A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Phenotypic Characterization
2.1.1. Culture Growth
2.1.2. Microbial Characterization
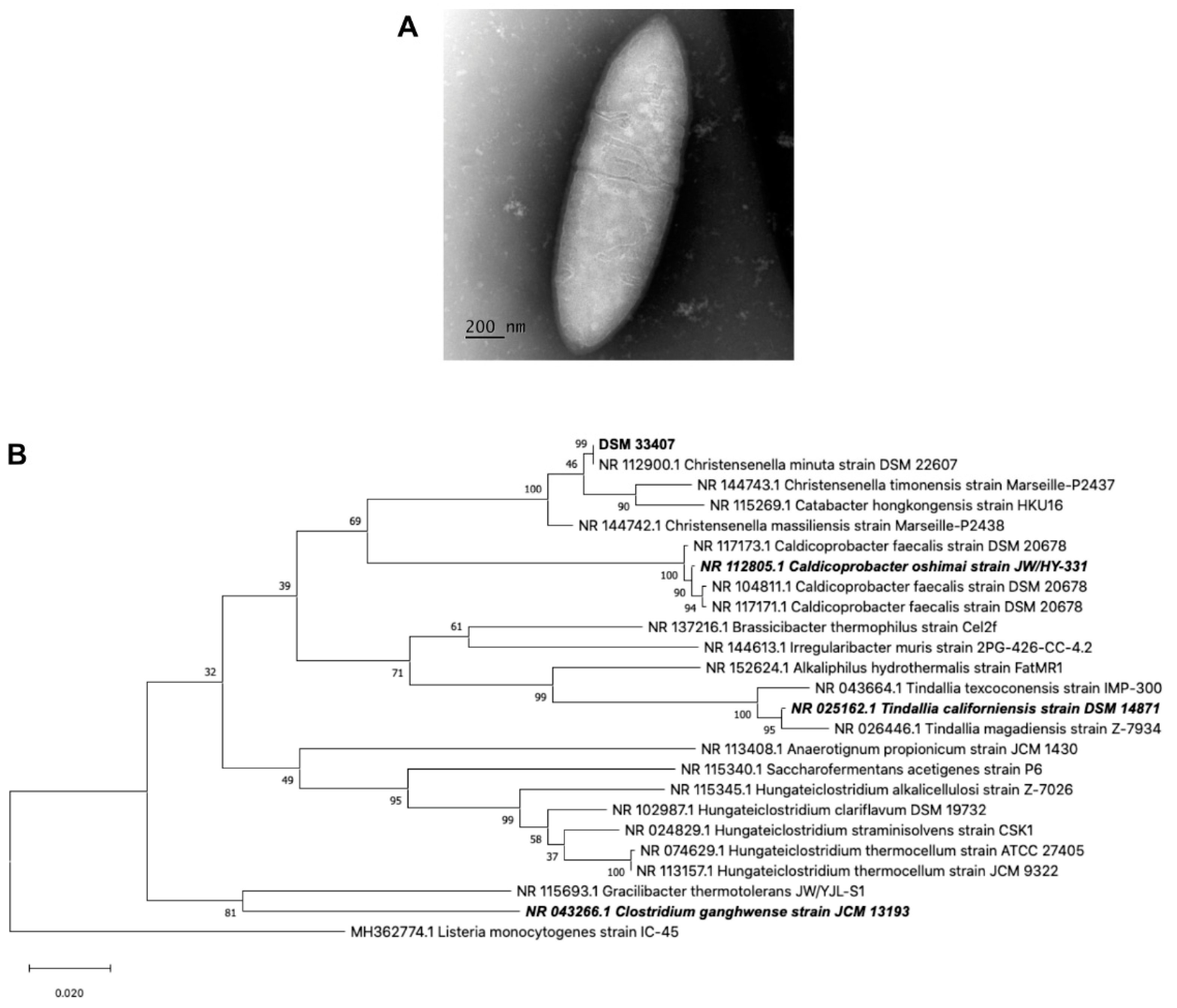
2.1.3. 16S Genotyping and Phylogenetic Analysis
2.2. Animal Assays
2.3. Triple SHIME® Model
2.4. Shotgun Metagenome Sequencing
2.4.1. DNA Extraction Procedure
2.4.2. DNA Library Preparation
2.4.3. Sequencing on Illumina HiSeq4000
2.4.4. Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Strain Isolation and Characterization
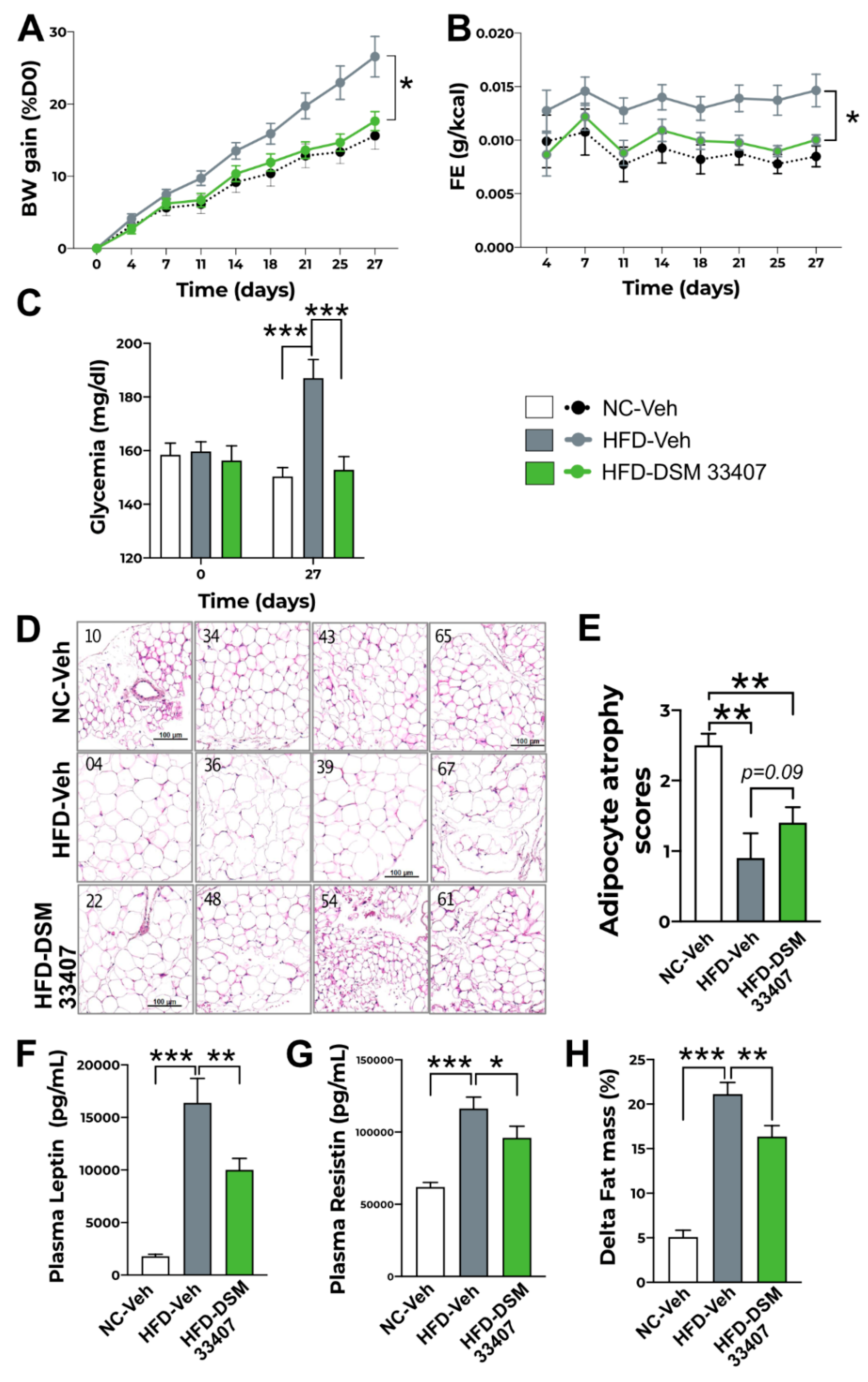
3.2. DSM33407 Protects from Diet-Induced Obesity and Regulates Associated Metabolic Markers
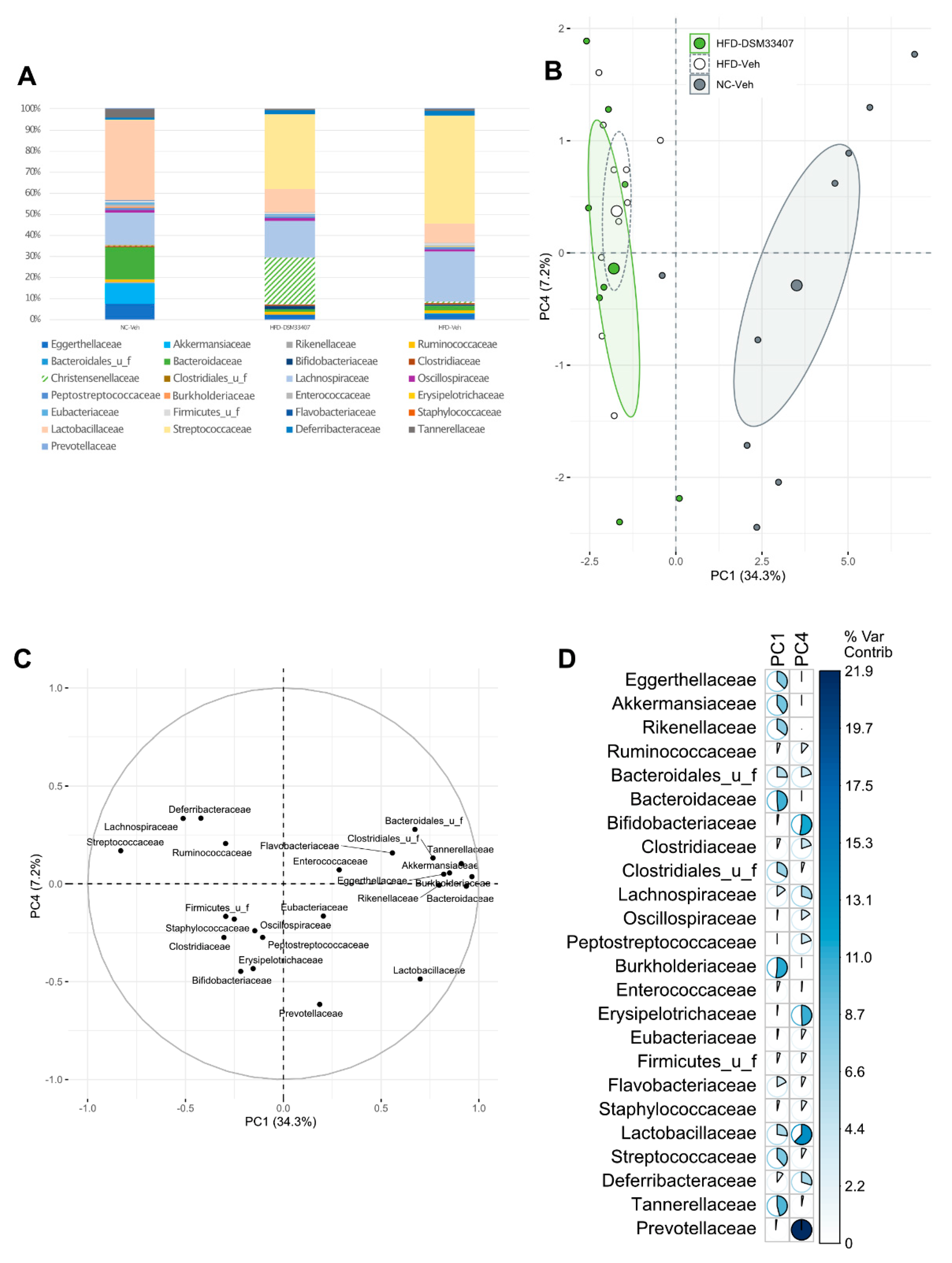
3.3. DSM33407 Modulates the Gut Microbiota and Their Metabolic Activity
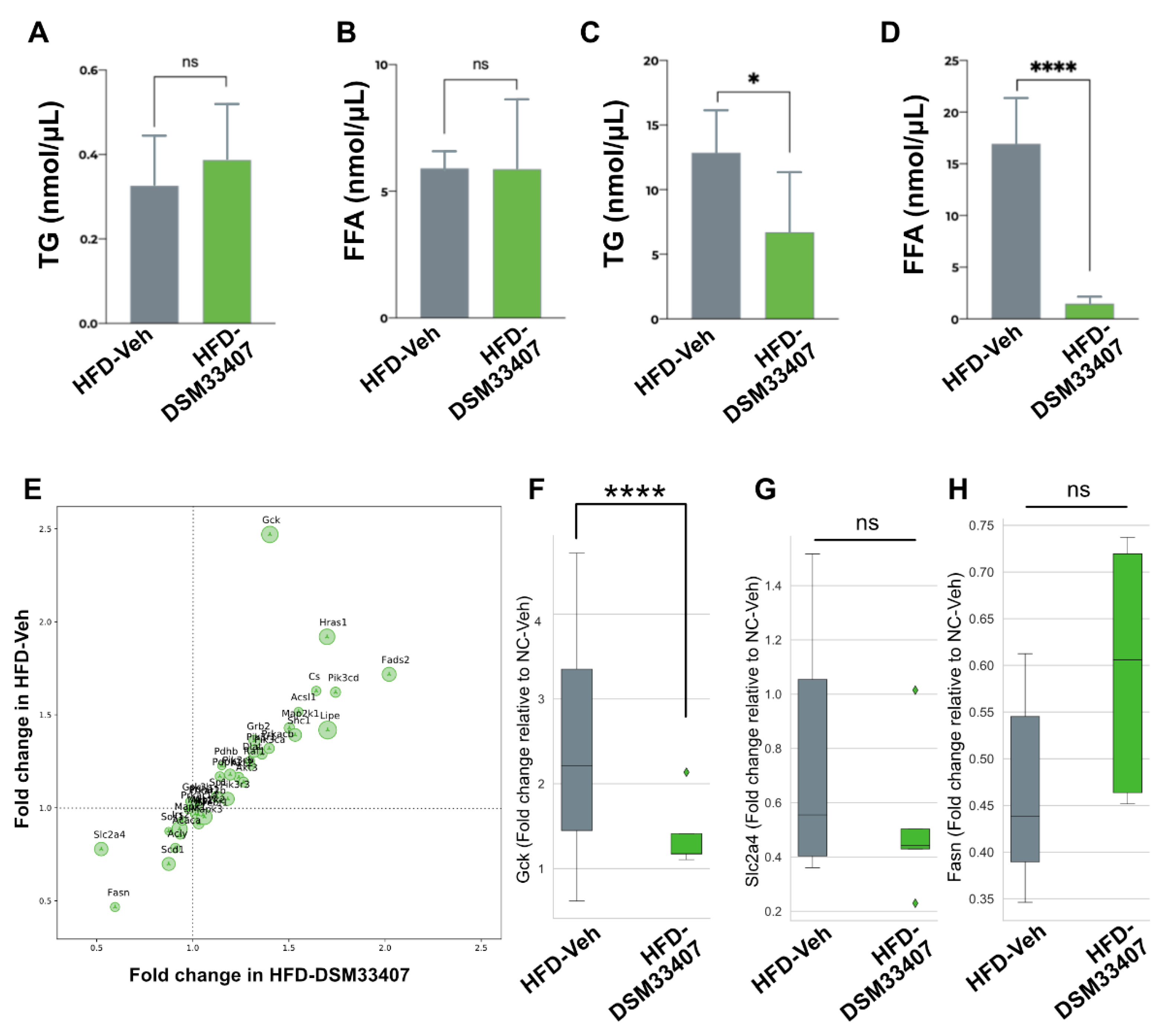
3.4. DSM33407 Associates with Modulations of Hepatic Lipid Metabolism
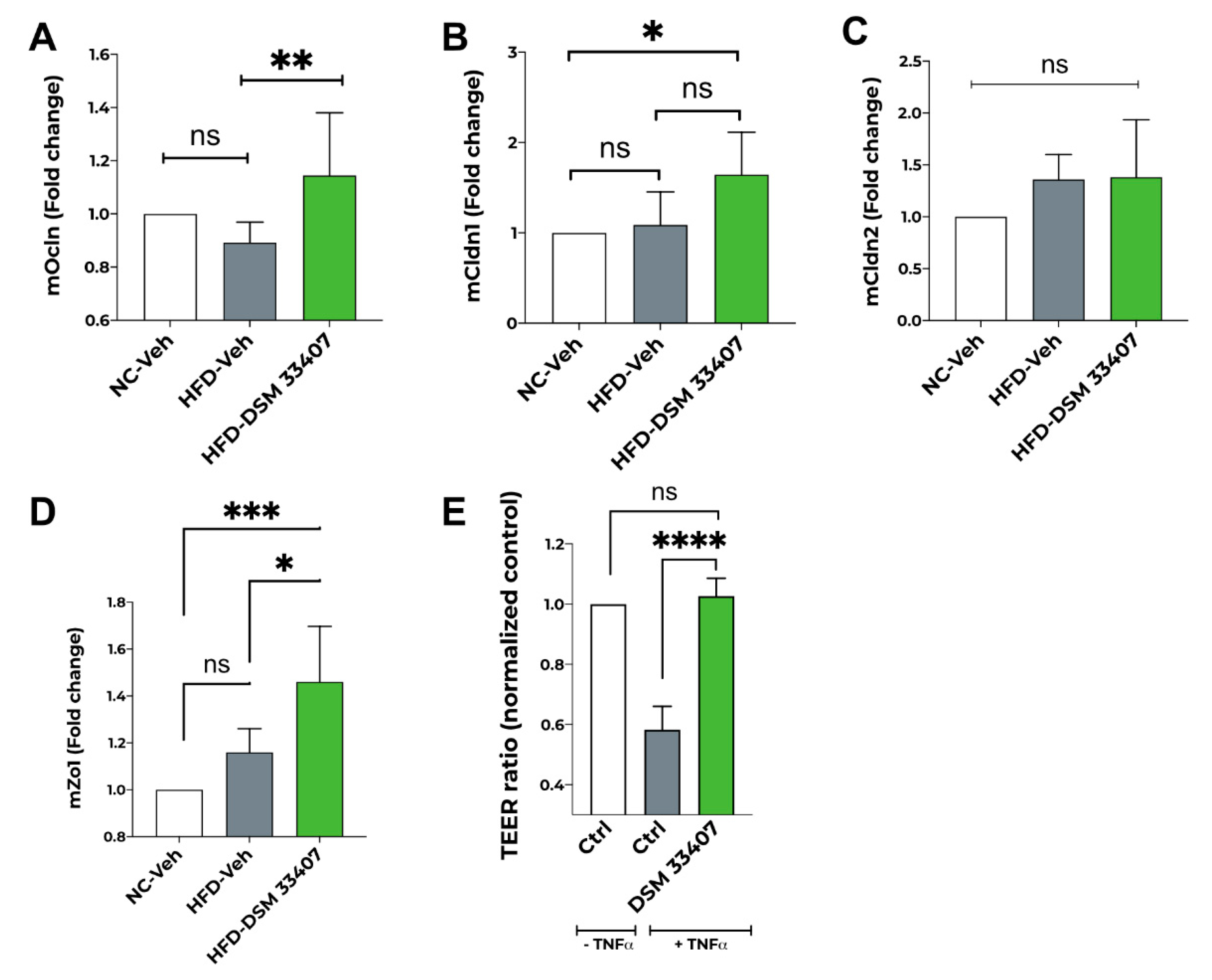
3.5. DSM33407 Maintains Gut Epithelial Integrity
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morotomi, M.; Nagai, F.; Watanabe, Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 144–149. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Micro-biome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; He, Y.; Li, P.; Zhou, H.; Zeng, N. Regional distribution of Christensenellaceae and its associations with metabolic syndrome based on a population-level analysis. PeerJ 2020, 8, e9591. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; De Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J. Transl. Med. 2018, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Rampelli, S.; Turroni, S.; Quercia, S.; Candela, M.; Brigidi, P. The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech. Ageing Dev. 2017, 165, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Choi, C.W.; Shin, H.; Jin, S.-P.; Bae, J.-S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. J. Microbiol. Biotechnol. 2019, 29, 429–440. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Braun, T.; Di Segni, A.; BenShoshan, M.; Neuman, S.; Levhar, N.; Bubis, M.; Picard, O.; Sosnovski, K.; Efroni, G.; Barhom, S.F.; et al. Individualized Dynamics in the Gut Microbiota Precede Crohn’s Disease Flares. Am. J. Gastroenterol. 2019, 114, 1142–1151. [Google Scholar] [CrossRef]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.-U.; Schrumpf, E.; Moum, B.; et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017, 66, 611–619. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Lim, M.Y.; You, H.J.; Yoon, H.S.; Kwon, B.; Lee, J.Y.; Lee, S.; Song, Y.-M.; Lee, K.; Sung, J.; Ko, G. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 2016, 66, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2019, 1461, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Funke, G.; Pfaller, M.A.; Caroll, K.C. Reagents, Stains and Media: Bacteriology; ASM Press: Washington, DC, USA, 2005. [Google Scholar]
- (CLSI), C.A.L.S.I. 2020 Performance Standards for Antimicrobial Susceptibility Testing. In CLSI Supplement M100, CLSI.-(CLSI), C.A.L.S.I.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- (CLSI), C.A.L.S.I. 2018 Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. In CLSI Standard M11, CLSI; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Abbeele, P.V.D.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; et al. Microbial Community Development in a Dynamic Gut Model Is Reproducible, Colon Region Specific, and Selective for Bacteroidetes and Clostridium Cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef]
- Ndongo, S.; Khelaifia, S.; Lagier, J.C.; Raoult, D. From anaerobes to aerointolerant prokaryotes. Hum. Microbiome J. 2020, 15, 100068. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Van den Abbeele, P.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, D., Swiatecka, D., Wichers, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- O’Doherty, R.M.; Lehman, D.L.; Telemaque-Potts, S.; Newgard, C.B. Metabolic impact of glucokinase overexpression in liver: Lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 1999, 48, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Guerville, M.; Leroy, A.; Sinquin, A.; Laugerette, F.; Michalski, M.-C.; Boudry, G. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Am. J. Physiol. Metab. 2017, 313, E107–E120. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Ferre, T.; Riu, E.; Franckhauser, S.; Agudo, J.; Bosch, F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia 2003, 46, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Golozoubova, V.; Matthias, A.; Asadi, A.; Jacobsson, A.; Cannon, B. UCP1: The only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta (BBA) Bioenerg. 2001, 1504, 82–106. [Google Scholar] [CrossRef]
- Tsukita, S.; Yamada, T.; Uno, K.; Takahashi, K.; Kaneko, K.; Ishigaki, Y.; Imai, J.; Hasegawa, Y.; Sawada, S.; Ishihara, H.; et al. Hepatic Glucokinase Modulates Obesity Predisposition by Regulating BAT Thermogenesis via Neural Signals. Cell Metab. 2012, 16, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nat. Cell Biol. 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Bleau, C.; Karelis, A.D.; St-Pierre, D.H.; Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab. Res. Rev. 2015, 31, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]

| Strain Characteristics | |
| Morphology | Short Rods with Tapered ends; Single, Pairs, or Rosettes |
| Growth condition | Anaerobic |
| Gram stain | Negative |
| Motility | None |
| Spore formation | None |
| Catalase | Negative |
| Oxidase | Negative |
| pH range | 6.0 to 9.0 |
| Bile resistance | Up to 80% |
| API 20A Gallery Results | |
| Subtract | Growth |
| Indole (IND) | − |
| Urea (URE) | − |
| Glucose (GLU) | + |
| Mannitol (MAN) | − |
| Lactose (LAC) | − |
| Saccharose (SAC) | − |
| Maltose (MAL) | − |
| Salicin (SAL) | + |
| Xylose (XYL) | + |
| Arabinose (ARA) | + |
| Gelatin (GEL) | − |
| Esculin (ESC) | − |
| Glycerol (GLY) | − |
| Cellobiose (CEL) | − |
| Mannose (MNE) | − |
| Melezitose (MLZ) | − |
| Raffinose (RAF) | − |
| Sorbitol (SOR) | − |
| Rhamnose (RHA) | +/− |
| Trehalose (TRE) | − |
| Antibiotic Name | MIC (μg/mL) | Conclusion |
|---|---|---|
| Ampicillin | 4 | Resistant |
| Tetracycline | >32 | Resistant |
| Chloramphenicol | 8 | Sensitive |
| Clindamycin | 0.03 | Sensitive |
| Meropenem | 0.25 | Sensitive |
| Metronidazole | 0.12 | Sensitive |
| Moxifloxacin | 0.25 | Sensitive |
| Piperacillin/Tazobactam | 1/4 | Sensitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazier, W.; Le Corf, K.; Martinez, C.; Tudela, H.; Kissi, D.; Kropp, C.; Coubard, C.; Soto, M.; Elustondo, F.; Rawadi, G.; et al. A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases. Cells 2021, 10, 823. https://doi.org/10.3390/cells10040823
Mazier W, Le Corf K, Martinez C, Tudela H, Kissi D, Kropp C, Coubard C, Soto M, Elustondo F, Rawadi G, et al. A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases. Cells. 2021; 10(4):823. https://doi.org/10.3390/cells10040823
Chicago/Turabian StyleMazier, Wilfrid, Katy Le Corf, Ccori Martinez, Héloïse Tudela, Déborah Kissi, Camille Kropp, Chrislain Coubard, Marion Soto, Frédéric Elustondo, Georges Rawadi, and et al. 2021. "A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases" Cells 10, no. 4: 823. https://doi.org/10.3390/cells10040823
APA StyleMazier, W., Le Corf, K., Martinez, C., Tudela, H., Kissi, D., Kropp, C., Coubard, C., Soto, M., Elustondo, F., Rawadi, G., & Claus, S. P. (2021). A New Strain of Christensenella minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases. Cells, 10(4), 823. https://doi.org/10.3390/cells10040823






