The Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Development and/or Progression of Endometriosis-State of the Art
Abstract
1. Introduction
2. Immunophenotype of Myeloid-Derived Suppressor Cells (MDSCs)
2.1. Monocytic MDSCs (M-MDSCs)
2.2. Polymorphonuclear MDSCs (PMN-MDSCs)
3. Immunosuppressive Activity of MDSCs in the Immune System
3.1. MDSCs Influence Monocytes/Macrophages
3.2. MDSCs Influence NK Cells
3.3. MDSCs Influence T Cell Activity
3.4. MDSCs Influence B Cell Function
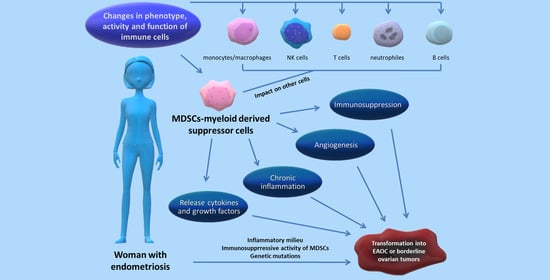
4. Relation between MDSCs and the Progression of Endometriosis—State of the Art
5. Link between Endometriosis and Ovarian Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Tang, S.; Feng, F.; Liu, C.; Wang, L.; Zhao, W.; Zhang, T.; Yao, Y.; Wang, X.; Sun, C. Impact of endometriosis on risk of ovarian, endometrial and cervical cancers: A meta-analysis. Arch. Gynecol. Obstet. 2019, 299, 35–46. [Google Scholar] [CrossRef]
- Chiang, A.J.; Chang, C.; Huang, C.H.; Huang, W.C.; Kan, Y.Y.; Chen, J. Risk factors in progression from endometriosis to ovarian cancer: A cohort study based on medical insurance data. J. Gynecol. Oncol. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Tamauchi, S.; Yoshikawa, N.; Niimi, K.; Shibata, K.; Kikkawa, F. Endometriosis and cancer. Free Radic Biol. Med. 2019, 133, 186–192. [Google Scholar] [CrossRef]
- Torng, P.L. Clinical implication for endometriosis associated with ovarian cancer. Gynecol. Minim. Invasive Ther. 2017, 6, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Králíčková, M.; Laganà, A.S.; Ghezzi, F.; Vetvicka, V. Endometriosis and risk of ovarian cancer: What do we know? Arch. Gynecol. Obstet. 2019, 301, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef]
- Chen, H.; Qin, S.; Lei, A.; Li, X.; Gao, Q.; Dong, J.; Xiao, Q.; Zhou, J. Expansion of monocytic myeloid-derived suppressor cells in endometriosis patients: A pilot study. Int. Immunopharmacol. 2017, 47, 150–158. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Xu, Y.; Guo, P.; Hong, B.; Cao, Y.; Wei, Z.; Xue, R.; Wang, C.; Jiang, H. Alteration of myeloid-derived suppressor cells, chronic inflammatory cytokines, and exosomal miRNA contribute to the peritoneal immune disorder of patients with endometriosis. Reprod. Sci. 2019, 26, 1130–1138. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, J.; Man, G.C.W.; Leung, K.T.; Liang, B.; Xiao, B.; Ma, X.; Huang, S.; Huang, H.; Hegde, V.L.; et al. MDSCs drive the process of endometriosis by enhancing angiogenesis and are a new potential therapeutic target. Eur. J. Immunol. 2018, 48, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shao, J.; Jiang, F.; Wang, Y.; Yan, Q.; Yu, N.; Zhang, J.; Zhang, J.; Li, M.; He, Y. CD33+ CD14+ CD11b+ HLA-DR− monocytic myeloid-derived suppressor cells recruited and activated by CCR9/CCL25 are crucial for the pathogenic progression of endometriosis. Am. J. Reprod. Immunol. 2019, 81, e13067. [Google Scholar] [CrossRef]
- Wu, L.; Deng, Z.; Peng, Y.; Han, L.; Liu, J.; Wang, L.; Li, B.; Zhao, J.; Jiao, S.; Wei, H. Ascites-derived IL-6 and IL-10 synergistically expand CD14+ HLA-DR-/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget 2017, 8, 76843–76856. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020, 10, 200111. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Pawelec, G.; Verschoor, C.P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: Not only in tumor immunity. Front. Immunol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Schouppe, E.; De Baetselier, P.; Van Ginderachter, J.A.; Sarukhan, A. Instruction of myeloid cells by the tumor microenvironment: Open questions on the dynamics and plasticity of different tumor-associated myeloid cell populations. Oncoimmunology 2012, 1, 1135–1145. [Google Scholar] [CrossRef]
- Zhou, J.; Nefedova, Y.; Lei, A.; Gabrilowich, D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin. Immunol. 2018, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Demircan, N.C.; Boussios, S.; Tasci, T.; Öztürk, A.A. Current and future immunotherapy approaches in ovarian cancer. Ann. Transl. Med. 2020, 8, 1714. [Google Scholar] [CrossRef]
- Zhu, H.; Gu, Y.; Xue, Y.; Yuan, M.; Cao, X.; Liu, Q. CXCR2+ MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget 2017, 8, 114554. [Google Scholar] [CrossRef]
- Law, A.M.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef]
- Cassetta, L.; Bruderek, K.; Skrzeczynska-Moncznik, J.; Osiecka, O.; Hu, X.; Rundgren, I.M.; Lin, A.; Santegoets, K.; Horzum, U.; Godinho-Santos, A.; et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Okla, K.; Wertel, I.; Wawruszak, A.; Bobiński, M.; Kotarski, J. Blood-based analyses of cancer: Circulating myeloid-derived suppressor cells–is a new era coming? Crit. Rev. Clin. Lab. Sci. 2018, 55, 376–407. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Role of Tumor-Associated Myeloid Cells in Breast Cancer. Cells 2020, 9, 1785. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P.; Beury, D.W.; Clements, V.K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 2012, 22, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Bun, S.K.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006, 176, 284–290. [Google Scholar] [CrossRef]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; Van den Bossche, J.; Mack, M.; Pipeleers, D.; In’t Veld, P.; De Baetselier, P.; et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef]
- Beury, D.W.; Parker, K.H.; Nyandjo, M.; Sinha, P.; Carter, K.A.; Ostrand-Rosenberg, S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J. Leukoc. Biol. 2014, 96, 1109–1118. [Google Scholar] [CrossRef]
- Bunt, S.K.; Clements, V.K.; Hanson, E.M.; Sinha, P.; Ostrand-Rosenberg, S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 2009, 85, 996–1004. [Google Scholar] [CrossRef]
- Parker, K.H.; Sinha, P.; Horn, L.A.; Clements, V.K.; Yang, H.; Li, J.; Tracey, K.J.; Ostrand-Rosenberg, S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014, 74, 5723–5733. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef]
- Huang, B.; Pan, P.Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021, 359, 104254. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019, 35, 588–602. [Google Scholar] [CrossRef]
- Vankerckhoven, A.; Wouters, R.; Mathivet, T.; Ceusters, J.; Baert, T.; Van Hoylandt, A.; Gerhardt, H.; Vergote, I.; Coosemans, A. Opposite Macrophage Polarization in Different Subsets of Ovarian Cancer: Observation from a Pilot Study. Cells 2020, 9, 305. [Google Scholar] [CrossRef]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 1–16. [Google Scholar] [CrossRef]
- Laganà, A.S.; Salmeri, F.M.; Ban Frangež, H.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. 2020, 36, 441–444. [Google Scholar] [CrossRef]
- Nie, M.F.; Xie, Q.; Wu, Y.H.; He, H.; Zou, L.J.; She, X.L.; Wu, X.Q. Serum and Ectopic Endometrium from Women with Endometriosis Modulate Macrophage M1/M2 Polarization via the Smad2/Smad3 Pathway. J. Immunol. Res. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Bruno, A.; Mortara, L.; Baci, D.; Noonan, D.M.; Albini, A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front. Immunol. 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Liao, Y.J.; Chiou, T.J.; Huang, H.T.; Lin, Y.H.; Twu, Y.C. TGF-β regulated leukemia cell susceptibility against NK targeting through the down-regulation of the CD48 expression. Immunobiology 2019, 224, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Adema, G.J.; Baran, J.; Brandau, S.; Van Ginderachter, J.A.; Hu, X.; Jablonska, J.; Mojsilovic, S.; Papadaki, H.A.; Pico de Coaña, Y.; et al. Interactions among myeloid regulatory cells in cancer. Cancer Immunol. Immunother. 2019, 68, 645–660. [Google Scholar] [CrossRef]
- Fleming, V.; Hu, X.; Weber, R.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front. Immunol. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Özkan, B.; Lim, H.; Park, S.G. Immunomodulatory function of myeloid-derived suppressor cells during B cell-mediated immune response. Int. J. Mol. Sci. 2018, 19, 1468. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Immunosenescence: The potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell Mol. Life Sci. 2019, 76, 1901–1918. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Bi, K.; Wang, K.; Lu, Z.; Xu, Y.; Guo, P.; Li, C.; Wei, Z.; Chen, Y.; Cao, Y. Reduction of myeloid derived suppressor cells by inhibiting Notch pathway prevents the progression of endometriosis in mice model. Int. Immunopharmacol. 2020, 82, 106352. [Google Scholar] [CrossRef]
- Gupta, P.; Chen, C.; Chaluvally-Raghavan, P.; Pradeep, S. B cells as an immune-regulatory signature in ovarian cancer. Cancers 2019, 11, 894. [Google Scholar] [CrossRef]
- Lee, W.; Wang, P. Immunology and ovarian cancers. J. Chin. Med. Assoc. 2020, 83, 425–432. [Google Scholar] [CrossRef]
- Lelis, F.J.N.; Jaufmann, J.; Singh, A.; Fromm, K.; Teschner, A.C.; Pöschel, S.; Schäfer, I.; Beer-Hammer, S.; Rieber, N.; Hartl, D. Myeloid-derived suppressor cells modulate B-cell responses. Immunol. Lett. 2017, 188, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.E.; Knight, K.L. Inhibition of B lymphopoiesis by adipocytes and IL-1–producing myeloid-derived suppressor cells. J. Immunol. 2015, 195, 2666–2674. [Google Scholar] [CrossRef]
- Riccio, L.G.C.; Baracat, E.C.; Chapron, C.; Batteux, F.; Abrão, M.S. The role of the B lymphocytes in endometriosis: A systematic review. J. Reprod. Immunol. 2017, 123, 29–34. [Google Scholar] [CrossRef]
- Porpora, M.G.; Scaramuzzino, S.; Sangiuliano, C.; Piacenti, I.; Bonanni, V.; Piccioni, M.G.; Ostuni, R.; Masciullo, L.; Benedetti Panici, P.L. High prevalence of autoimmune diseases in women with endometriosis: A case-control study. Gynecol Endocrinol. 2020, 36, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Balduit, A.; Mangogna, A.; Zito, G.; Romano, F.; Ricci, G.; Kishore, U.; Bulla, R. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front. Immunol 2021, 11, 3454. [Google Scholar] [CrossRef] [PubMed]
- Bungum, H.F.; Nygaard, U.; Vestergaard, C.; Martensen, P.M.; Knudsen, U.B. Increased IL-25 levels in the peritoneal fluid of patients with endometriosis. J. Reprod. Immunol. 2016, 114, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Chen, H.Y.; Chen, W.; Liu, Y.N.; Fu, Y.; Wang, L.N. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol. Endocrinol. 2018, 34, 507–512. [Google Scholar] [CrossRef]
- Matalliotakis, M.; Goulielmos, G.N.; Kalogiannidis, I.; Koumantakis, G.; Matalliotakis, I.; Arici, A. Extra pelvic endometriosis: Retrospective analysis on 200 cases in two different countries. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 217, 34–37. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.; Souza, C.C.; Fernandes, L.F.C.; Abrao, M.S.; Kho, R.M. Extrapelvic endometriosis: A systematic review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef]
- Mignemi, G.; Facchini, C.; Raimondo, D.; Montanari, G.; Ferrini, G.; Seracchioli, R. A case report of nasal endometriosis in a patient affected by Behcet’s disease. J. Minim. Invasive Gynecol. 2012, 19, 514–516. [Google Scholar] [CrossRef]
- Braun, D.P.; Dmowski, W.P. Endometriosis: Abnormal endometrium and dysfunctional immune response. Curr. Opin. Obstet. Gynecol. 1998, 10, 365–369. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, A. Endometriosis: New concepts in the pathogenesis. Int. J. Biochem. Cell Biol. 2010, 42, 778–780. [Google Scholar] [CrossRef]
- Nisolle, M.; Alvarez, M.L.; Colombo, M.; Foidart, J.M. Pathogenèse de l’endométriose. Gynecol. Obstet. Fertil. 2007, 35, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Figueira, P.G.M.; Abrão, M.S.; Krikun, G.; Taylor, H. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2011, 1221, 10. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Koga, K.; Osuga, Y. Extra-pelvic endometriosis: A review. Reprod. Med. Biol. 2020, 19, 323–333. [Google Scholar] [CrossRef]
- Machairiotis, N.; Stylianaki, A.; Dryllis, G.; Zarogoulidis, P.; Kouroutou, P.; Tsiamis, N.; Katsikogiannis, N.; Sarika, E.; Courcoutsakis, N.; Tsiouda, T.; et al. Extrapelvic endometriosis: A rare entity or an under diagnosed condition? Diagn Pathol. 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Sinaii, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Crook, K.R.; Liu, P. Role of myeloid-derived suppressor cells in autoimmune disease. World J. Immunol. 2014, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Bi, K.; Lu, Z.; Wang, K.; Xu, Y.; Wu, H.; Cao, Y.; Jiang, H. CCR5/CCR5 ligands induced myeloid-derived suppressor cells are related to the progression of endometriosis. Reprod. Biomed. Online 2019, 39, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Körbel, C.; Gerstner, M.D.; Menger, M.D.; Laschke, M.W. Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis 2018, 21, 37–46. [Google Scholar] [CrossRef]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5+ myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. 2018, 78, 157–167. [Google Scholar] [CrossRef]
- Melin, A.; Sparen, P.; Persson, I.; Bergqvist, A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum. Reprod. 2006, 21, 1237–1242. [Google Scholar] [CrossRef]
- Mogensen, J.B.; Kjær, S.K.; Mellemkjær, L.; Jensen, A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol. Oncol. 2016, 143, 87–92. [Google Scholar] [CrossRef]
- Dawson, A.; Fernandez, M.L.; Anglesio, M.; Yong, P.J.; Carey, M.S. Endometriosis and endometriosis-associated cancers: New insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018, 12, 803. [Google Scholar] [CrossRef]
- Robinson, K.A.; Menias, C.O.; Chen, L.; Schiappacasse, G.; Shaaban, A.M.; Caserta, M.P.; Elsayes, K.M.; VanBuren, W.M.; Bolan, C.W. Understanding malignant transformation of endometriosis: Imaging features with pathologic correlation. Abdom. Radiol. 2020, 1–14. [Google Scholar] [CrossRef]
- Nezhat, F.; Apostol, R.; Nezhat, C.; Pejovic, T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am. J. Obstet. Gynecol. 2015, 213, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Rovere Querini, P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 1–14. [Google Scholar] [CrossRef]
- Jiang, X.; Morland, S.J.; Hitchcock, A.; Thomas, E.J.; Campbell, I.G. Allelotyping of Endometriosis with Adjacent Ovarian Carcinoma Reveals Evidence of a Common Lineage. Cancer Res. 1998, 58, 1707–1712. [Google Scholar] [PubMed]
- Brilhante, A.V.; Augusto, K.L.; Portela, M.C.; Sucupira, L.C.; Oliveira, L.A.; Pouchaim, A.J.; Nóbrega, L.R.; Magalhães, T.F.; Sobreira, L.R. Endometriosis and ovarian cancer: An integrative review (endometriosis and ovarian cancer). Asian Pac. J. Cancer Prev. 2017, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Mangili, G.; Bergamini, A.; Taccagni, G.; Gentile, C.; Panina, P.; Viganò, P.; Candiani, M. Unraveling the two entities of endometrioid ovarian cancer: A single center clinical experience. Gynecol. Oncol. 2012, 126, 403–407. [Google Scholar] [CrossRef]
- Pierson, W.E.; Peters, P.N.; Chang, M.T.; Chen, L.M.; Quigley, D.A.; Ashworth, A.; Chapman, J.S. An integrated molecular profile of endometrioid ovarian cancer. Gynecol. Oncol. 2020, 157, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Labidi-Galy, S.I.; Moschetta, M.; Uccello, M.; Kalaitzopoulos, D.R.; Perez-Fidalgo, J.A.; Boussios, S. Endometriosis-associated ovarian carcinomas: Insights into pathogenesis, diagnostics, and therapeutic targets—A narrative review. Ann. Transl. Med. 2020, 8, 1712. [Google Scholar] [CrossRef]
- Howitt, B.E.; Strickland, K.C.; Sholl, L.M.; Rodig, S.; Ritterhouse, L.L.; Chowdhury, D.; Matulonis, U.A.; D’Andrea, A.D.; Konstantinopoulos, P.A. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology 2017, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Tanaka, N.; Ohira, M.; Itami, M.; Hippo, Y.; Nagase, H. Identification of novel mutations in Japanese ovarian clear cell carcinoma patients using optimized targeted NGS for clinical diagnosis. Gynecol. Oncol. 2017, 144, 377–383. [Google Scholar] [CrossRef]
- Boussios, S.; Mikropoulos, C.; Samartzis, E.; Karihtala, P.; Moschetta, M.; Sheriff, M.; Pavlidis, N. Wise management of ovarian cancer: On the cutting edge. J. Pers. Med. 2020, 10, 41. [Google Scholar] [CrossRef]
- Ricciardi, E.; Baert, T.; Ataseven, B.; Heitz, F.; Prader, S.; Bommert, M.; Harter, P. Low-grade serous ovarian carcinoma. Geburtshilfe Frauenheilkd. 2018, 78, 972. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, S.; Friedrich, K.; Redline, R.; Avril, S. Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2017, 470, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Chauleur, C.; Corsini, T.; Venet, M.; Habougit, C.; Honeyman, F.; Forest, F.; Peoc’h, M. Seromucinous ovarian tumor A comparison with the rest of ovarian epithelial tumors. Ann. Diagn. Pathol. 2017, 27, 28–33. [Google Scholar] [CrossRef]
- Verta, S.; Kipp, B. Ultraconservative, Fertility Sparing Treatment of Bilateral Borderline Ovarian Tumors: A Case Report of a 26-Year-Old, 0-Gravida with an Endometrioid Borderline Ovarian Tumor of the Right Ovary and a Sero-Mucinous Borderline Ovarian Tumor of the Left Ovary and a Review of the Literature. Int. J. Womens Health 2020, 12, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Matias-Guiu, X.; Stewart, C.J. Endometriosis-associated ovarian neoplasia. Pathology 2018, 50, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.E.; Abrams, S.I.; Moysich, K.B. A Call for Epidemiological Research on Myeloid-Derived Suppressor Cells in Ovarian Cancer: A Review of the Existing Immunological Evidence and Suggestions for Moving Forward. Front. Immunol. 2019, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Endometriosis | Ovarian Cancer |
|---|---|---|
| Uncontrolled growth | ✓ | ✓ |
| Ability to invade other tissues | ✓ | ✓ |
| Proliferation via blood and lymphatic vessels | ✓ | ✓ |
| Neoangiogenesis | ✓ | ✓ |
| Lower ability to undergo apoptosis | ✓ | ✓ |
| Local inflammation | ✓ | ✓ |
| Increased volume of peritoneal fluid | ✓ | ✓ |
| Presence of LOH (loss of heterozygosity) | ✓ | ✓ |
| Mutation in genes TP53, KRAS, PTEN, PIK3CA, and ARID1A | ✓ | ✓ |
| High risk of return of the disease | ✓ | ✓ |
| Oral contraceptive pills—lower risk of development of the disease | ✓ | ✓ |
| Fallopian tube ligation—lower risk of development of the disease | ✓ | ✓ |
| Hysterectomy—lower risk of development of the disease | ✓ | ✓ |
| Multiple pregnancies—lower risk of development of the disease | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suszczyk, D.; Skiba, W.; Jakubowicz-Gil, J.; Kotarski, J.; Wertel, I. The Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Development and/or Progression of Endometriosis-State of the Art. Cells 2021, 10, 677. https://doi.org/10.3390/cells10030677
Suszczyk D, Skiba W, Jakubowicz-Gil J, Kotarski J, Wertel I. The Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Development and/or Progression of Endometriosis-State of the Art. Cells. 2021; 10(3):677. https://doi.org/10.3390/cells10030677
Chicago/Turabian StyleSuszczyk, Dorota, Wiktoria Skiba, Joanna Jakubowicz-Gil, Jan Kotarski, and Iwona Wertel. 2021. "The Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Development and/or Progression of Endometriosis-State of the Art" Cells 10, no. 3: 677. https://doi.org/10.3390/cells10030677
APA StyleSuszczyk, D., Skiba, W., Jakubowicz-Gil, J., Kotarski, J., & Wertel, I. (2021). The Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Development and/or Progression of Endometriosis-State of the Art. Cells, 10(3), 677. https://doi.org/10.3390/cells10030677