RhoGEF17—An Essential Regulator of Endothelial Cell Death and Growth
Abstract
1. Introduction
2. Materials and Methods
3. Results
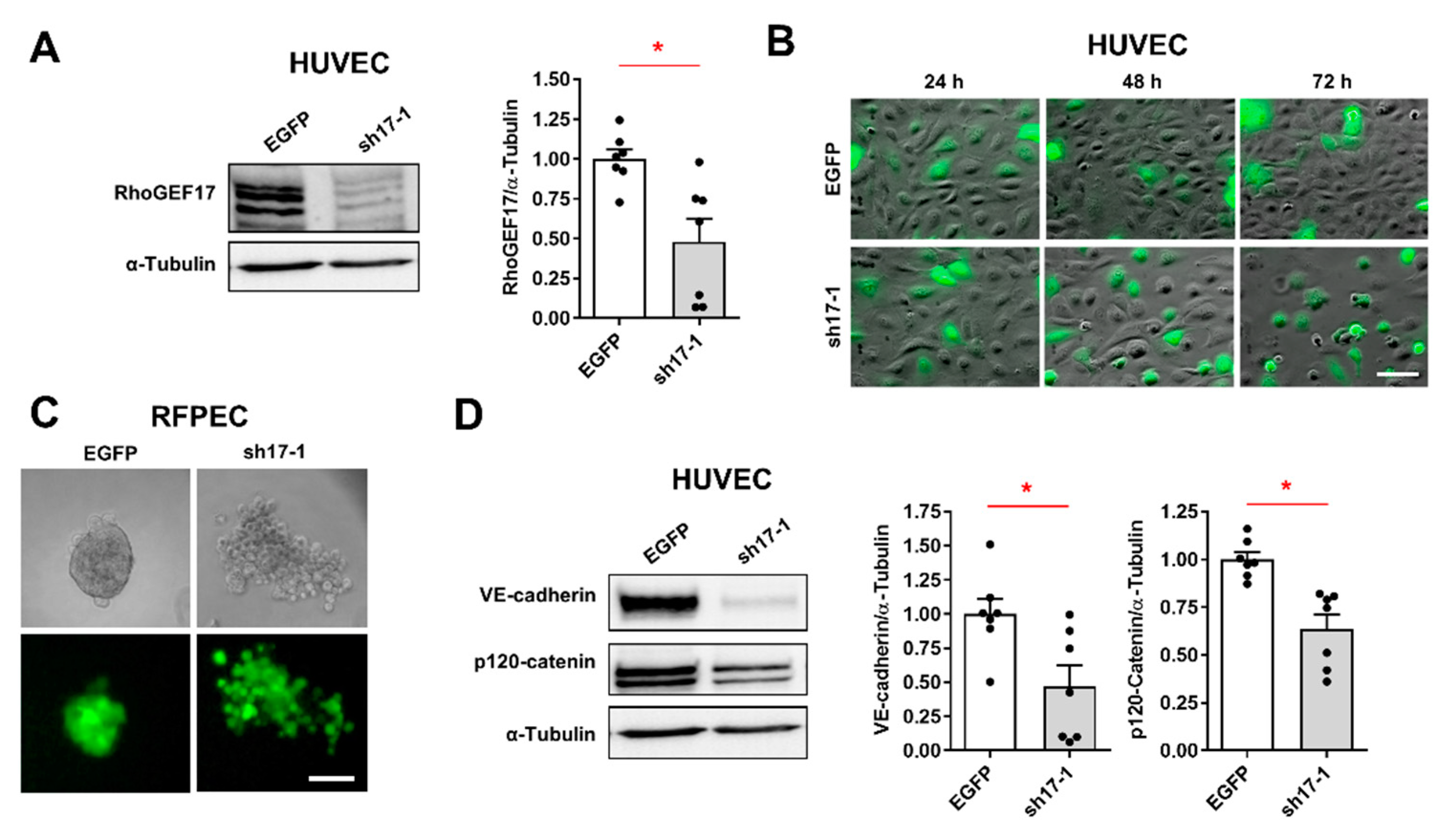
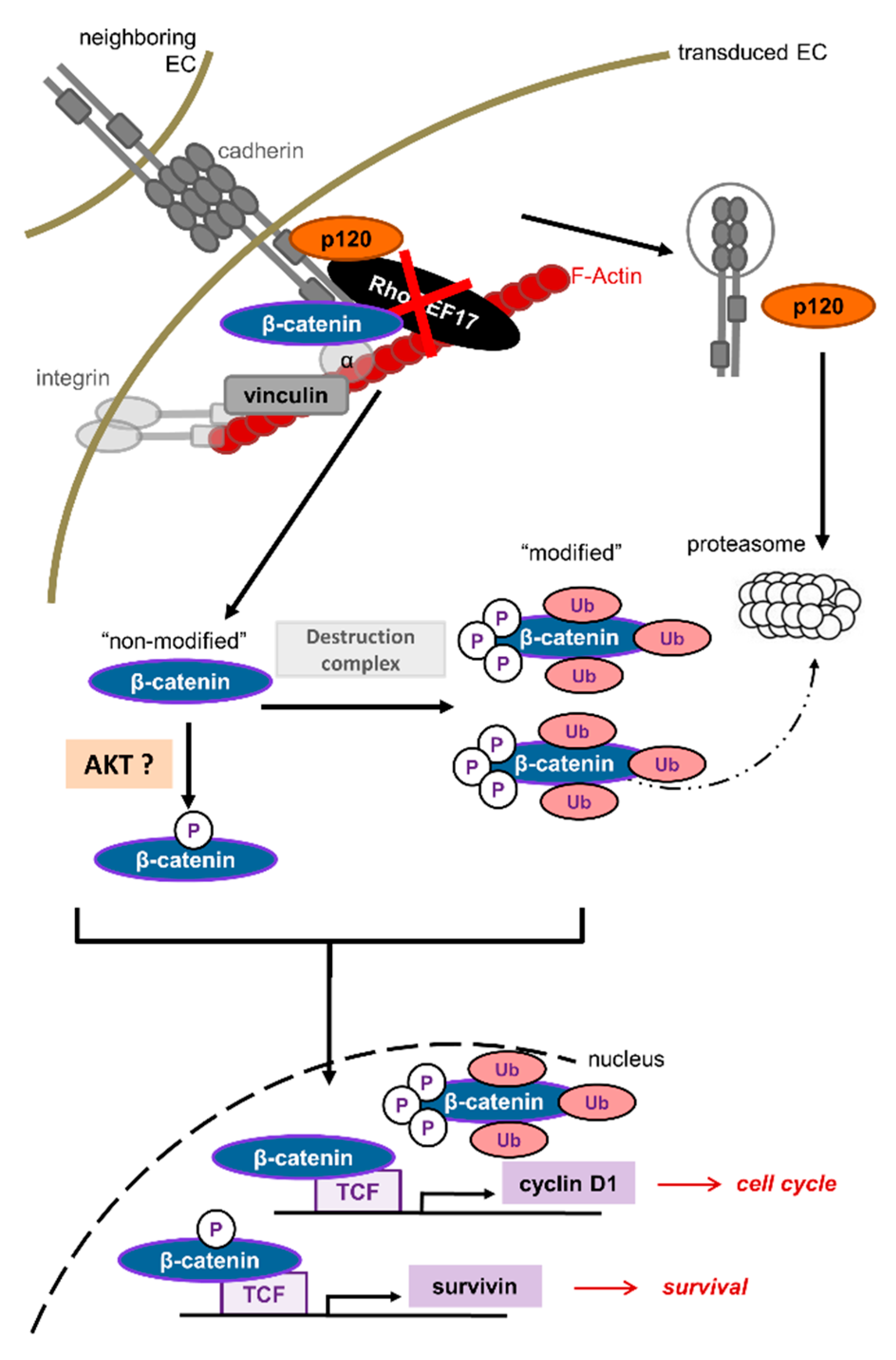
3.1. RhoGEF17 Is Essential for AJ Formation in EC
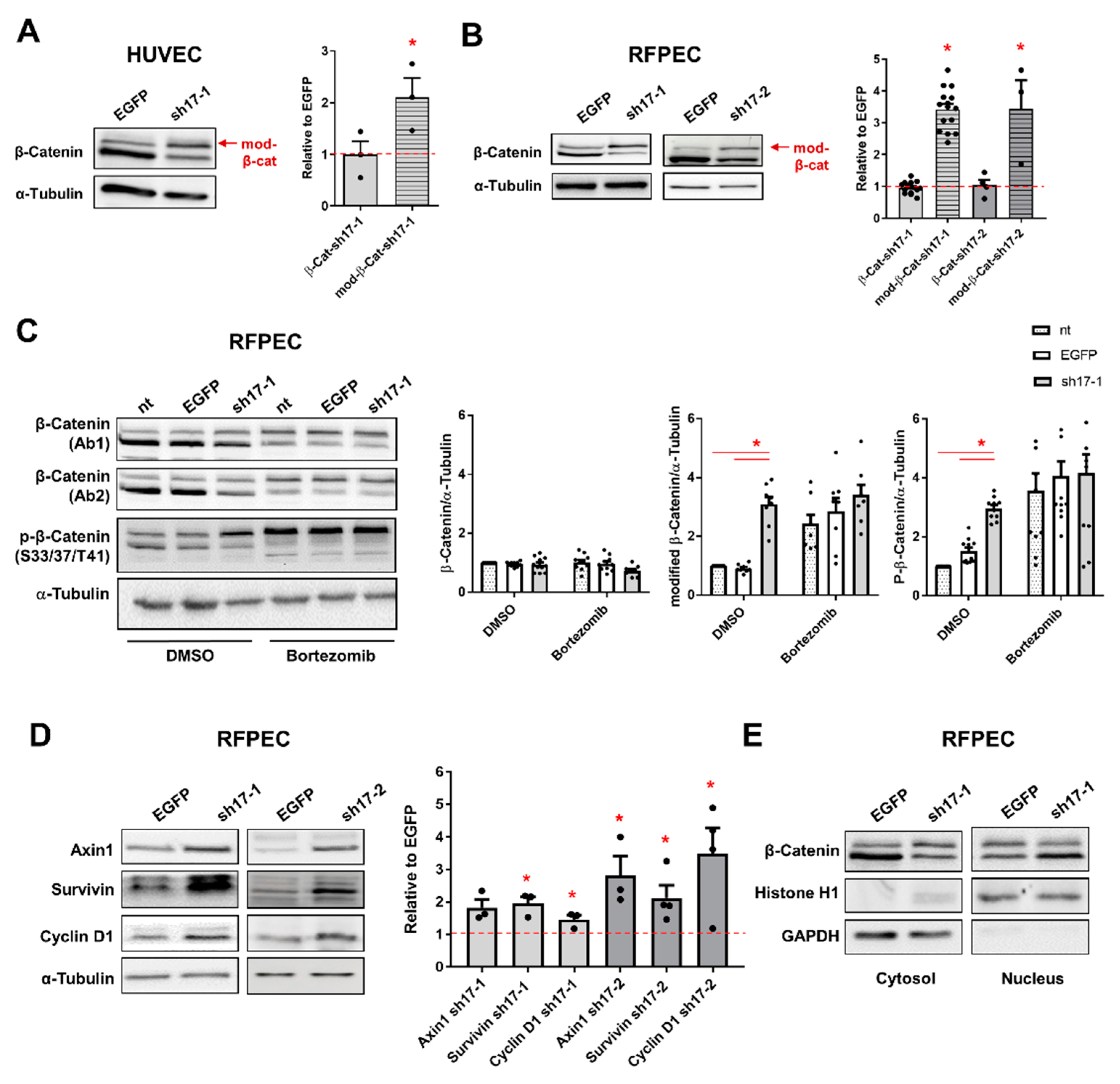
3.2. AJ Proteins Are Predominantly Lost by Proteasomal Degradation in EC with Low Expression of RhoGEF17
3.3. The Reduction of RhoGEF17 Is Accompanied by an Increased Protein Modification of β-Catenin and Changes in the Expression of Associated Genes
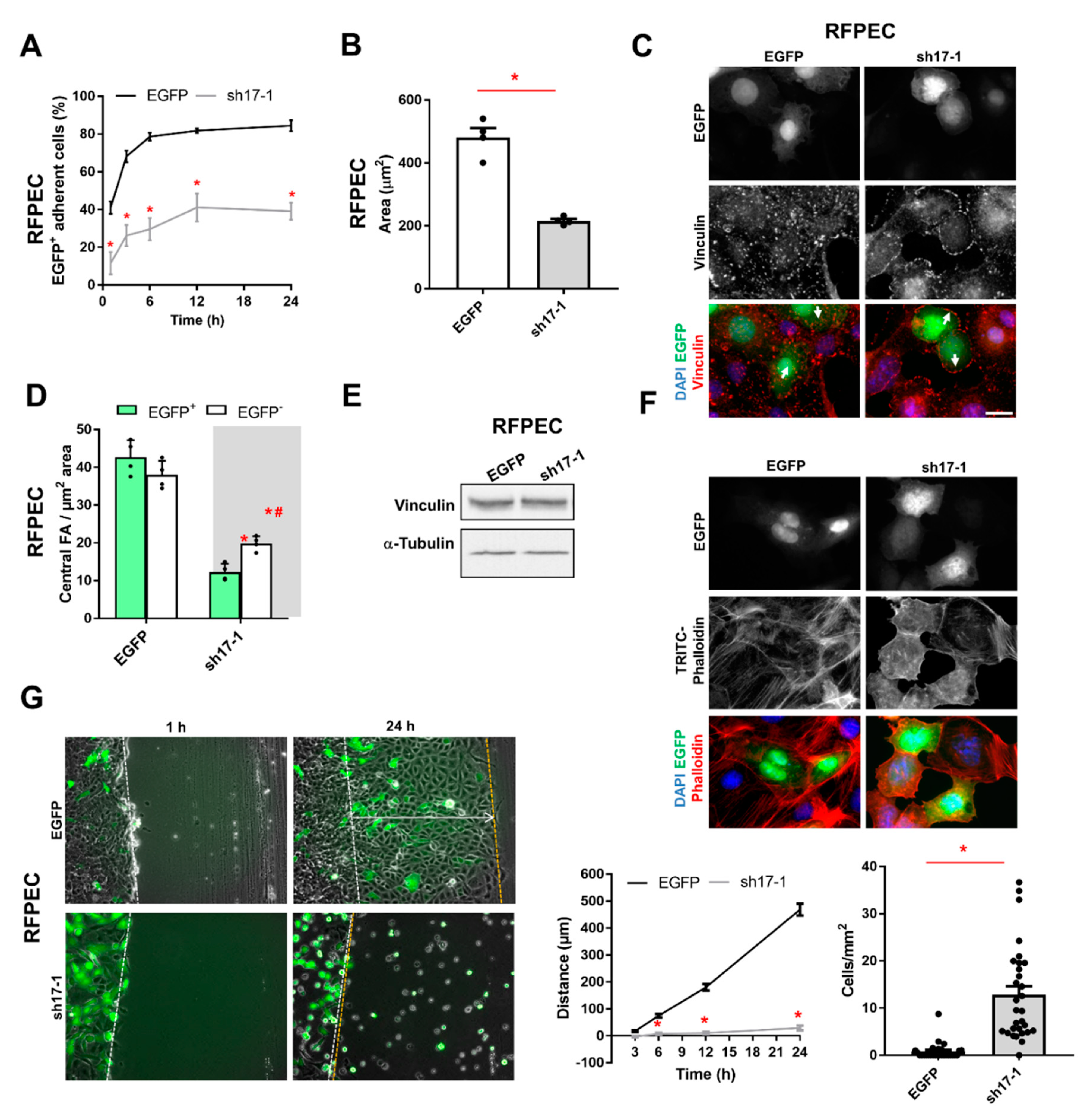
3.4. The Reduction of RhoGEF17 Impairs RFPEC Adhesion and Sheet Migration
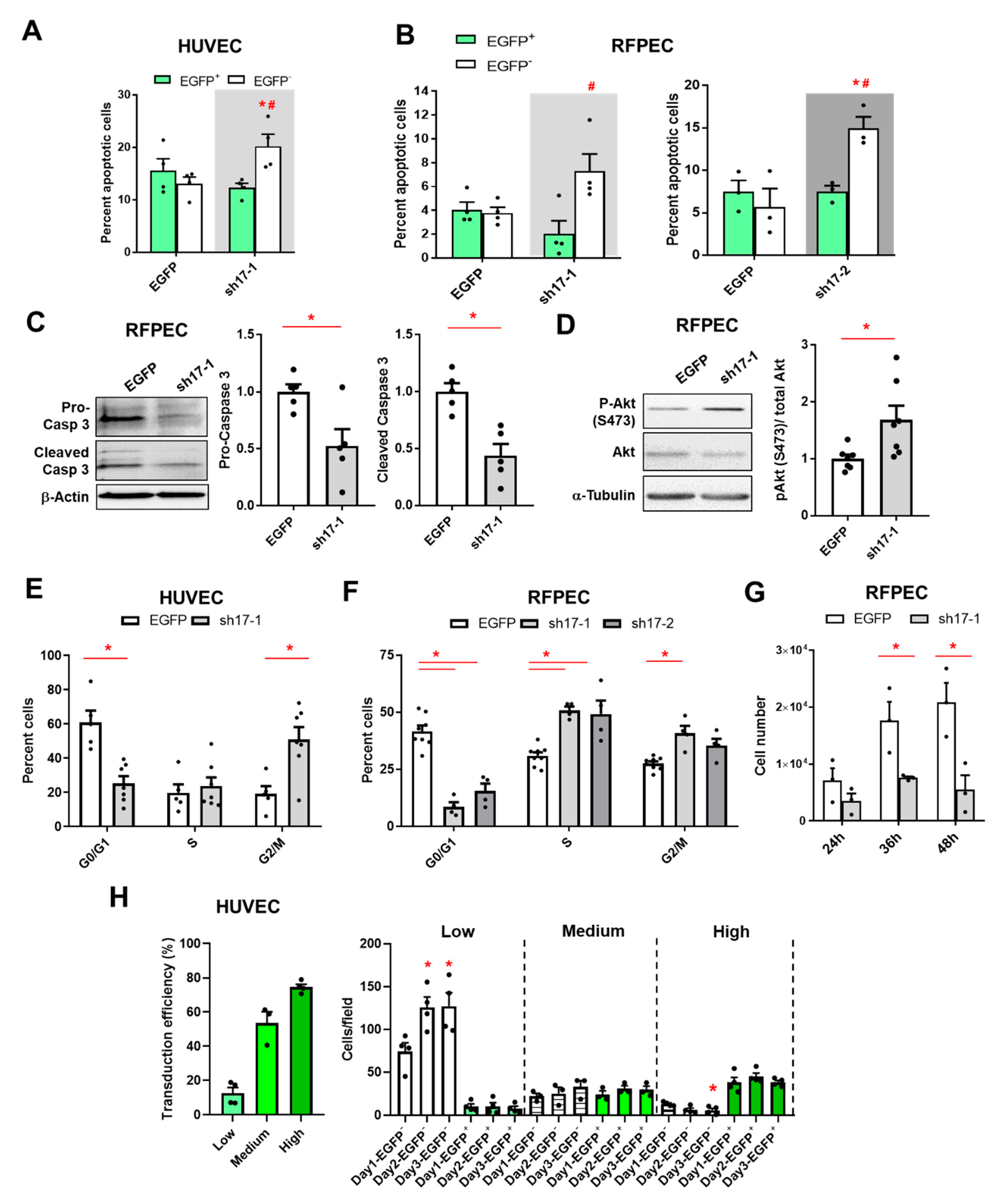
3.5. The Reduction of RhoGEF17 Prevents Apoptosis and Induces a Cell Cycle Block in EC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Nonstandard Abbreviations
| AJ | Adherens junctions |
| EC | Endothelial cells |
| FA | focal adhesion |
| HUVEC | Human umbilical vein endothelial cells |
| RFPEC | Rat fat pad endothelial cells |
| RhoGEF | Rho-specific guanine nucleotide exchange factor |
| TEM4 | Tumor endothelial marker 4 |
References
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Ferreri, D.M.; Minnear, F.L.; Yin, T.; Kowalczyk, A.P.; Vincent, P.A. N-cadherin levels in endothelial cells are regulated by monolayer maturity and p120 availability. Cell Commun. Adhes. 2008, 15, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Wolburg, H.; Redies, C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. 2000, 218, 472–479. [Google Scholar] [CrossRef]
- Paik, J.H.; Skoura, A.; Chae, S.S.; Cowan, A.E.; Han, D.K.; Proia, R.L.; Hla, T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004, 18, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Ruco, L.; Dejana, E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J. Cell Biol. 1998, 140, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Tillet, E.; Vittet, D.; Feraud, O.; Moore, R.; Kemler, R.; Huber, P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp. Cell Res. 2005, 310, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, K.; Simons, M.; Murakami, M. Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H162–H172. [Google Scholar] [CrossRef]
- Reynolds, A.; Daniel, J.; McCrea, P.; Wheelock, M.; Wu, J.; Zhang, Z. Identification of a new catenin: The tyrosine kinase substrate p120cat associates with E-cadherin complexes. Mol. Cell Biol. 1994, 14, 8333–8342. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, M.; Corada, M.; Caveda, L.; Breviario, F.; Ayalon, O.; Geiger, B.; Dejana, E. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J. Cell Biol. 1995, 129, 203–217. [Google Scholar] [CrossRef]
- Ikeda, S.; Kishida, S.; Yamamoto, H.; Murai, H.; Koyama, S.; Kikuchi, A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kato, Y.; Zhang, Z.; Do, V.M.; Yankner, B.A.; He, X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc. Natl. Acad. Sci. USA 1999, 96, 6273–6278. [Google Scholar] [CrossRef]
- Brunner, E.; Peter, O.; Schweizer, L.; Basler, K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 1997, 385, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, M.; van de Wetering, M.; Oosterwegel, M.; Peterson-Maduro, J.; Godsave, S.; Korinek, V.; Roose, J.; Destree, O.; Clevers, H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86, 391–399. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Q.Z.; Wang, S.; Xu, H.T.; Miao, Y.; Wang, L.; Wang, E.H. Kaiso interacts with p120-catenin to regulate beta-catenin expression at the transcriptional level. PLoS ONE 2014, 9, e87537. [Google Scholar] [CrossRef]
- Park, J.I.; Kim, S.W.; Lyons, J.P.; Ji, H.; Nguyen, T.T.; Cho, K.; Barton, M.C.; Deroo, T.; Vleminckx, K.; Moon, R.T.; et al. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev. Cell 2005, 8, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Chervin-Petinot, A.; Courcon, M.; Almagro, S.; Nicolas, A.; Grichine, A.; Grunwald, D.; Prandini, M.H.; Huber, P.; Gulino-Debrac, D. Epithelial protein lost in neoplasm (EPLIN) interacts with alpha-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J. Biol. Chem. 2012, 287, 7556–7572. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, E.S.; Izard, T. Dimer asymmetry defines alpha-catenin interactions. Nat. Struct. Mol. Biol. 2013, 20, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Guerrera, D.; Spadaro, D.; Shah, J. Epithelial junctions and Rho family GTPases: The zonular signalosome. Small GTPases 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ngok, S.; Geyer, R.; Kourtidis, A.; Mitin, N.; Feathers, R.; Der, C.; Anastasiadis, P. TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J. Cell Sci. 2013, 126, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- St Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.; Montgomery, E.; Lal, A.; Riggins, G.; Lengauer, C.; Vogelstein, B.; et al. Genes expressed in human tumor endothelium. Science (Wash. DC) 2000, 289, 1197–1202. [Google Scholar] [CrossRef]
- Bloethner, S.; Mould, A.; Stark, M.; Hayward, N. Identification of ARHGEF17, DENND2D, FGFR3, and RB1 mutations in melanoma by inhibition of nonsense-mediated mRNA decay. Genes Chromosomes Cancer 2008, 47, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Mitin, N.; Rossman, K.; Der, C. Identification of a Novel Actin-Binding Domain within the Rho Guanine Nucleotide Exchange Factor TEM4. PLoS ONE 2012, 7, e41876. [Google Scholar] [CrossRef] [PubMed]
- Rümenapp, U.; Freichel-Blomquist, A.; Wittinghofer, B.; Jakobs, K.; Wieland, T. A mammalian Rho-specific guanine-nucleotide exchange factor (p164-RhoGEF) without a pleckstrin homology domain. Biochem. J. 2002, 366, 721–728. [Google Scholar] [CrossRef][Green Version]
- Mitin, N.; Rossman, K.; Currin, R.; Sandeep, A.; Marshall, T.; Bear, J.; Bautch, V.; Der, C. The RhoGEF TEM4 Regulates Endothelial Cell Migration by Suppressing Actomyosin Contractility. PLoS ONE 2013, 8. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Li, J.; Fang, Y.; Zhang, Z.; Jin, D.; Chen, X.; Zhao, Y.; Li, M.; Huan, L.; Kent, T.A.; et al. Rho Guanine Nucleotide Exchange Factor ARHGEF17 Is a Risk Gene for Intracranial Aneurysms. Circ. Genom. Precis. Med. 2018, 11, e002099. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhou, S.; da Costa, L.; Yu, J.; Kinzler, K.; Vogelstein, B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 1998, 95, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Ongherth, A.; Pasch, S.; Wuertz, C.M.; Nowak, K.; Kittana, N.; Weis, C.A.; Jatho, A.; Vettel, C.; Tiburcy, M.; Toischer, K.; et al. p63RhoGEF regulates auto- and paracrine signaling in cardiac fibroblasts. J. Mol. Cell Cardiol. 2015, 88, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, C.M.; Lorincz, A.; Vettel, C.; Thomas, M.A.; Wieland, T.; Lutz, S. p63RhoGEF--a key mediator of angiotensin II-dependent signaling and processes in vascular smooth muscle cells. FASEB J. 2010, 24, 4865–4876. [Google Scholar] [CrossRef] [PubMed]
- Marcum, J.A.; Rosenberg, R.D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem. Biophys. Res. Commun. 1985, 126, 365–372. [Google Scholar] [CrossRef]
- Kroll, J.; Epting, D.; Kern, K.; Dietz, C.; Feng, Y.; Hammes, H.; Wieland, T.; Augustin, H. Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, 893–899. [Google Scholar] [CrossRef]
- Korff, T.; Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 1998, 143, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat. Commun. 2015, 6, 6725. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.; Quaranta, V. Cadherin-bound beta-catenin feeds into the Wnt pathway upon adherens junctions dissociation: Evidence for an intersection between beta-catenin pools. PLoS ONE 2009, 4, e4580. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Mesquita, A.P.; de Araujo Lopes, S.; Pernambuco Filho, P.C.A.; Nader, H.B.; Lopes, C.C. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis Int. J. Program. Cell Death 2017, 22, 1116–1137. [Google Scholar] [CrossRef]
- Hernandez-Garcia, R.; Iruela-Arispe, M.L.; Reyes-Cruz, G.; Vazquez-Prado, J. Endothelial RhoGEFs: A systematic analysis of their expression profiles in VEGF-stimulated and tumor endothelial cells. Vasc. Pharmacol. 2015, 74, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Beutelspacher, S.C.; Serbecic, N.; Tan, P.; McClure, M.O. [Comparison of several viral vectors for gene therapy of corneal endothelial cells]. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2005, 102, 1168–1174. [Google Scholar] [CrossRef]
- Sakoda, T.; Kasahara, N.; Kedes, L.; Ohyanagi, M. Lentiviral vector-mediated gene transfer to endotherial cells compared with adenoviral and retroviral vectors. Prep. Biochem. Biotechnol. 2007, 37, 1–11. [Google Scholar] [CrossRef]
- Carmeliet, P.; Lampugnani, M.G.; Moons, L.; Breviario, F.; Compernolle, V.; Bono, F.; Balconi, G.; Spagnuolo, R.; Oosthuyse, B.; Dewerchin, M.; et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 1999, 98, 147–157. [Google Scholar] [CrossRef]
- Corada, M.; Liao, F.; Lindgren, M.; Lampugnani, M.G.; Breviario, F.; Frank, R.; Muller, W.A.; Hicklin, D.J.; Bohlen, P.; Dejana, E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 2001, 97, 1679–1684. [Google Scholar] [CrossRef]
- Meredith, J.E., Jr.; Fazeli, B.; Schwartz, M.A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef]
- Brooks, P.C.; Montgomery, A.M.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Maubant, S.; Saint-Dizier, D.; Boutillon, M.; Perron-Sierra, F.; Casara, P.J.; Hickman, J.A.; Tucker, G.C.; Van Obberghen-Schilling, E. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood 2006, 108, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Zanetti, A.; Sironi, M.; Polentarutti, N.; Lanfrancone, L.; Dejana, E.; Colotta, F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J. Cell Biol. 1994, 127, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Saha, T.; Bose, S.; Chakraborty, S.; Dhar, S.; Khan, P.; Adhikary, A.; Das, T.; Sa, G. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis Int. J. Program. Cell Death 2019. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.S.; Yung, M.M.; Hui, L.M.; Leung, L.L.; Liang, R.; Chen, K.; Liu, S.S.; Qin, Y.; Leung, T.H.; Lee, K.F.; et al. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol. Cancer 2017, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Fujio, Y.; Walsh, K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J. Biol. Chem. 1999, 274, 16349–16354. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, M.; Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 1994, 14, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.W.; Theodoras, A.M.; Shay, J.W.; Draetta, G.F.; Pagano, M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: Association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene 1994, 9, 2663–2674. [Google Scholar]
- Tamura, D.; Arao, T.; Tanaka, K.; Kaneda, H.; Matsumoto, K.; Kudo, K.; Aomatsu, K.; Fujita, Y.; Watanabe, T.; Saijo, N.; et al. Bortezomib potentially inhibits cellular growth of vascular endothelial cells through suppression of G2/M transition. Cancer Sci. 2010, 101, 1403–1408. [Google Scholar] [CrossRef]
- Isokane, M.; Walter, T.; Mahen, R.; Nijmeijer, B.; Heriche, J.K.; Miura, K.; Maffini, S.; Ivanov, M.P.; Kitajima, T.S.; Peters, J.M.; et al. ARHGEF17 is an essential spindle assembly checkpoint factor that targets Mps1 to kinetochores. J. Cell Biol. 2016, 212, 647–659. [Google Scholar] [CrossRef]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Otevrel, T.; Gao, Z.; Gao, Z.; Ehrlich, S.M.; Fields, J.Z.; Boman, B.M. Evidence that APC regulates survivin expression: A possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001, 61, 8664–8667. [Google Scholar] [PubMed]
- Yost, C.; Torres, M.; Miller, J.R.; Huang, E.; Kimelman, D.; Moon, R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996, 10, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Hart, M.; Concordet, J.P.; Lassot, I.; Albert, I.; del los Santos, R.; Durand, H.; Perret, C.; Rubinfeld, B.; Margottin, F.; Benarous, R.; et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. CB 1999, 9, 207–210. [Google Scholar] [CrossRef]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, Y.N.; Li, J.; Lee, Y.T.; Rios-Esteves, J.; Friedman, D.B.; Choi, H.J.; Weis, W.I.; Wang, C.Y.; Chazin, W.J. Direct ubiquitination of beta-catenin by Siah-1 and regulation by the exchange factor TBL1. J. Biol. Chem. 2010, 285, 13507–13516. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Feetham, M.C.; Tao, W.A.; He, X.C.; Li, L.; Aebersold, R.; Hood, L. Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc. Natl. Acad. Sci. USA 2004, 101, 15370–15375. [Google Scholar] [CrossRef]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef]
- Khaidakov, M.; Wang, X.; Mehta, J.L. Potential involvement of LOX-1 in functional consequences of endothelial senescence. PLoS ONE 2011, 6, e20964. [Google Scholar] [CrossRef]
- Krouwer, V.J.; Hekking, L.H.; Langelaar-Makkinje, M.; Regan-Klapisz, E.; Post, J.A. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc. Cell 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zheng, H.; Huang, A.; Mai, L.; Huang, X.; Hu, Y.; Huang, Y. Piwi-interacting RNAs play a role in vitamin C-mediated effects on endothelial aging. Int. J. Med. Sci. 2020, 17, 946–952. [Google Scholar] [CrossRef]
- Korybalska, K.; Kawka, E.; Kusch, A.; Aregger, F.; Dragun, D.; Jorres, A.; Breborowicz, A.; Witowski, J. Recovery of senescent endothelial cells from injury. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Cattelino, A.; Gallini, R.; Rudini, N.; Iurlaro, M.; Piccolo, S.; Dejana, E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 2004, 166, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Volkmann, I.; Jazbutyte, V.; Dangwal, S.; Park, D.H.; Thum, T. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arter. Thromb. Vasc. Biol. 2012, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, P.; Baltus, D.; Jatho, A.; Drews, O.; Zelarayan, L.C.; Wieland, T.; Lutz, S. RhoGEF17—An Essential Regulator of Endothelial Cell Death and Growth. Cells 2021, 10, 741. https://doi.org/10.3390/cells10040741
Weber P, Baltus D, Jatho A, Drews O, Zelarayan LC, Wieland T, Lutz S. RhoGEF17—An Essential Regulator of Endothelial Cell Death and Growth. Cells. 2021; 10(4):741. https://doi.org/10.3390/cells10040741
Chicago/Turabian StyleWeber, Pamina, Doris Baltus, Aline Jatho, Oliver Drews, Laura C. Zelarayan, Thomas Wieland, and Susanne Lutz. 2021. "RhoGEF17—An Essential Regulator of Endothelial Cell Death and Growth" Cells 10, no. 4: 741. https://doi.org/10.3390/cells10040741
APA StyleWeber, P., Baltus, D., Jatho, A., Drews, O., Zelarayan, L. C., Wieland, T., & Lutz, S. (2021). RhoGEF17—An Essential Regulator of Endothelial Cell Death and Growth. Cells, 10(4), 741. https://doi.org/10.3390/cells10040741






