Hyaluronic Acid as Macromolecular Crowder in Equine Adipose-Derived Stem Cell Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stem Cell Extraction, Expansion, Cryopreservation and Thawing
2.3. Cell Morphology Analysis
2.4. Stem Cell Characterisation Analysis
2.4.1. Osteogenic Analysis
2.4.2. Adipogenic Analysis
2.4.3. Chondrogenic Analysis
2.4.4. FACS Analysis
2.5. Solubility Analysis
2.6. Dynamic Light Scattering Analysis
2.7. Stem Cell Culture
2.8. Stem Cell Viability Analysis
2.9. Stem Cell Proliferation Analysis
2.10. Stem Cell Metabolic Activity Analysis
2.11. Gel Electrophoresis Analysis
2.12. Immunocytochemistry Analysis
2.13. Statistical Analysis
3. Results
3.1. Stem Cell Characterisation
3.2. HA Solubility Assessment
3.3. Dynamic Light Scattering Assessment
3.4. Cell Morphology Assessment
3.5. Cell Viability, Proliferation and Metabolic Activity Assessment
3.6. Gel Electrophoresis Assessment
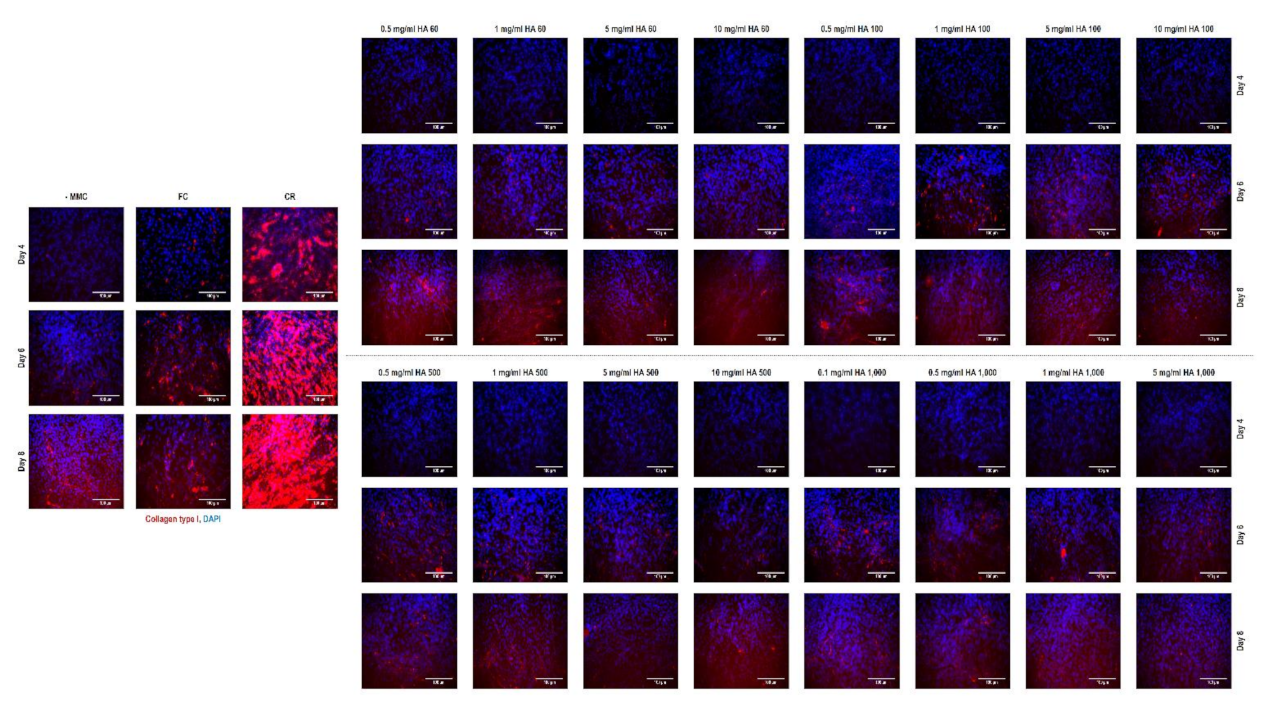
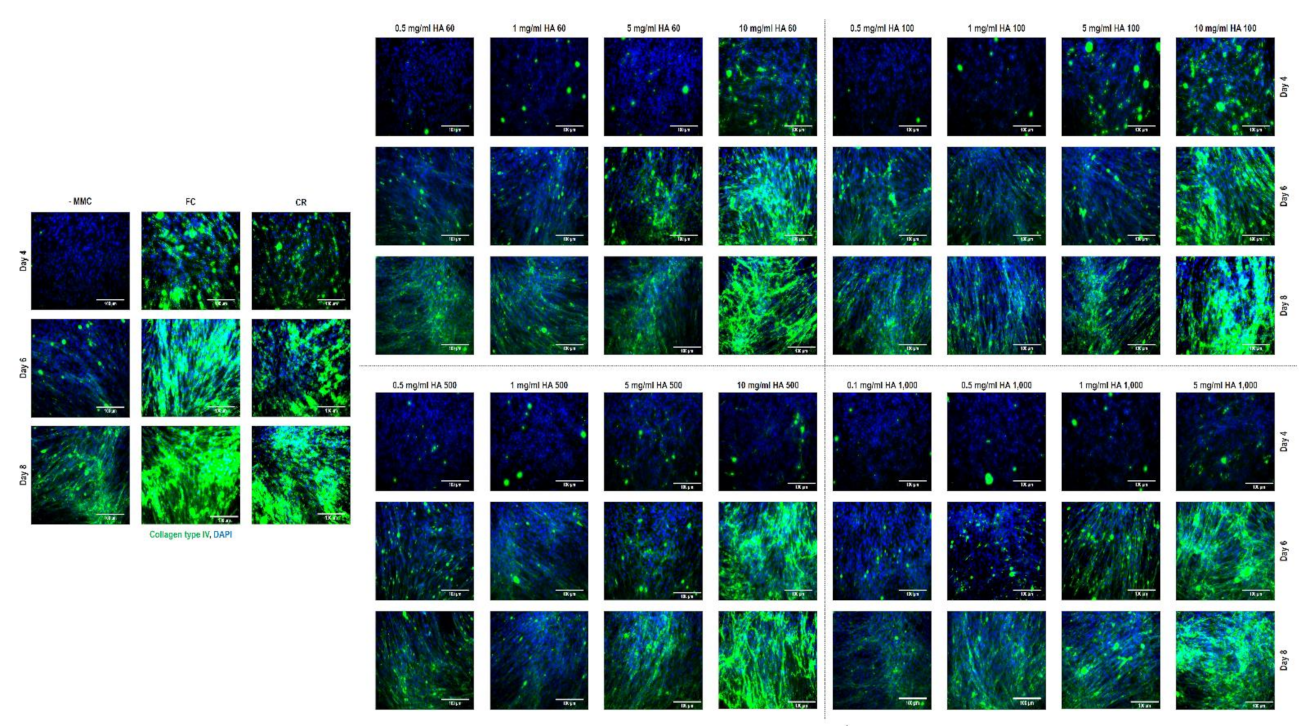
3.7. Immunocytochemistry Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e003918. [Google Scholar] [CrossRef]
- Sato, M.; Yamato, M.; Mitani, G.; Takagaki, T.; Hamahashi, K.; Nakamura, Y.; Ishihara, M.; Matoba, R.; Kobayashi, H.; Okano, T.; et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen Med. 2019, 4, 4. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.; Ahmado, A.; Vernon, A.; Daniels, J.; Nommiste, B.; Hasan, S.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Dusserre, N.; Konig, G.; Victor, B.; Keire, P.; Wight, T.; Chronos, N.; Kyles, A.; Gregory, C.; Hoyt, G.; et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006, 12, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Ten Ham, R.; Hövels, A.; Hoekman, J.; Frederix, G.; Leufkens, H.; Klungel, O.; Jedema, I.; Veld, S.; Nikolic, T.; Van Pel, M.; et al. What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy 2020, 22, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.; Langer, R.; Borenstein, J. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. Engl. 2009, 48, 5406–5415. [Google Scholar] [CrossRef]
- Nemir, S.; West, J. Synthetic materials in the study of cell response to substrate rigidity. Ann. Biomed. Eng. 2010, 38, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef]
- Delaine-Smith, R.; Reilly, G. The effects of mechanical loading on mesenchymal stem cell differentiation and matrix production. Vitam. Horm. 2011, 87, 417–480. [Google Scholar]
- Lee, D.; Kim, D.; Cho, J. Role of growth factors in hematopoietic stem cell niche. Cell Biol. Toxicol. 2020, 36, 131–144. [Google Scholar] [CrossRef]
- Satyam, A.; Kumar, P.; Fan, X.; Gorelov, A.; Rochev, Y.; Joshi, L.; Peinado, H.; Lyden, D.; Thomas, B.; Rodriguez, B.; et al. Macromolecular crowding meets tissue engineering by self-assembly: A paradigm shift in regenerative medicine. Adv. Mater. 2014, 26, 3024–3034. [Google Scholar] [CrossRef]
- Gaspar, D.; Fuller, K.P.; Zeugolis, D.I. Polydispersity and negative charge are key modulators of extracellular matrix deposition under macromolecular crowding conditions. Acta Biomater. 2019, 88, 197–210. [Google Scholar] [CrossRef]
- De Pieri, A.; Rana, S.; Korntner, S.; Zeugolis, D. Seaweed polysaccharides as macromolecular crowding agents. Int. J. Biol. Macromol. 2020, 164, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, M.; Njieha, F.; Prockop, D. Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J. Biol. Chem. 1982, 257, 8442–8448. [Google Scholar] [CrossRef]
- Zeugolis, D. Bioinspired in vitro microenvironments to control cell fate: Focus on macromolecular crowding. Am. J. Physiol. Cell Physiol. 2021, submitted. [Google Scholar]
- Kuznetsova, I.; Zaslavsky, B.; Breydo, L.; Turoverov, K.; Uversky, V. Beyond the excluded volume effects: Mechanistic complexity of the crowded milieu. Molecules 2015, 20, 1377–1409. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Skolnick, J. Crowding and hydrodynamic interactions likely dominate in vivo macromolecular motion. Proc. Natl. Acad. Sci. USA 2010, 107, 18457–18462. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, D.; Sugimoto, N. Molecular crowding effects on structure and stability of DNA. Biochimie 2008, 90, 1040–1051. [Google Scholar] [CrossRef]
- Ramisetty, S.; Langlete, P.; Lale, R.; Dias, R. In vitro studies of DNA condensation by bridging protein in a crowding environment. Int. J. Biol. Macromol. 2017, 103, 845–853. [Google Scholar] [CrossRef]
- Akabayov, B.; Akabayov, S.; Lee, S.; Wagner, G.; Richardson, C. Impact of macromolecular crowding on DNA replication. Nat. Commun. 2013, 4, 1615. [Google Scholar] [CrossRef]
- Chung, S.; Lerner, E.; Jin, Y.; Kim, S.; Alhadid, Y.; Grimaud, L.; Zhang, I.; Knobler, C.; Gelbart, W.; Weiss, S. The effect of macromolecular crowding on single-round transcription by Escherichia coli RNA polymerase. Nucleic Acids Res. 2019, 47, 1440–1450. [Google Scholar] [CrossRef]
- Wirth, A.; Gruebele, M. Quinary protein structure and the consequences of crowding in living cells: Leaving the test-tube behind. Bioessays 2013, 35, 984–993. [Google Scholar] [CrossRef]
- Christiansen, A.; Wang, Q.; Cheung, M.; Wittung-Stafshede, P. Effects of macromolecular crowding agents on protein folding in vitro and in silico. Biophys. Rev. 2013, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Guseman, A.; Speer, S.; Perez Goncalves, G.; Pielak, G. Surface charge modulates protein-protein interactions in physiologically relevant environments. Biochemistry 2018, 57, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Toan, N.; Hyeon, C.; Thirumalai, D. Unexpected swelling of stiff DNA in a polydisperse crowded environment. J. Am. Chem. Soc. 2015, 137, 10970–10978. [Google Scholar] [CrossRef] [PubMed]
- Sharp, K.A. Analysis of the size dependence of macromolecular crowding shows that smaller is better. Proc. Natl. Acad. Sci. USA 2015, 112, 7990–7995. [Google Scholar] [CrossRef] [PubMed]
- Skóra, T.; Vaghefikia, F.; Fitter, J.; Kondrat, S. Macromolecular crowding: How shape and interactions affect diffusion. J. Phys. Chem. B 2020, 124, 7537–7543. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, S.; Siggel, M.; Linke, M.; Hummer, G. Dynamic cluster formation determines viscosity and diffusion in dense protein solutions. Proc. Natl. Acad. Sci. USA 2019, 116, 9843–9852. [Google Scholar] [CrossRef]
- Kumar, P.; Satyam, A.; Fan, X.; Collin, E.; Rochev, Y.; Rodriguez, B.J.; Gorelov, A.; Dillon, S.; Joshi, L.; Raghunath, M.; et al. Macromolecularly crowded in vitro microenvironments accelerate the production of extracellular matrix-rich supramolecular assemblies. Sci. Rep. 2015, 5, 8729. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Lim, N.; Chee, S.; Png, S.; Wohland, T.; Raghunath, M. Novel use for polyvinylpyrrolidone as a macromolecular crowder for enhanced extracellular matrix deposition and cell proliferation. Tissue Eng. Part C Methods 2014, 20, 994–1002. [Google Scholar] [CrossRef]
- McKim, J.M.; Willoughby, J.A.; Blakemore, W.R.; Weiner, M.L. A critical review of “A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity (Nutr. Healthy Aging. 2017, 4, 181–192).”. J. Nutr. Health Aging 2019, 5, 149–158. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Shendi, D.; Marzi, J.; Linthicum, W.; Rickards, A.J.; Dolivo, D.M.; Keller, S.; Kauss, M.A.; Wen, Q.; McDevitt, T.C.; Dominko, T.; et al. Hyaluronic acid as a macromolecular crowding agent for production of cell-derived matrices. Acta Biomater. 2019, 100, 292–305. [Google Scholar] [CrossRef]
- Majewski, G.; Rodan, K.; Fields, K.; Falla, T. Characterization of bound water in skin hydrators prepared with and without a 3D3P interpenetrating polymer network. Skin Res. Technol. 2019, 25, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K. Hyaluronan and hyaluronan fragments. Adv. Carbohydr. Chem. Biochem. 2017, 74, 1–59. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Detection of Mycoplasma contamination in cell cultures. Curr. Protoc. Mol. Biol. 2014, 106, 1–14. [Google Scholar] [CrossRef]
- Ranera, B.; Remacha, A.R.; Alvarez-Arguedas, S.; Romero, A.; Vazquez, F.J.; Zaragoza, P.; Martin-Burriel, I.; Rodellar, C. Effect of hypoxia on equine mesenchymal stem cells derived from bone marrow and adipose tissue. BMC Vet. Res. 2012, 8, 142. [Google Scholar] [CrossRef]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vazquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martin-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef]
- Maia, L.; Landim-Alvarenga, F.C.; Da Mota, L.S.; De Assis Golim, M.; Laufer-Amorim, R.; De Vita, B.; Barberini, D.J.; Listoni, A.J.; De Moraes, C.N.; Heckler, M.C.; et al. Immunophenotypic, immunocytochemistry, ultrastructural, and cytogenetic characterization of mesenchymal stem cells from equine bone marrow. Microsc. Res. Tech. 2013, 76, 618–624. [Google Scholar] [CrossRef]
- Barberini, D.J.; Freitas, N.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; da Cruz Landim-Alvarenga, F.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 25. [Google Scholar] [CrossRef]
- Fulber, J.; Maria, D.A.; da Silva, L.C.; Massoco, C.O.; Agreste, F.; Baccarin, R.Y. Comparative study of equine mesenchymal stem cells from healthy and injured synovial tissues: An in vitro assessment. Stem Cell Res. Ther. 2016, 7, 35. [Google Scholar] [CrossRef]
- Shikh Alsook, M.K.; Gabriel, A.; Piret, J.; Waroux, O.; Tonus, C.; Connan, D.; Baise, E.; Antoine, N. Tissues from equine cadaver ligaments up to 72 hours of post-mortem: A promising reservoir of stem cells. Stem Cell Res. Ther 2015, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Loe, F.; Blocki, A.; Peng, Y.; Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Deliv. Rev. 2011, 63, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Capella-Monsonis, H.; Coentro, J.Q.; Graceffa, V.; Wu, Z.; Zeugolis, D.I. An experimental toolbox for characterization of mammalian collagen type I in biological specimens. Nat. Protoc. 2018, 13, 507–529. [Google Scholar] [CrossRef] [PubMed]
- Cigognini, D.; Gaspar, D.; Kumar, P.; Satyam, A.; Alagesan, S.; Sanz-Nogues, C.; Griffin, M.; O’Brien, T.; Pandit, A.; Zeugolis, D.I. Macromolecular crowding meets oxygen tension in human mesenchymal stem cell culture—A step closer to physiologically relevant in vitro organogenesis. Sci. Rep. 2016, 6, 30746. [Google Scholar] [CrossRef]
- Chen, C.Z.; Peng, Y.X.; Wang, Z.B.; Fish, P.V.; Kaar, J.L.; Koepsel, R.R.; Russell, A.J.; Lareu, R.R.; Raghunath, M. The Scar-in-a-Jar: Studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br. J. Pharmacol. 2009, 158, 1196–1209. [Google Scholar] [CrossRef]
- Prewitz, M.C.; Stißel, A.; Friedrichs, J.; Träber, N.; Vogler, S.; Bornhäuser, M.; Werner, C. Extracellular matrix deposition of bone marrow stroma enhanced by macromolecular crowding. Biomaterials 2015, 73, 60–69. [Google Scholar] [CrossRef]
- Graceffa, V.; Zeugolis, D.I. Macromolecular crowding as a means to assess the effectiveness of chondrogenic media. J. Tissue Eng. Regen Med. 2019, 13, 217–231. [Google Scholar] [CrossRef]
- FDA. Food Additives Permitted for Direct Addition to Food for Human Consumption. Section 172.620: Carrageenan; US Food & Drug Administration: Washingtong, DC, USA, 2019.
- Papalia, R.; Russo, F.; Torre, G.; Albo, E.; Grimaldi, V.; Papalia, G.; Sterzi, S.; Vadalà, G.; Bressi, F.; Denaro, V. Hybrid hyaluronic acid versus high molecular weight hyaluronic acid for the treatment of osteoarthritis in obese patients. J. Biol. Regul Homeost. Agents 2017, 31, 103–109. [Google Scholar]
- Awartani, F.; Tatakis, D. Interdental papilla loss: Treatment by hyaluronic acid gel injection: A case series. Clin. Oral Investig. 2016, 20, 1775–1780. [Google Scholar] [CrossRef]
- Cavallini, M.; Papagni, M.; Ryder, T.; Patalano, M. Skin quality improvement with VYC-12, a new injectable hyaluronic acid: Objective results using digital analysis. Dermatol. Surg. 2019, 45, 1598–1604. [Google Scholar] [CrossRef]
- Wang, F.; Garza, L.A.; Kang, S.; Varani, J.; Orringer, J.S.; Fisher, G.J.; Voorhees, J.J. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch. Dermatol. 2007, 143, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; El Haj, A.; Alini, M.; Stoddart, M. Sodium hyaluronate supplemented culture media as a new hMSC chondrogenic differentiation media-model for in vitro/ex vivo screening of potential cartilage repair therapies. Front. Bioeng. Biotechnol. 2020, 8, 243. [Google Scholar] [CrossRef]
- Gallorini, M.; Berardi, A.C.; Berardocco, M.; Gissi, C.; Maffulli, N.; Cataldi, A.; Oliva, F. Hyaluronic acid increases tendon derived cell viability and proliferation in vitro: Comparative study of two different hyaluronic acid preparations by molecular weight. Muscles Ligaments Tendons J. 2017, 7, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Osti, L.; Berardocco, M.; di Giacomo, V.; Di Bernardo, G.; Oliva, F.; Berardi, A. Hyaluronic acid increases tendon derived cell viability and collagen type I expression in vitro: Comparative study of four different hyaluronic acid preparations by molecular weight. BMC Musculoskelet Disord. 2015, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Bernard, G.W. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res. 1998, 294, 323–333. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Palka, J.A.; Jarzabek, K.; Wolczynski, S. Hyaluronic acid counteracts interleukin-1-induced inhibition of collagen biosynthesis in cultured human chondrocytes. Pharmacol. Res. 2006, 54, 275–281. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Y.Y.; Koo, P.L.; Lee, K.M.; Qin, L.; Cheng, J.C.; Kumta, S.M. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mater. Res. A 2003, 66, 880–884. [Google Scholar] [CrossRef]
- Hegewald, A.A.; Ringe, J.; Bartel, J.; Kruger, I.; Notter, M.; Barnewitz, D.; Kaps, C.; Sittinger, M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: A preliminary study. Tissue Cell 2004, 36, 431–438. [Google Scholar] [CrossRef]
- Zou, L.; Zou, X.; Chen, L.; Li, H.; Mygind, T.; Kassem, M.; Bunger, C. Effect of hyaluronan on osteogenic differentiation of porcine bone marrow stromal cells in vitro. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 713–720. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Zhu, Y.; Potschka, M.; Dubin, P.; Cai, C.H. A method for the quantitation of charge by size exclusion chromatography demonstrated with components of ficoll 400. Macromol. Chem. Phys. 2001, 202, 61–72. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.; Trotter, J.; Perminova, A.; Donnelly, R. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic acid: The influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Hascall, V.; Esko, J. Hyaluronan. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R., Esko, J., Stanley, P., Hart, G., Aebi, M., Darvill, A., Kinoshita, T., Packer, N., Prestegard, J., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release Off. J. Control. Release Soc. 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Panchal, J.; Kotarek, J.; Marszal, E.; Topp, E.M. Analyzing subvisible particles in protein drug products: A comparison of dynamic light scattering (DLS) and resonant mass measurement (RMM). AAPS J. 2014, 16, 440–451. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Chapter 11—Characterization of nanomaterials: Tools and challenges. In Nanomaterials for Food Applications; López Rubio, A., Fabra Rovira, M.J., Martínez Sanz, M., Gómez-Mascaraque, L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar] [CrossRef]
- Lareu, R.R.; Subramhanya, K.H.; Peng, Y.; Benny, P.; Chen, C.; Wang, Z.; Rajagopalan, R.; Raghunath, M. Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: The biological relevance of the excluded volume effect. FEBS Lett. 2007, 581, 2709–2714. [Google Scholar] [CrossRef]
- Shahid, S.; Hassan, M.; Islam, A.; Ahmad, F. Size-dependent studies of macromolecular crowding on the thermodynamic stability, structure and functional activity of proteins: In vitro and in silico approaches. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Ryan, C.N.M.; Zeugolis, D.I. Multifactorial bottom-up bioengineering approaches for the development of living tissue substitutes. FASEB J. 2019, 33, 5741–5754. [Google Scholar] [CrossRef]
- Graceffa, V.; Zeugolis, D.I. Carrageenan enhances chondrogenesis and osteogenesis in human bone marrow stem cell culture. Eur. Cell Mater. 2019, 37, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, A.S.; Loe, F.C.; Li, R.; Raghunath, M.; Van Vliet, K.J. Macromolecular crowding directs extracellular matrix organization and mesenchymal stem cell behavior. PLoS ONE 2012, 7, e37904. [Google Scholar] [CrossRef] [PubMed]
- Patrikoski, M.; Lee, M.H.C.; Mäkinen, L.; Ang, X.M.; Mannerström, B.; Raghunath, M.; Miettinen, S. Effects of macromolecular crowding on human adipose stem cell culture in fetal bovine serum, human serum, and defined xeno-free/serum-free conditions. Stem Cells Int. 2017, 2017, 6909163. [Google Scholar] [CrossRef]
- Kumar, P.; Satyam, A.; Fan, X.; Rochev, Y.; Rodriguez, B.J.; Gorelov, A.; Joshi, L.; Raghunath, M.; Pandit, A.; Zeugolis, D.I. Accelerated development of supramolecular corneal stromal-like assemblies from corneal fibroblasts in the presence of macromolecular crowders. Tissue Eng. Part C Methods 2015, 21, 660–670. [Google Scholar] [CrossRef]
- Tsiapalis, D.; De Pieri, A.; Spanoudes, K.; Sallent, I.; Kearns, S.; Kelly, J.L.; Raghunath, M.; Zeugolis, D.I. The synergistic effect of low oxygen tension and macromolecular crowding in the development of extracellular matrix-rich tendon equivalents. Biofabrication 2020, 12, 025018. [Google Scholar] [CrossRef]
- Moreno, A.; Martínez, A.; Olmedillas, S.; Bello, S.; de Miguel, F. Hyaluronic acid effect on adipose-derived stem cells. Biological in vitro evaluation. Rev. Esp. Cirugía Ortop. Traumatol. 2015, 59, 215–221. [Google Scholar] [CrossRef]
- Kawasaki, K.; Ochi, M.; Uchio, Y.; Adachi, N.; Matsusaki, M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J. Cell Physiol. 1999, 179, 142–148. [Google Scholar] [CrossRef]
- Mast, B.A.; Diegelmann, R.F.; Krummel, T.M.; Cohen, I.K. Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix 1993, 13, 441–446. [Google Scholar] [CrossRef]
- Goldberg, R.L.; Toole, B.P. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987, 30, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Wiig, M.; Abrahamsson, S.O.; Lundborg, G. Effects of hyaluronan on cell proliferation and collagen synthesis: A study of rabbit flexor tendons in vitro. J. Hand. Surg. Am. 1996, 21, 599–604. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Velez, A.; Howard, M. Collagen IV in normal skin and in pathological processes. N. Am. J. Med. Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Raghunath, M.; Vogel, V. Fibrillar fibronectin plays a key role as nucleator of collagen I polymerization during macromolecular crowding-enhanced matrix assembly. Biomater. Sci. 2019, 7, 4519–4535. [Google Scholar] [CrossRef]
- Lee, C.; Bird, S.; Shaw, M.; Jean, L.; Vaux, D. Combined effects of agitation, macromolecular crowding, and interfaces on amyloidogenesis. J. Biol. Chem. 2012, 287, 38006–38019. [Google Scholar] [CrossRef]
- Chen, E.; Kliger, D. Time-resolved linear dichroism measurements of carbonmonoxy myoglobin as a probe of the microviscosity in crowded environments. J. Phys. Chem. B 2017, 121, 7064–7074. [Google Scholar] [CrossRef]
- Damodarasamy, M.; Johnson, R.S.; Bentov, I.; MacCoss, M.J.; Vernon, R.B.; Reed, M.J. Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair Regen 2014, 22, 521–526. [Google Scholar] [CrossRef] [PubMed]
- David-Raoudi, M.; Tranchepain, F.; Deschrevel, B.; Vincent, J.C.; Bogdanowicz, P.; Boumediene, K.; Pujol, J.P. Differential effects of hyaluronan and its fragments on fibroblasts: Relation to wound healing. Wound Repair Regen 2008, 16, 274–287. [Google Scholar] [CrossRef]
- Donejko, M.; Przylipiak, A.; Rysiak, E.; Głuszuk, K.; Surażyński, A. Influence of caffeine and hyaluronic acid on collagen biosynthesis in human skin fibroblasts. Drug Des. Devel. Ther. 2014, 8, 1923–1928. [Google Scholar] [PubMed]
- Croce, M.; Dyne, K.; Boraldi, F.; Quaglino, D.; Cetta, G.; Tiozzo, R.; Pasquali Ronchetti, I. Hyaluronan affects protein and collagen synthesis by in vitro human skin fibroblasts. Tissue Cell 2001, 33, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Satyam, A.; Kumar, P.; Cigognini, D.; Pandit, A.; Zeugolis, D.I. Low, but not too low, oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human dermal fibroblast culture. Acta Biomater. 2016, 44, 221–231. [Google Scholar] [CrossRef]
- Tsiapalis, D.; Kearns, S.; Kelly, J.L.; Zeugolis, D.I. Growth factor and macromolecular crowding supplementation in human tenocyte culture. Biomater. Biosyst. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Huang, L.; Gu, H.; Burd, A. A reappraisal of the biological effects of hyaluronan on human dermal fibroblast. J. Biomed. Mater. Res. A 2009, 90, 1177–1185. [Google Scholar] [CrossRef]
- Ang, X.M.; Lee, M.H.; Blocki, A.; Chen, C.; Ong, L.L.; Asada, H.H.; Sheppard, A.; Raghunath, M. Macromolecular crowding amplifies adipogenesis of human bone marrow-derived mesenchymal stem cells by enhancing the pro-adipogenic microenvironment. Tissue Eng. Part A 2014, 20, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Goralczyk, A.G.; Kriszt, R.; Ang, X.M.; Badowski, C.; Li, Y.; Summers, S.A.; Toh, S.A.; Yassin, M.S.; Shabbir, A.; et al. ECM microenvironment unlocks brown adipogenic potential of adult human bone marrow-derived MSCs. Sci. Rep. 2016, 6, 21173. [Google Scholar] [CrossRef]
- Obrink, B. A study of the interactions between monomeric tropocollagen and glycosaminoglycans. Eur. J. Biochem. 1973, 33, 387–400. [Google Scholar] [CrossRef]
- Obrink, B. The influence of glycosaminoglycans on the formation of fibers from monomeric tropocollagen in vitro. Eur. J. Biochem. 1973, 34, 129–137. [Google Scholar] [CrossRef] [PubMed]


| Polymer (Concentration) | Zeta Potential (mV) | Hydrodynamic Radius (nm) | Polydispersity Index | Fractional Volume Occupancy (%) |
|---|---|---|---|---|
| FC 70 (25 mg/mL) + FC 400 (37.5 mg/mL) | −2.2 ± 1.1 # | 15 ± 1 # | 0.77 ± 0.11 | (111 ± 13) |
| CR (75 µg/mL) | −26.5 ± 6.7 * | 140 ± 29 | 0.68 ± 0.11 | (1019 ± 570) |
| HA 60 (0.5 mg/mL) | −11.8 ± 1.9 | 246 ± 68 | 0.88 ± 0.12 | (36,140 ± 29,701) |
| HA 60 (1 mg/mL) | −10.8 ± 1.6 | 302 ± 60 | 0.89 ± 0.12 | (124,894 ± 61,980) |
| HA 60 (5 mg/mL) | −13.3 ± 0.8 | 249 ± 133 | 0.87 ± 0.11 | (516,151 ± 631,032) |
| HA 60 (10 mg/mL) | −13.1 ± 2.7 | 262 ± 108 | 0.86 ± 0.12 | (995,364 ± 771,048 +) |
| HA 100 (0.5 mg/mL) | −2.6 ± 2.3 # | 85 ± 10 | 0.51 ± 0.06 | (784 ± 264) |
| HA 100 (1 mg/mL) | −12.8 ± 1.6 | 194 ± 113 | 0.68 ± 0.37 | (5448 ± 116) |
| HA 100 (5 mg/mL) | −16.0 ± 4.9 | 570 ± 17 | 0.60 ± 0.11 | (2,341,046 ± 211,149) |
| HA 100 (10 mg/mL) | −23.2 ± 2.1 *, + | 1886 ± 123 *, + | 0.35 ± 0.02 # | (170,651,055 ± 33,934,948 *, +) |
| HA 500 (0.5 mg/mL) | −3.7 ± 0.7 # | 59 ± 15 | 0.86 ± 0.12 | 59 ± 37 |
| HA 500 (1 mg/mL) | −8.8 ± 2.6 | 46 ± 8 | 0.92 ± 0.08 | 51 ± 26 |
| HA 500 (5 mg/mL) | −18.0 ± 3.1 | 213 ± 36 | 1.00 ± 0.00 | (25,933 ± 12,345) |
| HA 500 (10 mg/mL) | −25.8 ± 3.3 *, + | 604 ± 68 + | 1.00 ± 0.00 | (1,140,733 ± 384,037 +) |
| HA 1000 (0.1 mg/mL) | −7.1 ± 4.0 | 70 ± 24 | 0.97 ± 0.03 | 11 ± 8 # |
| HA 1000 (0.5 mg/mL) | −6.9 ± 4.2 | 99 ± 53 | 0.96 ± 0.08 | (193 ± 226) |
| HA 1000 (1 mg/mL) | −9.0 ± 2.5 | 95 ± 29 | 0.78 ± 0.18 | (256 ± 204) |
| HA 1000 (5 mg/mL) | −12.4 ± 1.5 | 133 ± 40 | 0.64 ± 0.09 | (3484 ± 2294 +) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnica-Galvez, S.; Korntner, S.H.; Skoufos, I.; Tzora, A.; Diakakis, N.; Prassinos, N.; Zeugolis, D.I. Hyaluronic Acid as Macromolecular Crowder in Equine Adipose-Derived Stem Cell Cultures. Cells 2021, 10, 859. https://doi.org/10.3390/cells10040859
Garnica-Galvez S, Korntner SH, Skoufos I, Tzora A, Diakakis N, Prassinos N, Zeugolis DI. Hyaluronic Acid as Macromolecular Crowder in Equine Adipose-Derived Stem Cell Cultures. Cells. 2021; 10(4):859. https://doi.org/10.3390/cells10040859
Chicago/Turabian StyleGarnica-Galvez, Sergio, Stefanie H. Korntner, Ioannis Skoufos, Athina Tzora, Nikolaos Diakakis, Nikitas Prassinos, and Dimitrios I. Zeugolis. 2021. "Hyaluronic Acid as Macromolecular Crowder in Equine Adipose-Derived Stem Cell Cultures" Cells 10, no. 4: 859. https://doi.org/10.3390/cells10040859
APA StyleGarnica-Galvez, S., Korntner, S. H., Skoufos, I., Tzora, A., Diakakis, N., Prassinos, N., & Zeugolis, D. I. (2021). Hyaluronic Acid as Macromolecular Crowder in Equine Adipose-Derived Stem Cell Cultures. Cells, 10(4), 859. https://doi.org/10.3390/cells10040859








