Role of Decorin in Posterior Capsule Opacification and Eye Lens Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure for Generation of PCO Animal Model
2.3. RNA Extraction
2.4. Microarray and Gene Ontology Analysis
2.5. RT-qPCR Validation
2.6. Construction of Green Fluorescent Protein (GFP)-hDCN Vector and Lentivirus Production
2.7. Cell Culture
2.8. Western Blotting
2.9. Constructs for the hDCN-Tg Line
2.10. Generation of hDCN-Tg
2.11. Histochemical Staining for LacZ with X-Gal
2.12. Microscopic, Histological, and Immunohistochemical Analyses
2.13. In Vivo Wound Healing Assay
2.14. Obtaining LEC and Aqueous Humor Samples from Patients with Cataract
2.15. Enzyme-Linked Immunosorbent Assay (ELISA) for DCN in the Aqueous Humor
2.16. Statistical Methods
3. Results
3.1. Screening of Gene Expression Profile
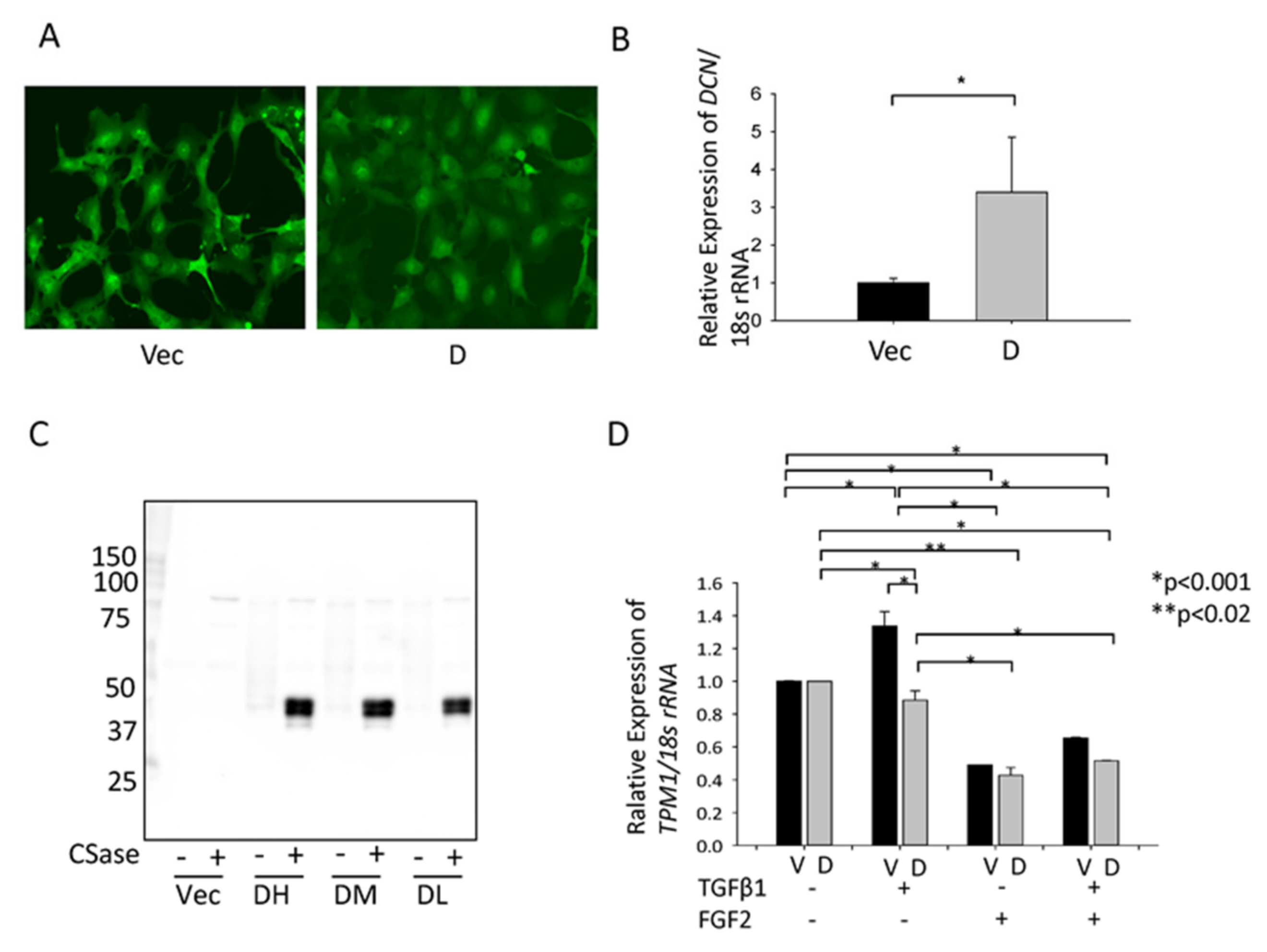
3.2. Confirmation of Gene Upregulation Using RT-qPCR and Western Blotting in Rat and Mouse PCO Models
3.3. Effect of TGFβ and FGF2 on the Expression of Dcn and Tpm1 in MLECs and SRA-HLECs
3.4. Effect of hDCN Overexpression on TGFβ-Induced Tpm1 Upregulation
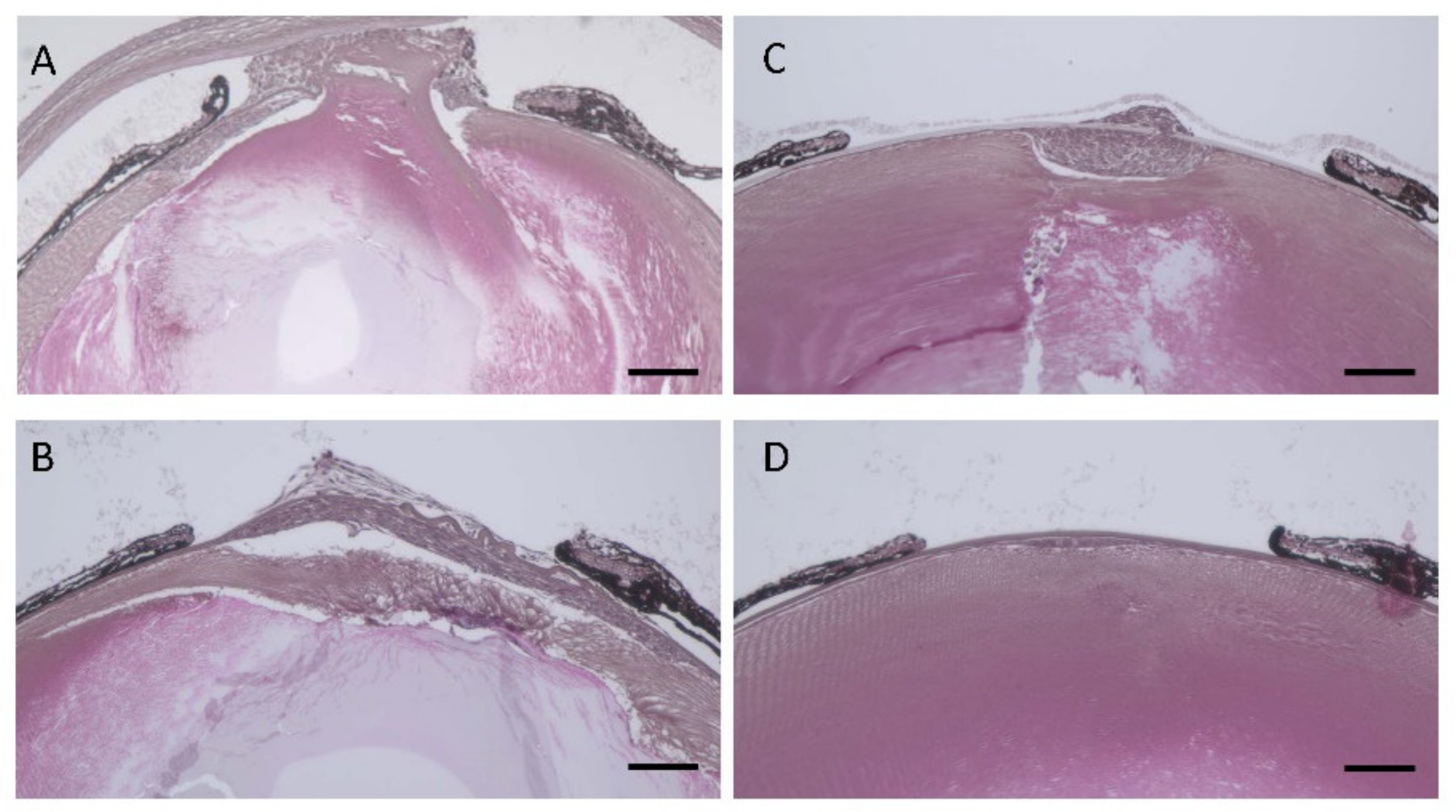
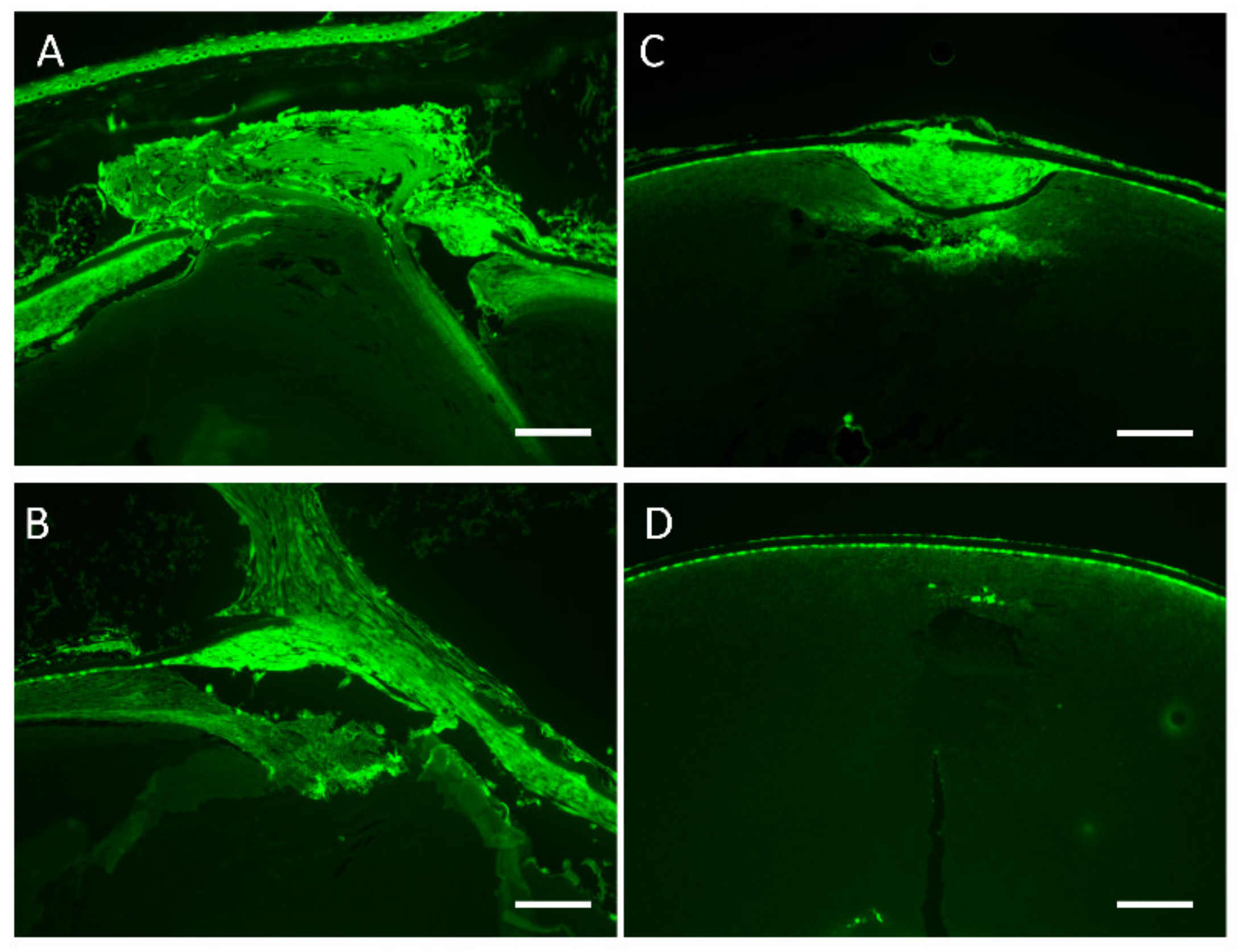
3.5. Lens Morphology of hDCN-Tg Mice
3.6. Effect of hDCN Overexpression on Wound Healing on Lens Surface In Vivo
3.7. Relationship between Expression of DCN, Severity of Cataract, and Age in Aqueous Humor or Human LECs of Patients with Cataract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wormstone, I.M. Posterior capsule opacification: A cell biological perspective. Exp. Eye Res. 2002, 74, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, Y.; Lu, G.; Wang, M. Primary posterior capsulorhexis with anterior vitrectomy in preventing posterior capsule opacification in pediatric cataract microsurgery. Microsurgery 2008, 28, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Ben Ezra, D.; Cohen, E. Posterior capsulectomy in pediatric cataract surgery: The necessity of a choice. Ophthalmology 1997, 104, 2168–2174. [Google Scholar] [CrossRef]
- Nibourg, L.M.; Sharma, P.K.; van Kooten, T.G.; Koopmans, S.A. Changes in lens stiffness due to capsular opacification in accommodative lens refilling. Exp. Eye Res. 2015, 134, 148–154. [Google Scholar] [CrossRef] [PubMed]
- De Iongh, R.U.; Lovicu, F.J.; Overbeek, P.A.; Schneider, M.D.; Joya, J.; Hardeman, E.D.; McAvoy, J.W. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development 2001, 128, 3995–4010. [Google Scholar] [PubMed]
- De Iongh, R.U.; Wederell, E.; Lovicu, F.J.; McAvoy, J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs 2005, 179, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Seomun, Y.; Hwang, K.H.; Kim, J.E.; Kim, I.S.; Kim, J.H.; Joo, C.K. Overexpression of the transforming growth factor-beta-inducible gene betaig-h3 in anterior polar cataracts. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1840–1845. [Google Scholar]
- Lovicu, F.J.; Schulz, M.W.; Hales, A.M.; Vincent, L.N.; Overbeek, P.A.; Chamberlain, C.G.; McAvoy, J.W. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br. J. Ophthalmol. 2002, 86, 220–226. [Google Scholar] [CrossRef]
- Saika, S.; Miyamoto, T.; Ishida, I.; Shirai, K.; Ohnishi, Y.; Ooshima, A.; McAvoy, J.W. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br. J. Ophthalmol. 2002, 86, 1428–1433. [Google Scholar] [CrossRef]
- Medvedovic, M.; Tomlinson, C.R.; Call, M.K.; Grogg, M.; Tsonis, P.A. Gene expression and discovery during lens regeneration in mouse: Regulation of epithelial to mesenchymal transition and lens differentiation. Mol. Vis. 2006, 12, 422–440. [Google Scholar]
- Border, W.A.; Noble, N.A.; Yamamoto, T.; Harper, J.R.; Yamaguchi, Y.; Pierschbacher, M.D.; Ruoslahti, E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 1992, 360, 361–364. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, S.; Shibata, T.; Kiyokawa, E.; Sasaki, H.; Singh, D.P. FGF2 antagonizes aberrant TGFbeta regulation of tropomyosin: Role for posterior capsule opacity. J. Cell. Mol. Med. 2017, 21, 916–928. [Google Scholar] [CrossRef]
- Mincione, G.; Esposito, D.L.; Di Marcantonio, M.C.; Piccirelli, A.; Cama, A.; Colletta, G. TGF-beta 1 modulation of IGF-I signaling pathway in rat thyroid epithelial cells. Exp. Cell Res. 2003, 287, 411–423. [Google Scholar] [CrossRef]
- Seomun, Y.; Kim, J.; Lee, E.H.; Joo, C.K. Overexpression of matrix metalloproteinase-2 mediates phenotypic transformation of lens epithelial cells. Biochem. J. 2001, 358, 41–48. [Google Scholar] [CrossRef]
- Chamberlain, C.G.; McAvoy, J.W. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1989, 1, 125–134. [Google Scholar] [CrossRef]
- McAvoy, J.W.; Chamberlain, C.G. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 1989, 107, 221–228. [Google Scholar]
- Nishi, O.; Nishi, K.; Fujiwara, T.; Shirasawa, E.; Ohmoto, Y. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. Br. J. Ophthalmol. 1996, 80, 63–68. [Google Scholar] [CrossRef]
- Kubo, E.; Hasanova, N.; Fatma, N.; Sasaki, H.; Singh, D.P. Elevated tropomyosin expression is associated with epithelial-mesenchymal transition of lens epithelial cells. J. Cell. Mol. Med. 2013, 17, 212–221. [Google Scholar] [CrossRef]
- Bhargavan, B.; Chhunchha, B.; Fatma, N.; Kubo, E.; Kumar, A.; Singh, D.P. Epigenetic repression of LEDGF during UVB exposure by recruitment of SUV39H1 and HDAC1 to the Sp1-responsive elements within LEDGF promoter CpG island. Epigenetics 2013, 8, 268–280. [Google Scholar] [CrossRef][Green Version]
- Maniatis, T.; Fritsch, E.F.; Sambrook, J. Molecular Cloning. A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1982. [Google Scholar]
- Fatma, N.; Kubo, E.; Sharma, P.; Beier, D.R.; Singh, D.P. Impaired homeostasis and phenotypic abnormalities in Prdx6-/-mice lens epithelial cells by reactive oxygen species: Increased expression and activation of TGFbeta. Cell Death Differ. 2005, 12, 734–750. [Google Scholar] [CrossRef]
- Kubo, E.; Fatma, N.; Akagi, Y.; Beier, D.R.; Singh, S.P.; Singh, D.P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol. Cell Physiol 2008, 294, C842–C855. [Google Scholar] [CrossRef]
- Kubo, E.; Singh, D.P.; Fatma, N.; Shinohara, T.; Zelenka, P.; Reddy, V.N.; Chylack, L.T. Cellular distribution of lens epithelium-derived growth factor (LEDGF) in the rat eye: Loss of LEDGF from nuclei of differentiating cells. Histochem. Cell Biol. 2003, 119, 289–299. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Rizo, C.M.; Overbeek, P.A.; Robinson, M.L. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1930–1939. [Google Scholar] [CrossRef]
- Kubo, E.; Hasanova, N.; Sasaki, H.; Singh, D.P. Dynamic and differential regulation in the microRNA expression in the developing and mature cataractous rat lens. J. Cell. Mol. Med. 2013, 17, 1146–1159. [Google Scholar] [CrossRef]
- Kubo, E.; Miyazawa, T.; Fatma, N.; Akagi, Y.; Singh, D.P. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech. Ageing Dev. 2006, 127, 249–256. [Google Scholar] [CrossRef]
- Shibata, T.; Shibata, S.; Ishigaki, Y.; Kiyokawa, E.; Ikawa, M.; Singh, D.P.; Sasaki, H.; Kubo, E. Tropomyosin 2 heterozygous knockout in mice using CRISPR-Cas9 system displays the inhibition of injury-induced epithelial-mesenchymal transition, and lens opacity. Mech. Ageing Dev. 2018, 171, 24–30. [Google Scholar] [CrossRef]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2004, 15, 124–129. [Google Scholar]
- Thylefors, B.; Chylack, L.T., Jr.; Konyama, K.; Sasaki, K.; Sperduto, R.; Taylor, H.R.; West, S.; The WHO Cataract Grading Group. A simplified cataract grading system. Ophthalmic Epidemiol. 2002, 9, 83–95. [Google Scholar] [CrossRef]
- Azuma, N.; Hara, T.; Hara, T. Extracellular matrix of opacified anterior capsule after endocapsular cataract surgery. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 531–536. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, T.; Singh, D.P.; Sasaki, H. Roles of TGF beta and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity. Int. J. Mol. Sci. 2018, 19, 3093. [Google Scholar] [CrossRef]
- Goldoni, S.; Humphries, A.; Nystrom, A.; Sattar, S.; Owens, R.T.; McQuillan, D.J.; Ireton, K.; Iozzo, R.V. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009, 185, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Iozzo, R.V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 1998, 67, 609–652. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.S.; Yenisey, C.; Rose, R.W.; Tootell, M.; Santra, M.; Iozzo, R.V. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene 2002, 21, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.M.; Rak, J.; Hung, M.C.; Rockwell, P.; Goldstein, N.; Fendly, B.; Kerbel, R.S. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: Angiogenic implications for signal transduction therapy of solid tumors. Am. J. Pathol. 1997, 151, 1523–1530. [Google Scholar] [PubMed]
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J. Biol. Chem. 1998, 273, 28116–28121. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.; Deakin, J.A.; Rahmoune, H.; Fernig, D.G.; Nakamura, T.; Gallagher, J.T. Hepatocyte growth factor/scatter factor binds with high affinity to dermatan sulfate. J. Biol. Chem. 1998, 273, 271–278. [Google Scholar] [CrossRef]
- Kurosaka, D.; Kato, K.; Nagamoto, T.; Negishi, K. Growth factors influence contractility and alpha-smooth muscle actin expression in bovine lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 1995, 36, 1701–1708. [Google Scholar]
- Meacock, W.R.; Spalton, D.J.; Stanford, M.R. Role of cytokines in the pathogenesis of posterior capsule opacification. Br. J. Ophthalmol. 2000, 84, 332–336. [Google Scholar] [CrossRef]
- Grisanti, S.; Szurman, P.; Warga, M.; Kaczmarek, R.; Ziemssen, F.; Tatar, O.; Bartz-Schmidt, K.U. Decorin modulates wound healing in experimental glaucoma filtration surgery: A pilot study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 191–196. [Google Scholar] [CrossRef]
- Hill, L.J.; Mead, B.; Blanch, R.J.; Ahmed, Z.; De Cogan, F.; Morgan-Warren, P.J.; Mohamed, S.; Leadbeater, W.; Scott, R.A.; Berry, M.; et al. Decorin Reduces Intraocular Pressure and Retinal Ganglion Cell Loss in Rodents Through Fibrolysis of the Scarred Trabecular Meshwork. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3743–3757. [Google Scholar] [CrossRef]
- Vial, C.; Gutierrez, J.; Santander, C.; Cabrera, D.; Brandan, E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J. Biol. Chem. 2011, 286, 24242–24252. [Google Scholar] [CrossRef]
- Davies, J.E.; Tang, X.; Denning, J.W.; Archibald, S.J.; Davies, S.J. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur. J. Neurosci. 2004, 19, 1226–1242. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Haylor, J.L.; El Nahas, A.M.; Johnson, T.S. Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int. 2007, 71, 755–763. [Google Scholar] [CrossRef]
- Nassar, K.; Luke, J.; Luke, M.; Kamal, M.; Abd El-Nabi, E.; Soliman, M.; Rohrbach, M.; Grisanti, S. The novel use of decorin in prevention of the development of proliferative vitreoretinopathy (PVR). Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1649–1660. [Google Scholar] [CrossRef]
- Isaka, Y.; Brees, D.K.; Ikegaya, K.; Kaneda, Y.; Imai, E.; Noble, N.A.; Border, W.A. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat. Med. 1996, 2, 418–423. [Google Scholar] [CrossRef]
- Davies, J.E.; Tang, X.; Bournat, J.C.; Davies, S.J. Decorin promotes plasminogen/plasmin expression within acute spinal cord injuries and by adult microglia in vitro. J. Neurotrauma 2006, 23, 397–408. [Google Scholar] [CrossRef]
- Fernandez, V.; Fragoso, M.A.; Billotte, C.; Lamar, P.; Orozco, M.A.; Dubovy, S.; Willcox, M.; Parel, J.M. Efficacy of various drugs in the prevention of posterior capsule opacification: Experimental study of rabbit eyes. J. Cataract Refract. Surg. 2004, 30, 2598–2605. [Google Scholar] [CrossRef]
- D’Antin, J.C.; Barraquer, R.I.; Tresserra, F.; Michael, R. Prevention of posterior capsule opacification through intracapsular hydrogen peroxide or distilled water treatment in human donor tissue. Sci. Rep. 2018, 8, 12739. [Google Scholar] [CrossRef]
- Boswell, B.A.; Korol, A.; West-Mays, J.A.; Musil, L.S. Dual function of TGFbeta in lens epithelial cell fate: Implications for secondary cataract. Mol. Biol. Cell 2017, 28, 907–921. [Google Scholar] [CrossRef]
- Wertheimer, C.; Siedlecki, J.; Kook, D.; Mayer, W.J.; Wolf, A.; Klingenstein, A.; Kampik, A.; Eibl-Lindner, K. EGFR inhibitor Gefitinib attenuates posterior capsule opacification in vitro and in the ex vivo human capsular bag model. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 409–417. [Google Scholar] [CrossRef] [PubMed]
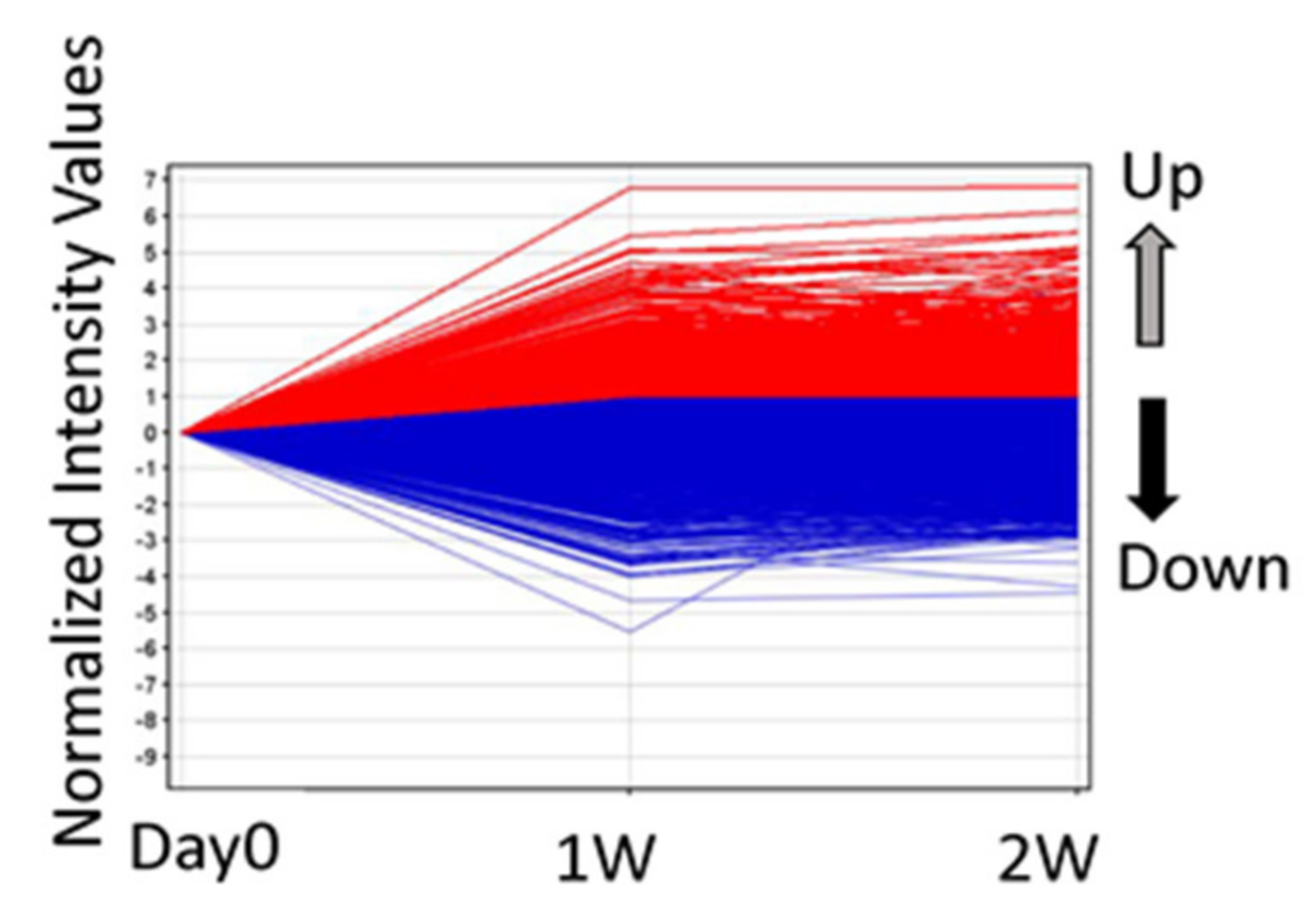




| Gene Symbol | Gene Description | Gene Symbol | Gene Description | ||
|---|---|---|---|---|---|
| 1 | Dcn | decorin | 16 | RT1-Da | RT1 class II, locus Da |
| 2 | Col5a2 | collagen, type V, alpha 2 | 17 | Cybb | cytochrome b-245, beta polypeptide |
| 3 | Fn1 | fibronectin 1 | 18 | Col6a3 | collagen, type VI, alpha 3 |
| 4 | Cyr61 | cysteine-rich, angiogenic inducer, 61 | 19 | Rnase4 | ribonuclease, RNase A family 4 |
| 5 | Col5a2 | collagen, type V, alpha 2 | 20 | Runx1 | runt-related transcription factor 1 |
| 6 | Col1a1 | collagen, type I, alpha 1 | 21 | Tpm2 | tropomyosin 2, beta |
| 7 | Mylk | myosin light chain kinase | 22 | Ptgs2 | prostaglandin-endoperoxide synthase 2 |
| 8 | Col3a1 | collagen, type III, alpha 1 | 23 | Tgfbi | transforming growth factor, beta-induced |
| 9 | Actg2 | actin, gamma 2, smooth muscle, enteric | 24 | Mgp | matrix Gla protein |
| 10 | Gbp2 | guanylate binding protein 2, interferon-inducible | 25 | Ednrb | endothelin receptor type B |
| 11 | Emp1 | epithelial membrane protein 1 | 26 | Lyz2 | lysozyme 2 |
| 12 | Gpnmb | glycoprotein (transmembrane) nmb | 27 | Car3 | carbonic anhydrase 3 |
| 13 | Cd74 | Cd74 molecule, major histocompatibility complex | 28 | Sfrp2 | secreted frizzled-related protein 2 |
| 14 | Dct | dopachrome tautomerase | 29 | Fcrls | Fc receptor-like S, scavenger receptor |
| 15 | Scn7a | sodium channel, voltage-gated, type VII, alpha | 30 | Ifitm1 | interferon-induced transmembrane protein 1 |
| GO Accession Number | GO Term |
|---|---|
| GO:0006952|GO:0002217|GO:0042829 | Defense response |
| GO:0009605 | Response to external stimulus |
| GO:0051707|GO:0009613|GO:0042828 | Response to other organisms |
| GO:0009607 | Response to biotic stimulus |
| GO:0050896|GO:0051869 | Response to stimulus |
| GO:0043207 | Response to external biotic stimulus |
| GO:0001944 | Vasculature development |
| GO:0031012 | Extracellular matrix |
| GO:0030334 | Regulation of cell migration |
| GO:0006950 | Response to stress |
| Gene Symbol | Gene Description | 1 W | 2 W |
|---|---|---|---|
| Crygb | crystallin, gamma B | 0.021 | 2.292 |
| Crygc | crystallin, gamma C | 0.139 | 4.448 |
| Crygd | crystallin, gamma D | 0.148 | 2.450 |
| Colq | collagen-like tail subunit (single strand of homotrimer) of asymmetric acetylcholinesterase | 0.300 | 2.324 |
| Bfsp1 | beaded filament structural protein 1, filensin | 0.321 | 11.611 |
| Slc24a2 | solute carrier family 24 (sodium/potassium/calcium exchanger), member 2 | 0.323 | 5.878 |
| Snhg11 | small nucleolar RNA host gene 11 (non-protein coding) | 0.380 | 6.667 |
| Slc46a3 | solute carrier family 46, member 3 | 0.423 | 2.121 |
| Anxa9 | annexin A9 | 0.466 | 2.296 |
| GO Accession | GO Term |
|---|---|
| GO:0005212 | Structural constituent of eye |
| GO:0002088 | Lens development in camera-type eye |
| GO:0007601 | Visual perception |
| GO:0070306 | Lens fiber cell differentiation |
| GO:0050953 | Sensory perception of light stimulus |
| GO:0005198 | Structural molecule activity |
| GO:0043010|GO:0001747|GO0031075 | Camera-type eye development |
| GO:0001654|GO:0042460 | Eye development |
| GO:0070307 | Lens fiber cell development |
| GO:0007600 | Sensory perception |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, S.; Shibata, N.; Ohtsuka, S.; Yoshitomi, Y.; Kiyokawa, E.; Yonekura, H.; Singh, D.P.; Sasaki, H.; Kubo, E. Role of Decorin in Posterior Capsule Opacification and Eye Lens Development. Cells 2021, 10, 863. https://doi.org/10.3390/cells10040863
Shibata S, Shibata N, Ohtsuka S, Yoshitomi Y, Kiyokawa E, Yonekura H, Singh DP, Sasaki H, Kubo E. Role of Decorin in Posterior Capsule Opacification and Eye Lens Development. Cells. 2021; 10(4):863. https://doi.org/10.3390/cells10040863
Chicago/Turabian StyleShibata, Shinsuke, Naoko Shibata, Satoshi Ohtsuka, Yasuo Yoshitomi, Etsuko Kiyokawa, Hideto Yonekura, Dhirendra P. Singh, Hiroshi Sasaki, and Eri Kubo. 2021. "Role of Decorin in Posterior Capsule Opacification and Eye Lens Development" Cells 10, no. 4: 863. https://doi.org/10.3390/cells10040863
APA StyleShibata, S., Shibata, N., Ohtsuka, S., Yoshitomi, Y., Kiyokawa, E., Yonekura, H., Singh, D. P., Sasaki, H., & Kubo, E. (2021). Role of Decorin in Posterior Capsule Opacification and Eye Lens Development. Cells, 10(4), 863. https://doi.org/10.3390/cells10040863








