoon, E.J.; Xing, X.; et al. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 2020, 9. [Google Scholar] [CrossRef]
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K.; Kabayama, Y.; Suyama, M.; Sasaki, H.; Arima, T. Derivation of human trophoblast stem cells. Cell Stem Cell 2018, 22, 50–63. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Subramaniana, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Sahakyan, A.; Kim, R.; Chronis, C.; Sabri, S.; Bonora, G.; Theunissen, T.W.; Kuoy, E.; Langerman, J.; Clark, A.T.; Jaenisch, R.; et al. Human naive pluripotent stem cells model X chromosome dampening and X inactivation. Cell Stem Cell 2017, 20, 1–15. [Google Scholar] [CrossRef]
- Guo, G.; von Meyenn, F.; Santos, F.; Chen, Y.; Reik, W.; Bertone, P.; Smith, A.; Nichols, J. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis Hadley; Springer: Berlin, Germany, 2009; Volume 35. [Google Scholar]
- The R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Volume 2, Available online: http://www.R-project.org/ (accessed on 19 March 2021).
- Wang RY, H.; Gehrke, C.W.; Ehrlich, M. Comparison of bisulfite modification of 5-methyldeoxycytidine and deoxycytidine residues. Nucleic Acids Res. 1980, 8, 4777–4790. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Vilella, A.J.; Cuppen, E.; Nijman, I.J.; Prins, P. Sambamba: Fast processing of NGS alignment formats. Bioinformatics 2015, 31, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Poplin, R.; Ruano-Rubio, V.; DePristo, M.A.; Fennell, T.J.; Carneiro, M.O.; Van der Auwera, G.A.; Kling, D.E.; Gauthier, L.D.; Levy-Moonshine, A.; Roazen, D.; et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 2017. [Google Scholar] [CrossRef]
- Rugg-Gunn, P.J.; Ferguson-Smith, A.C.; Pedersen, R.A. Status of genomic imprinting in human embryonic stem cells as revealed by a large cohort of independently derived and maintained lines. Hum. Mol. Genet. 2007, 16, 243–251. [Google Scholar] [CrossRef]
- Choi, J.; Huebner, A.J.; Clement, K.; Walsh, R.M.; Savol, A.; Lin, K.; Gu, H.; Di Stefano, B.; Brumbaugh, J.; Kim, S.Y.; et al. Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells. Nature 2017, 548, 219–223. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Odajima, J.; Yawata, S.; Shioda, K.; Owa, C.; Isselbacher, K.J.; Hanna, J.H.; Shioda, T. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc. Natl. Acad. Sci. USA 2017, 114, E9913–E9922. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, P.; Yan, L.; Li, R.; Hu, B.; Lian, Y.; Yan, J.; Ren, X.; Lin, S.; Li, J.; et al. The DNA methylation landscape of human early embryos. Nature 2014, 511, 606–610. [Google Scholar] [CrossRef]
- Branco, M.R.; Oda, M.; Reik, W. Safeguarding parental identity: Dnmt1 maintains imprints during epigenetic reprogramming in early embryogenesis. Genes Dev. 2008, 22, 1567–1571. [Google Scholar] [CrossRef]
- Hirasawa, R.; Chiba, H.; Kaneda, M.; Tajima, S.; Li, E.; Jaenisch, R.; Sasaki, H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008, 22, 1607–1616. [Google Scholar] [CrossRef]
- Lynch, C.J.; Bernad, R.; Calvo, I.; Serrano, M. Manipulating the Mediator complex to induce naïve pluripotency. Exp. Cell Res. 2020, 395, 112215. [Google Scholar] [CrossRef]
- Yagi, M.; Kishigami, S.; Tanaka, A.; Semi, K.; Mizutani, E.; Wakayama, S.; Wakayama, T.; Yamamoto, T.; Yamada, Y. Derivation of ground-state female ES cells maintaining gamete-derived DNA methylation. Nature 2017, 548, 224–227. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 1–16. [Google Scholar] [CrossRef]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef]
- Pastor, W.A.; Liu, W.; Chen, D.; Ho, J.; Kim, R.; Hunt, T.J.; Lukianchikov, A.; Liu, X.; Polo, J.M.; Jacobsen, S.E.; et al. TFAP2C regulates transcription in human naive pluripotency by opening enhancers. Nat. Cell Biol. 2018, 20, 553–564. [Google Scholar] [CrossRef]
- Qin, H.; Hejna, M.; Liu, Y.; Percharde, M.; Wossidlo, M.; Blouin, L.; Durruthy-Durruthy, J.; Wong, P.; Qi, Z.; Yu, J.; et al. YAP induces human naive pluripotency. Cell Rep. 2016, 14, 2301–2312. [Google Scholar] [CrossRef]
- Cinkornpumin, J.K.; Kwon, S.Y.; Guo, Y.; Hossain, I.; Sirois, J.; Russett, C.S.; Tseng, H.W.; Okae, H.; Arima, T.; Duchaine, T.F.; et al. Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Rep. 2020, 15, 198–213. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernad, R.; Lynch, C.J.; Urdinguio, R.G.; Stephan-Otto Attolini, C.; Fraga, M.F.; Serrano, M. Stability of Imprinting and Differentiation Capacity in Naïve Human Cells Induced by Chemical Inhibition of CDK8 and CDK19. Cells 2021, 10, 876. https://doi.org/10.3390/cells10040876
Bernad R, Lynch CJ, Urdinguio RG, Stephan-Otto Attolini C, Fraga MF, Serrano M. Stability of Imprinting and Differentiation Capacity in Naïve Human Cells Induced by Chemical Inhibition of CDK8 and CDK19. Cells. 2021; 10(4):876. https://doi.org/10.3390/cells10040876
Chicago/Turabian StyleBernad, Raquel, Cian J. Lynch, Rocio G. Urdinguio, Camille Stephan-Otto Attolini, Mario F. Fraga, and Manuel Serrano. 2021. "Stability of Imprinting and Differentiation Capacity in Naïve Human Cells Induced by Chemical Inhibition of CDK8 and CDK19" Cells 10, no. 4: 876. https://doi.org/10.3390/cells10040876
APA StyleBernad, R., Lynch, C. J., Urdinguio, R. G., Stephan-Otto Attolini, C., Fraga, M. F., & Serrano, M. (2021). Stability of Imprinting and Differentiation Capacity in Naïve Human Cells Induced by Chemical Inhibition of CDK8 and CDK19. Cells, 10(4), 876. https://doi.org/10.3390/cells10040876





