One Brain—All Cells: A Comprehensive Protocol to Isolate All Principal CNS-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and EAE Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Active EAE Model
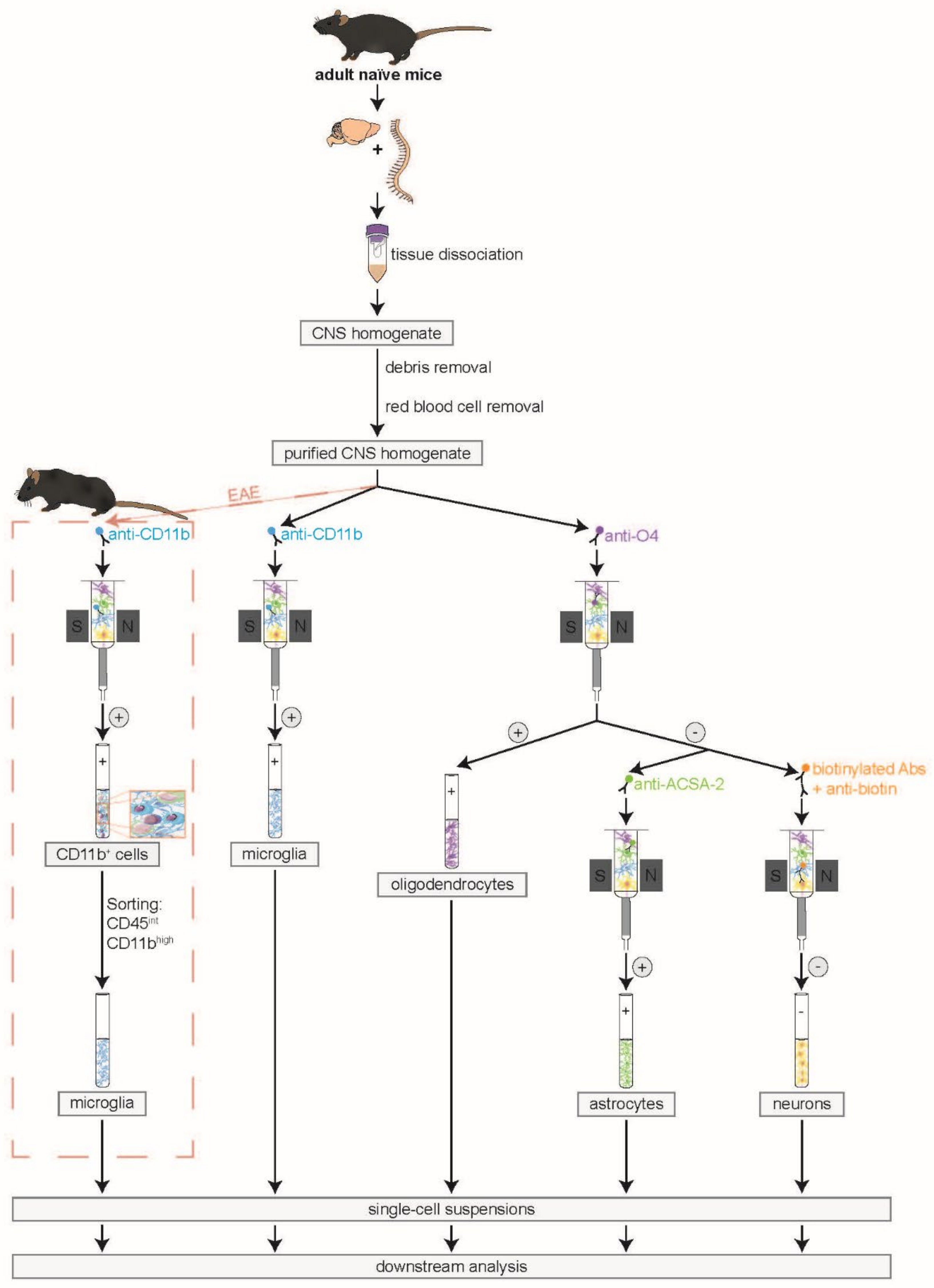
2.3. Isolation of Murine CNS-Resident Cells
2.3.1. Dissection of the CNS
2.3.2. CNS Tissue Dissociation
2.3.3. Debris and Red Blood Cell Removal
2.3.4. MACS in Naïve and EAE Mice
2.3.5. Amendment of the Protocol for Isolation of CNS-Resident Cells from EAE Mice
2.4. Purity Analyses of Isolated CNS-Resident Cells
2.5. Cultivation of Isolated CNS-Resident Cells
2.6. Validation of Isolated CNS-Resident Cells
2.6.1. Immunocytochemistry
2.6.2. Enzyme-Linked Immunosorbent Assays
2.6.3. Sholl Analysis
2.6.4. Ly6 Staining
2.7. Statistical Analysis
3. Results
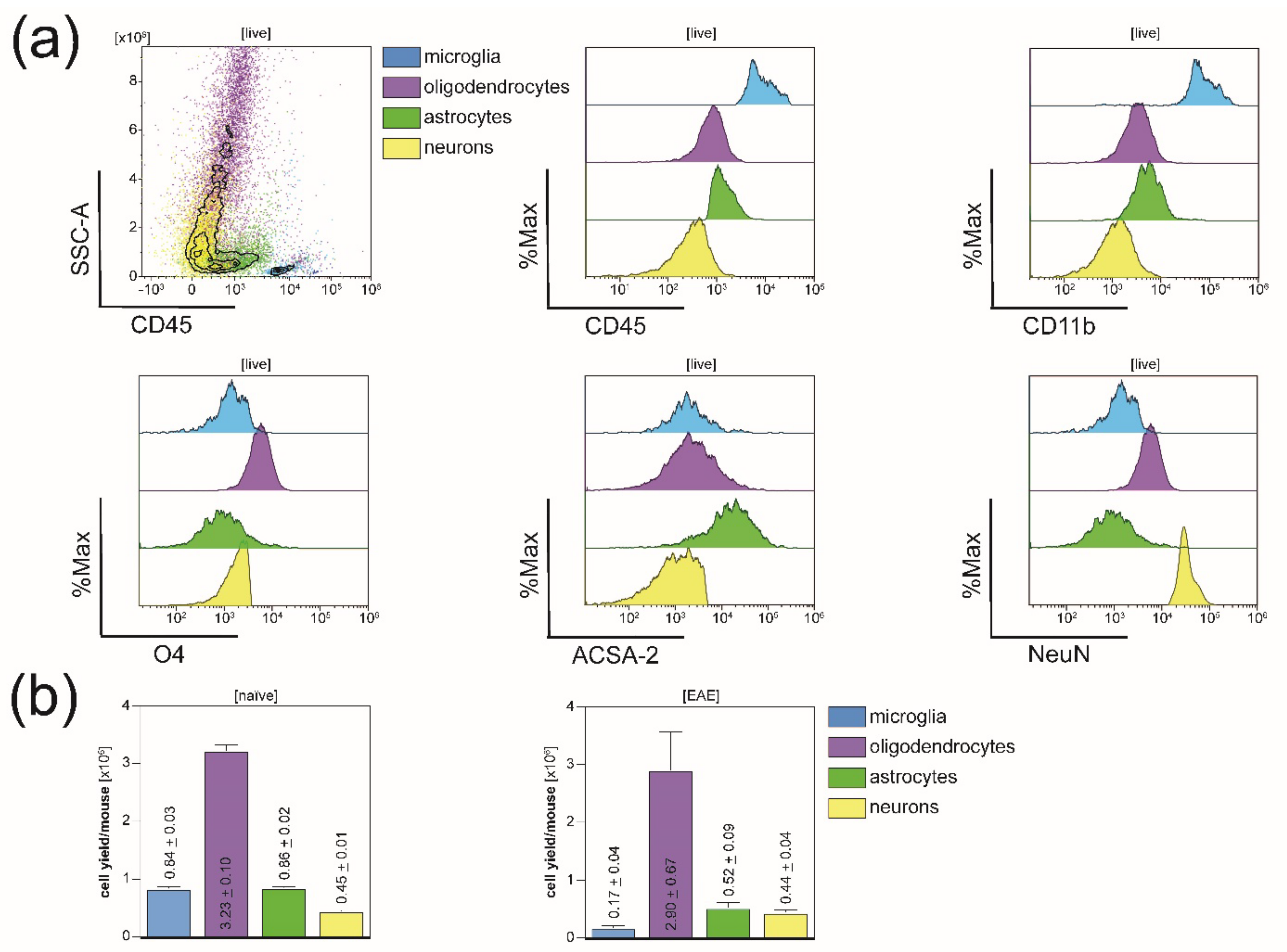
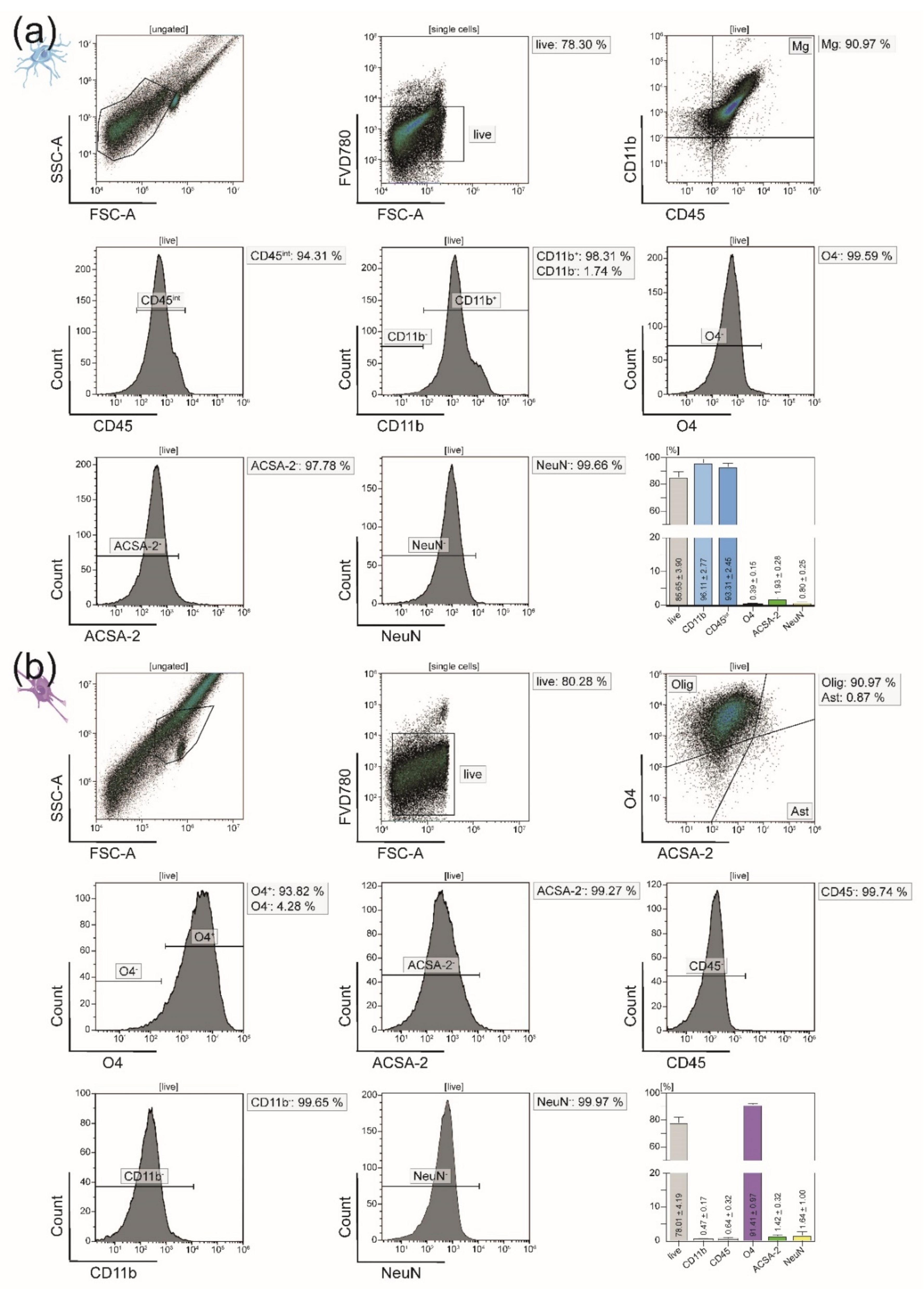
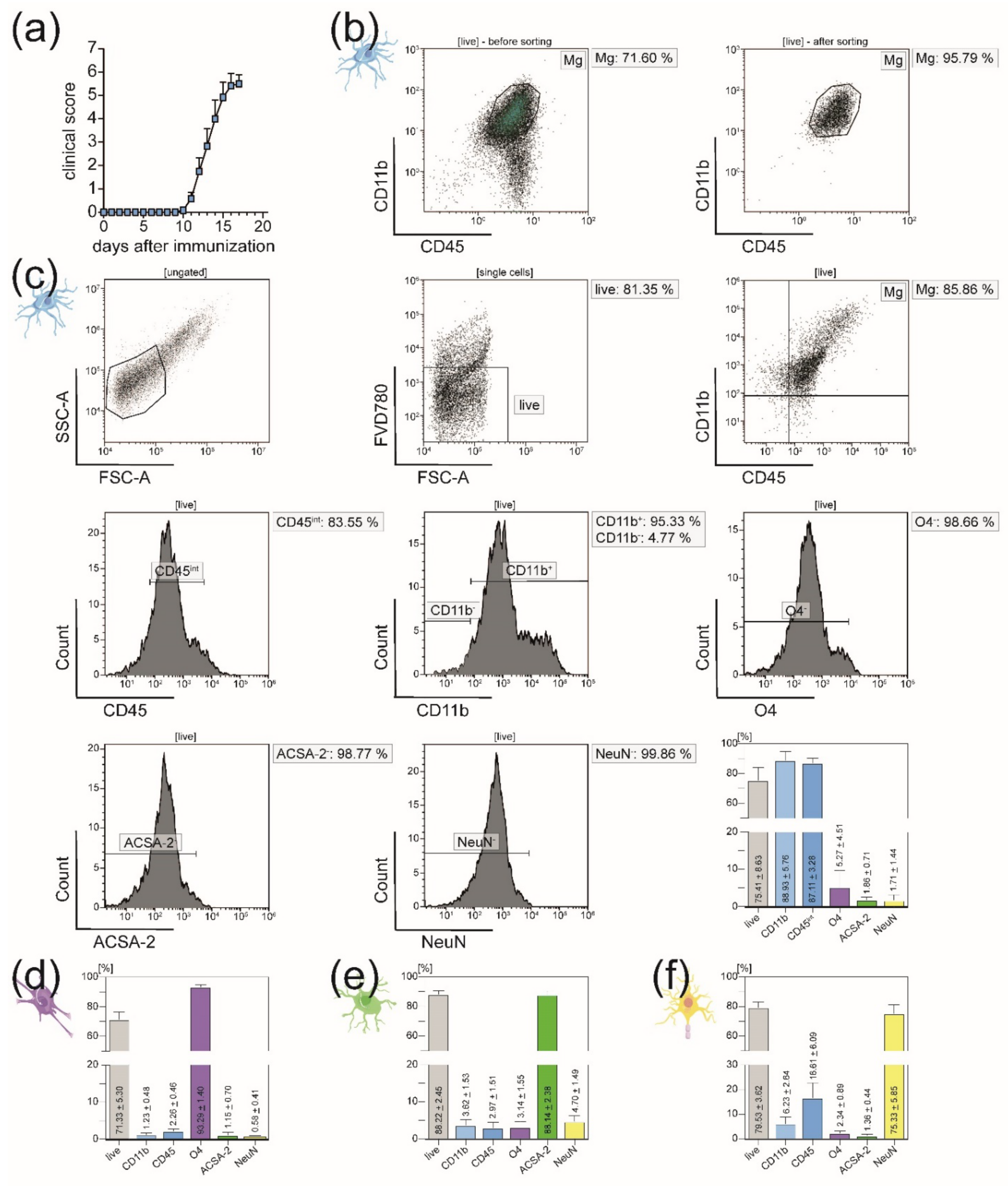
3.1. All Principal CNS-Resident Cell Types can Be Isolated Simultaneously from One CNS Homogenate with a Purity of Around 90%
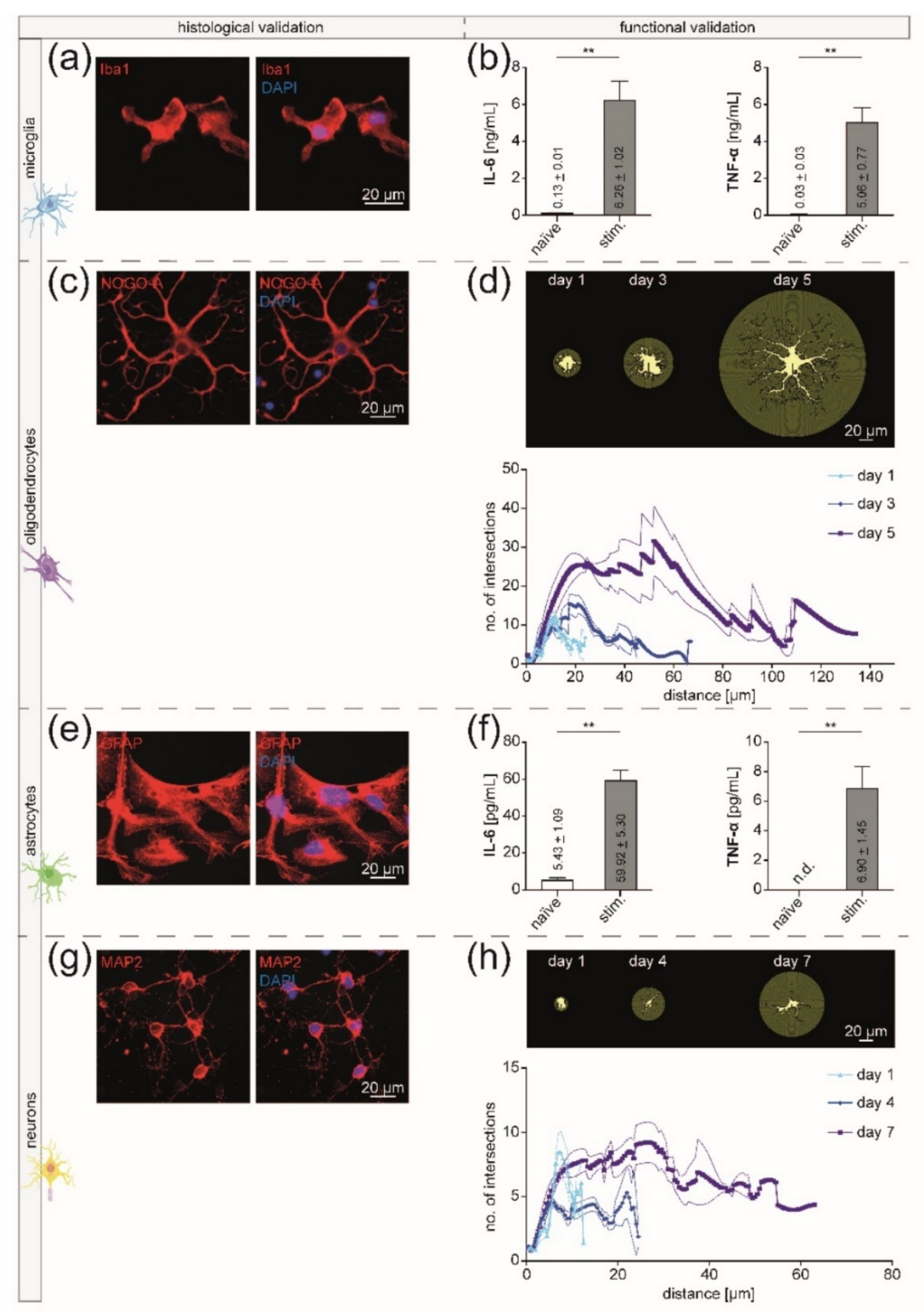
3.2. Isolated CNS-Resident Cells Showed a Cell-Type Specific Morphology and Functionality during Cultivation
3.3. In EAE Mice, Immigration of Peripheral Immune Cells into the CNS Necessitates Sorting of Microglia from the CD11b+ Cell Population after MACS
4. Discussion
- A positive influence on the three Rs of animal experiments: Replacement, Reduction, and Refinement.
- The assessment of cell-cell interactions and characteristics on an individual level. A way to overcome variability within groups: correlating phenotypic characteristics (e.g., behavioral scores) with comprehensive ex vivo analyses of all four major CNS cell types from the same individual animal.
- The chance to investigate complex cellular networks, e.g., neuronal networks and neuroinflammatory pathways ex vivo.
- The feasibility of multi-omic analyses from one individual CNS homogenate.
- The prospect of studying CNS-resident cells through different stages of a disease course, e.g., neuroinflammation, neurodegeneration, and remission in EAE or other disease models.
- The option of cultivating a fraction of the isolated adult CNS-resident cells in monocultures, allowing for further targeted functional assays.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trapp, B.D.; Nave, K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Stys, P.K.; Zamponi, G.W.; van Minnen, J.; Geurts, J.J.G. Will the Real Multiple Sclerosis Please Stand Up? Nat. Rev. Neurosci. 2012, 13, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Oksenberg, J.R. The Neurobiology of Multiple Sclerosis: Genes, Inflammation, and Neurodegeneration. Neuron 2006, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Korn, T. Pathophysiology of Multiple Sclerosis. J. Neurol. 2008, 255, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Afzali, A.M.; Wiendl, H.; Meuth, S.G. Myelin Oligodendrocyte Glycoprotein (MOG35–55) Induced Experimental Autoimmune Encephalomyelitis (EAE) in C57BL/6 Mice. J. Vis. Exp. 2014, 75, e51275. [Google Scholar] [CrossRef]
- Mendel, I.; de Rosbo, N.K.; Ben-Nun, A. A Myelin Oligodendrocyte Glycoprotein Peptide Induces Typical Chronic Experimental Autoimmune Encephalomyelitis in H-2b Mice: Fine Specificity and T Cell Receptor V Beta Expression of Encephalitogenic T Cells. Eur. J. Immunol. 1995, 25, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Lincoln, M.R.; Ramagopalan, S.V. Of Mice and Men: Experimental Autoimmune Encephalitis and Multiple Sclerosis. Eur. J. Clin. Investig. 2011, 41, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Bauer, M.A.; Ehling, P.; Bobak, N.; Breuer, J.; Herrmann, A.M.; Golfels, M.; Wiendl, H.; Budde, T.; Meuth, S.G. The TASK1 Channel Inhibitor A293 Shows Efficacy in a Mouse Model of Multiple Sclerosis. Exp. Neurol. 2012, 238, 149–155. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Wekerle, H. EAE: An Immunologist’s Magic Eye. Eur. J. Immunol. 2009, 39, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Asaridou, C.-M.; Merker, M.; Eichler, S.; Herrmann, A.M.; Geuß, E.; Ruck, T.; Schüngel, L.; Groeneweg, L.; Narayanan, V.; et al. Plasma Kallikrein Modulates Immune Cell Trafficking during Neuroinflammation via PAR2 and Bradykinin Release. Proc. Natl. Acad. Sci. USA 2019, 116, 271–276. [Google Scholar] [CrossRef]
- Hundehege, P.; Fernandez-Orth, J.; Römer, P.; Ruck, T.; Müntefering, T.; Eichler, S.; Cerina, M.; Epping, L.; Albrecht, S.; Menke, A.F.; et al. Targeting Voltage-Dependent Calcium Channels with Pregabalin Exerts a Direct Neuroprotective Effect in an Animal Model of Multiple Sclerosis. Neurosignals 2018, 26, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Galatro, T.F.; Vainchtein, I.D.; Brouwer, N.; Boddeke, E.W.G.M.; Eggen, B.J.L. Isolation of Microglia and Immune Infiltrates from Mouse and Primate Central Nervous System. Methods Mol. Biol. 2017, 1559, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tsatas, O.; Ghasemlou, N. Isolation and RNA Purification of Macrophages/Microglia from the Adult Mouse Spinal Cord. J. Immunol. Methods 2020, 477, 112678. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Torricelli, J.R. Isolation and Culture of Adult Neurons and Neurospheres. Nat. Protoc. 2007, 2, 1490–1498. [Google Scholar] [CrossRef]
- Berl, S.; Karram, K.; Scheller, A.; Jungblut, M.; Kirchhoff, F.; Waisman, A. Enrichment and Isolation of Neurons from Adult Mouse Brain for Ex Vivo Analysis. J. Neurosci. Methods 2017, 283, 15–22. [Google Scholar] [CrossRef]
- Sánchez-Gómez, M.V.; Serrano, M.P.; Alberdi, E.; Pérez-Cerdá, F.; Matute, C. Isolation, Expansion, and Maturation of Oligodendrocyte Lineage Cells Obtained from Rat Neonatal Brain and Optic Nerve. Methods Mol. Biol. 2018, 1791, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Tan, S.W.; Tong, C.K.; Vidyadaran, S. Isolation and Characterization of Primary Microglia from Post-Natal Murine Brain Tissues: A Comparison of Two Methods. Cell Biol. Int. 2015, 39, 1355–1363. [Google Scholar] [CrossRef]
- Feldmann, M.; Pathipati, P.; Sheldon, R.A.; Jiang, X.; Ferriero, D.M. Isolating Astrocytes and Neurons Sequentially from Postnatal Murine Brains with a Magnetic Cell Separation Technique. J. Biol. Methods 2014, 1, 11. [Google Scholar] [CrossRef]
- Lian, H.; Roy, E.; Zheng, H. Protocol for Primary Microglial Culture Preparation. Biol. Protoc. 2016, 6, e1989. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.G.; Herrmann, A.M.; Simon, O.J.; Siffrin, V.; Melzer, N.; Bittner, S.; Meuth, P.; Langer, H.F.; Hallermann, S.; Boldakowa, N.; et al. Cytotoxic CD8+ T Cell-Neuron Interactions: Perforin-Dependent Electrical Silencing Precedes but Is Not Causally Linked to Neuronal Cell Death. J. Neurosci. 2009, 29, 15397–15409. [Google Scholar] [CrossRef]
- Lafon, M.; Mégret, F.; Meuth, S.G.; Simon, O.; Romero, M.L.V.; Lafage, M.; Chen, L.; Alexopoulou, L.; Flavell, R.A.; Prehaud, C.; et al. Detrimental Contribution of the Immuno-Inhibitor B7-H1 to Rabies Virus Encephalitis. J. Immunol. 2008, 180, 7506–7515. [Google Scholar] [CrossRef] [PubMed]
- Holt, L.M.; Stoyanof, S.T.; Olsen, M.L. Magnetic Cell Sorting for In Vivo and In Vitro Astrocyte, Neuron, and Microglia Analysis. Curr. Protoc. Neurosci. 2019, 88, e71. [Google Scholar] [CrossRef]
- Swire, M.; Ffrench-Constant, C. Oligodendrocyte-Neuron Myelinating Coculture. Methods Mol. Biol. 2019, 1936, 111–128. [Google Scholar] [CrossRef]
- Park, J.; Koito, H.; Li, J.; Han, A. Microfluidic Compartmentalized Co-Culture Platform for CNS Axon Myelination Research. Biomed. Microdevices 2009, 11, 1145–1153. [Google Scholar] [CrossRef]
- Facci, L.; Barbierato, M.; Skaper, S.D. Astrocyte/Microglia Cocultures as a Model to Study Neuroinflammation. Methods Mol. Biol. 2018, 1727, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.V.; Cook, D.; Murai, K.K. A Neuron-Astrocyte Co-Culture System to Investigate Astrocyte-Secreted Factors in Mouse Neuronal Development. Methods Mol. Biol. 2012, 814, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Simpson, K.; Miguel-Hidalgo, J.J.; Savich, R. Neuron/Oligodendrocyte Myelination Coculture. Methods Mol. Biol. 2018, 1791, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Roqué, P.J.; Costa, L.G. Co-Culture of Neurons and Microglia. Curr. Protoc. Toxicol. 2017, 74, 1–17. [Google Scholar] [CrossRef]
- Speicher, A.M.; Wiendl, H.; Meuth, S.G.; Pawlowski, M. Generating Microglia from Human Pluripotent Stem Cells: Novel in Vitro Models for the Study of Neurodegeneration. Mol. Neurodegener. 2019, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, M.; Ortmann, D.; Bertero, A.; Tavares, J.M.; Pedersen, R.A.; Vallier, L.; Kotter, M.R.N. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Rep. 2017, 8, 803–812. [Google Scholar] [CrossRef]
- Moghadam, F.H.; Sadeghi-Zadeh, M.; Alizadeh-Shoorjestan, B.; Dehghani-Varnamkhasti, R.; Narimani, S.; Darabi, L.; Esfahani, A.K.; Esfahani, M.H.N. Isolation and Culture of Embryonic Mouse Neural Stem Cells. J. Vis. Exp. 2018, 74, e58874. [Google Scholar] [CrossRef]
- Pandya, H.; Shen, M.J.; Ichikawa, D.M.; Sedlock, A.B.; Choi, Y.; Johnson, K.R.; Kim, G.; Brown, M.A.; Elkahloun, A.G.; Maric, D.; et al. Differentiation of Human and Murine Induced Pluripotent Stem Cells to Microglia-like Cells. Nat. Neurosci. 2017, 20, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Vadodaria, K.C.; Jaeger, B.N.; Mei, A.; Lefcochilos-Fogelquist, S.; Mendes, A.P.D.; Erikson, G.; Shokhirev, M.; Randolph-Moore, L.; Fredlender, C.; et al. Differentiation of Inflammation-Responsive Astrocytes from Glial Progenitors Generated from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Tcw, J.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H.; et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High Gradient Magnetic Cell Separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjöström, P.J.; van Meyel, D.J. Neuronal Morphometry Directly from Bitmap Images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Haage, V.; Semtner, M.; Vidal, R.O.; Hernandez, D.P.; Pong, W.W.; Chen, Z.; Hambardzumyan, D.; Magrini, V.; Ly, A.; Walker, J.; et al. Comprehensive Gene Expression Meta-Analysis Identifies Signature Genes That Distinguish Microglia from Peripheral Monocytes/Macrophages in Health and Glioma. Acta Neuropathol. Commun. 2019, 7, 20. [Google Scholar] [CrossRef]
- Kosior, N.; Petkau, T.L.; Connolly, C.; Lu, G.; Leavitt, B.R. Isolating Cells from Adult Murine Brain for Validation of Cell-Type Specific Cre-Mediated Deletion. J. Neurosci. Methods 2019, 328, 108422. [Google Scholar] [CrossRef]
- Hickey, W.F.; Hsu, B.L.; Kimura, H. T-Lymphocyte Entry into the Central Nervous System. J. Neurosci. Res. 1991, 28, 254–260. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, Crawl, Cross: The T Cell Code to Breach the Blood-Brain Barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef]
- Man, S.; Ubogu, E.E.; Ransohoff, R.M. Inflammatory Cell Migration into the Central Nervous System: A Few New Twists on an Old Tale. Brain Pathol. 2007, 17, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/Macrophage-Specific Protein Iba1 Binds to Fimbrin and Enhances Its Actin-Bundling Activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Remington, L.; Maruschak, B.; Owens, T.; Brück, W. Nogo-A Is a Reliable Oligodendroglial Marker in Adult Human and Mouse CNS and in Demyelinated Lesions. J. Neuropathol. Exp. Neurol. 2007, 66, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, J.; Hol, E.M. GFAP in Health and Disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Soltani, M.H.; Pichardo, R.; Song, Z.; Sangha, N.; Camacho, F.; Satyamoorthy, K.; Sangueza, O.P.; Setaluri, V. Microtubule-Associated Protein 2, a Marker of Neuronal Differentiation, Induces Mitotic Defects, Inhibits Growth of Melanoma Cells, and Predicts Metastatic Potential of Cutaneous Melanoma. Am. J. Pathol. 2005, 166, 1841–1850. [Google Scholar] [CrossRef]
- Greter, M.; Lelios, I.; Croxford, A.L. Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation. Front. Immunol. 2015, 6, 249. [Google Scholar] [CrossRef]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, Evolution and Functions of the Human and Mouse Ly6/UPAR Family Genes. Hum. Genom. 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 Family Proteins in Neutrophil Biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.K.; Park, J.H. Characterization and Function of Human Ly-6/UPAR Molecules. BMB Rep. 2012, 45, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P. Replacement, Reduction and Refinement. ALTEX 2002, 19, 73–78. [Google Scholar]
- Sankowski, R.; Böttcher, C.; Masuda, T.; Geirsdottir, L.; Sagar, N.; Sindram, E.; Seredenina, T.; Muhs, A.; Scheiwe, C.; Shah, M.J.; et al. Mapping Microglia States in the Human Brain through the Integration of High-Dimensional Techniques. Nat. Neurosci. 2019, 22, 2098–2110. [Google Scholar] [CrossRef]
- Enright, H.A.; Lam, D.; Sebastian, A.; Sales, A.P.; Cadena, J.; Hum, N.R.; Osburn, J.J.; Peters, S.K.G.; Petkus, B.; Soscia, D.A.; et al. Functional and Transcriptional Characterization of Complex Neuronal Co-Cultures. Sci. Rep. 2020, 10, s41598. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.A.M.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Basri, S.M.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019, 56, 8489–8512. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.; Goh, E. Blood-Brain Barrier on a Chip. Methods Cell Biol. 2018, 146, 159–182. [Google Scholar] [CrossRef]
- Miccoli, B.; Braeken, D.; Li, Y.-C.E. Brain-on-a-Chip Devices for Drug Screening and Disease Modeling Applications. Curr. Pharm. Des. 2018, 24, 5419–5436. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral Organoids at the Air-Liquid Interface Generate Diverse Nerve Tracts with Functional Output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar] [CrossRef]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS Barrier-Forming Organoids with Cerebrospinal Fluid Production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.-F. Blood-Brain-Barrier Organoids for Investigating the Permeability of CNS Therapeutics. Nat. Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef]
- Bian, S.; Repic, M.; Guo, Z.; Kavirayani, A.; Burkard, T.; Bagley, J.A.; Krauditsch, C.; Knoblich, J.A. Author Correction: Genetically Engineered Cerebral Organoids Model Brain Tumor Formation. Nat. Methods 2018, 15, 748. [Google Scholar] [CrossRef]
- Chhibber, T.; Bagchi, S.; Lahooti, B.; Verma, A.; Al-Ahmad, A.; Paul, M.K.; Pendyala, G.; Jayant, R.D. CNS Organoids: An Innovative Tool for Neurological Disease Modeling and Drug Neurotoxicity Screening. Drug Discov. Today 2020, 25, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.-N.; Xu, T.-Y.; Miao, Z.-W.; Su, D.-F.; Miao, C.-Y. Organoid Technology for Brain and Therapeutics Research. CNS Neurosci. Ther. 2017, 23, 771–778. [Google Scholar] [CrossRef]
- Sutermaster, B.A.; Darling, E.M. Considerations for High-Yield, High-Throughput Cell Enrichment: Fluorescence versus Magnetic Sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wan, J. Methodological Comparison of FACS and MACS Isolation of Enriched Microglia and Astrocytes from Mouse Brain. J. Immunol. Methods 2020, 486, 112834. [Google Scholar] [CrossRef]
- Holt, L.M.; Olsen, M.L. Novel Applications of Magnetic Cell Sorting to Analyze Cell-Type Specific Gene and Protein Expression in the Central Nervous System. PLoS ONE 2016, 11, e0150290. [Google Scholar] [CrossRef] [PubMed]
- Shirao, T.; Sekino, Y. Clustering and Anchoring Mechanisms of Molecular Constituents of Postsynaptic Scaffolds in Dendritic Spines. Neurosci. Res. 2001, 40, 1–7. [Google Scholar] [CrossRef]
- Jacob, A.L.; Weinberg, R.J. The Organization of AMPA Receptor Subunits at the Postsynaptic Membrane. Hippocampus 2015, 25, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Mathiisen, T.M.; Ottersen, O.P. Aquaporin-4 in the Central Nervous System: Cellular and Subcellular Distribution and Coexpression with KIR4.1. Neuroscience 2004, 129, 905–913. [Google Scholar] [CrossRef]
- Nagy, J.I.; Rash, J.E. Astrocyte and Oligodendrocyte Connexins of the Glial Syncytium in Relation to Astrocyte Anatomical Domains and Spatial Buffering. Cell Commun. Adhes. 2003, 10, 401–406. [Google Scholar] [CrossRef]
- Bowles, K.R.; Tcw, J.; Qian, L.; Jadow, B.M.; Goate, A.M. Reduced Variability of Neural Progenitor Cells and Improved Purity of Neuronal Cultures Using Magnetic Activated Cell Sorting. PLoS ONE 2019, 14, e0213374. [Google Scholar] [CrossRef]
- Rangaraju, S.; Raza, S.A.; Li, N.X.; Betarbet, R.; Dammer, E.B.; Duong, D.; Lah, J.J.; Seyfried, N.T.; Levey, A.I. Differential Phagocytic Properties of CD45low Microglia and CD45high Brain Mononuclear Phagocytes—Activation and Age-Related Effects. Front. Immunol. 2018, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.L.; Goodsall, A.L.; Hickey, W.F.; Sedgwick, J.D. Normal Adult Ramified Microglia Separated from Other Central Nervous System Macrophages by Flow Cytometric Sorting. Phenotypic Differences Defined and Direct Ex Vivo Antigen Presentation to Myelin Basic Protein-Reactive CD4+ T Cells Compared. J. Immunol. 1995, 154, 4309–4321. [Google Scholar]
- DePaula-Silva, A.B.; Gorbea, C.; Doty, D.J.; Libbey, J.E.; Sanchez, J.M.S.; Hanak, T.J.; Cazalla, D.; Fujinami, R.S. Differential Transcriptional Profiles Identify Microglial- and Macrophage-Specific Gene Markers Expressed during Virus-Induced Neuroinflammation. J. Neuroinflamm. 2019, 16, 152. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Ji, K.; Min, K.; Joe, E.-H. Brain Inflammation and Microglia: Facts and Misconceptions. Exp. Neurobiol. 2013, 22, 59–67. [Google Scholar] [CrossRef]
- Li, Q.; Lan, X.; Han, X.; Wang, J. Expression of Tmem119/Sall1 and Ccr2/CD69 in FACS-Sorted Microglia- and Monocyte/Macrophage-Enriched Cell Populations After Intracerebral Hemorrhage. Front. Cell. Neurosci. 2019, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Jung, S. Monocytes: Subsets, Origins, Fates and Functions. Curr. Opin. Hematol. 2010, 17, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Misharin, A.; Perlman, H. A Novel Ly6C/Ly6G-Based Strategy to Analyze the Mouse Splenic Myeloid Compartment. Cytometry 2012, 81, 343–350. [Google Scholar] [CrossRef]
- Pyo, H.; Jou, I.; Jung, S.; Hong, S.; Joe, E. Mitogen-Activated Protein Kinases Activated by Lipopolysaccharide and β-Amyloid in Cultured Rat Microglia. NeuroReport 1998, 9, 871–874. [Google Scholar] [CrossRef]
- Pyo, H.; Joe, E.; Jung, S.; Lee, S.H.; Jou, I. Gangliosides Activate Cultured Rat Brain Microglia. J. Biol. Chem. 1999, 274, 34584–34589. [Google Scholar] [CrossRef]
- Ryu, J.; Pyo, H.; Jou, I.; Joe, E. Thrombin Induces NO Release from Cultured Rat Microglia via Protein Kinase C, Mitogen-Activated Protein Kinase, and NF-ΚB. J. Biol. Chem. 2000, 275, 29955–29959. [Google Scholar] [CrossRef]
- Min, K.; Jou, I.; Joe, E. Plasminogen-Induced IL-1β and TNF-α Production in Microglia Is Regulated by Reactive Oxygen Species. Biochem. Biophys. Res. Commun. 2003, 312, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of Microglia in Post-Mortem Brain Samples from Patients with Alzheimer’s Disease: A Systematic Review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Amann, L.; Sankowski, R.; Staszewski, O.; Lenz, M.; d’Errico, P.; Snaidero, N.; Jordão, M.J.C.; Böttcher, C.; Kierdorf, K.; et al. Novel Hexb-Based Tools for Studying Microglia in the CNS. Nat. Immunol. 2020, 21, 802–815, Erratum in 2020, 21, 1302, doi:10.1038/s41590-020-0774-6. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar, L.; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and Temporal Heterogeneity of Mouse and Human Microglia at Single-Cell Resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Mrdjen, D.; Pavlovic, A.; Hartmann, F.J.; Schreiner, B.; Utz, S.G.; Leung, B.P.; Lelios, I.; Heppner, F.L.; Kipnis, J.; Merkler, D.; et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 2018, 48, 380–395.e6. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Munoz-Manchado, A.B.; Codeluppi, S.; Lonnerberg, P.; La Manno, G.; Jureus, A.; Marques, S.; Munguba, H.; He, L.; Betsholtz, C.; et al. Cell Types in the Mouse Cortex and Hippocampus Revealed by Single-Cell RNA-Seq. Science 2015, 347, 1138–1142. [Google Scholar] [CrossRef]
- Van Wageningen, T.A.; Vlaar, E.; Kooij, G.; Jongenelen, C.A.M.; Geurts, J.J.G.; van Dam, A.-M. Regulation of Microglial TMEM119 and P2RY12 Immunoreactivity in Multiple Sclerosis White and Grey Matter Lesions Is Dependent on Their Inflammatory Environment. Acta Neuropathol. Commun. 2019, 7, 206. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F.C.; Liddelow, S.A.; Ajami, B.; Zamanian, J.L.; Fernhoff, N.B.; Mulinyawe, S.B.; Bohlen, C.J.; Adil, A.; Tucker, A.; et al. New Tools for Studying Microglia in the Mouse and Human CNS. Proc. Natl. Acad. Sci. USA 2016, 113, E1738–E1746. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a Unique TGF-β–Dependent Molecular and Functional Signature in Microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Kuil, L.E.; Martí, A.L.; Mascaro, A.C.; Bosch, J.C.; Berg, P.; Linde, H.C.; Schoonderwoerd, K.; Ruijter, G.J.G.; Ham, T.J. Hexb Enzyme Deficiency Leads to Lysosomal Abnormalities in Radial Glia and Microglia in Zebrafish Brain Development. Glia 2019, 67, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in Membrane Ruffling and Phagocytosis of Macrophages/Microglia. J. Cell Sci. 2000, 113 Pt 17, 3073–3084. [Google Scholar]
- Streit, W.J.; Braak, H.; Xue, Q.-S.; Bechmann, I. Dystrophic (Senescent) Rather than Activated Microglial Cells Are Associated with Tau Pathology and Likely Precede Neurodegeneration in Alzheimer’s Disease. Acta Neuropathol. 2009, 118, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Dal Bianco, A.; Bradl, M.; Frischer, J.; Kutzelnigg, A.; Jellinger, K.; Lassmann, H. Multiple Sclerosis and Alzheimer’s Disease. Ann. Neurol. 2008, 63, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhu, K.; Zhou, K.; Hakim, R.; Sankavaram, S.R.; Blomgren, K.; Lund, H.; Zhang, X.-M.; Harris, R.A. Sex-Specific Effects of Microglia-Like Cell Engraftment during Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2020, 21, 6824. [Google Scholar] [CrossRef] [PubMed]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e6. [Google Scholar] [CrossRef]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef] [PubMed]


| Microglia | Oligodendrocytes | |
|---|---|---|
| Positive Selection | Positive Selection | |
| 1. Blocking | Starting volume was adjusted to 90 µL PB buffer per 1 × 107 cells. Cells were resuspended carefully. | Starting volume was adjusted to 80 µL PB buffer per 1 × 107 cells. Cells were resuspended carefully. |
| Incubation for 10 min on ice. | FcR Blocking Reagent (mouse; Miltenyi Biotec) was added, 10 µL per 1 × 107 cells. Incubation for 10 min at 2–8 °C. | |
| 2. Magnetic labeling | CD11b MicroBeads (human, mouse; Miltenyi Biotec) were applied, 10 µL per 1 × 107 cells, and mixed with the cell suspension. | Anti-O4 MicroBeads (human, mouse, rat; Miltenyi Biotec) were applied, 10 µL per 1 × 107 cells, and mixed with the cell suspension. |
| Incubation for 15 min in the dark at 2–8 °C. | ||
| 3. Washing and preparation of columns | Cells were washed by adding 2 mL PB buffer per 1 × 107 cells followed by centrifugation at 300 g and 4 °C for 10 min. | |
| Meanwhile, one LS Column per 4 × 107 cells was placed with its column wings to the front in one of four gaps of a QuadroMACS Separator attached to a MultiStand (Miltenyi Biotec). Each column was equilibrated with 3 mL PB buffer. The flow-through was discarded. | ||
| 6. Magnetic separation—negative fraction | Upon termination of the centrifugation, the supernatant was aspirated completely. Up to 1 × 107 cells were resuspended in 500 µL PB buffer. The cell suspension was distributed among the utilized number of columns per cell type. The flow-through containing the unlabeled cells was collected in a new 15 mL falcon placed on ice underneath each column. | |
| The columns were washed three times with 3 mL PB buffer. The flow-through was pooled together with the unlabeled cells from the previous step in the 15 mL falcon. This negative fraction contained all non-targeted cells (CD11b−, O4− cells). | ||
| 7. Magnetic separation—positive fraction | The columns were removed from the magnetic field and placed on a new 15 mL falcon. PB buffer was added to each LS column (5 mL). The magnetically labeled cells were immediately flushed out by firmly pushing the provided plunger into the column. | |
| This positive fraction contained all targeted cells (microglia or CD11b+ cells and oligodendrocytes or O4+ cells). | ||
| 8. Counting of targeted cells | The total number of isolated cells was determined with the help of the electronic cell counter and analyzer system CASY TT (Roche Innovatis AG, Bielefeld, Germany). | |
| Settings for counting of microglia: Capillary: 150 µm, sample vol: 400 µL, x-axis: 30 µm, cycles: 3, dilution 2.01 × 102, y-axis: auto. Eval. Cursor: 8.03–30.00 µm, norm. cursor: 4.88–30.00 µm. % Calculation: % via, debris: off. | ||
| Settings for counting of oligodendrocytes: Capillary: 150 µm, sample vol: 400 µL, x-axis: 30 µm, cycles: 3, dilution 5.01 × 102, y-axis: auto. Eval. Cursor: 8.03–30.00 µm, norm. cursor: 6.00–30.00 µm. % Calculation: % via, debris: off. | ||
| Neurons | Astrocytes | |
|---|---|---|
| Negative Selection | Positive Selection | |
| 1. Blocking | Starting volume was adjusted to 80 µL PB buffer per 1 × 107 cells. Cells were carefully resuspended. | Starting volume was adjusted to 80 µL AstroMACS Separation buffer (Miltenyi Biotec) per 1 × 107 cells. Cells were carefully resuspended. |
| Incubation for 10 min on ice. | FcR Blocking Reagent was added, 10 µL per 1 × 107 cells. Incubation for 10 min at 2–8 °C. | |
| 2. Biotinylation of non-neuronal cells, magnetic labeling of astrocytes | Non-Neuronal Cell Biotin-Antibody Cocktail (mouse; Miltenyi Biotec) was applied, 20 µL per 1 × 107 cells, and mixed with the cell suspension. | Anti-ACSA-2 MicroBeads (mouse; Miltenyi Biotec) were applied, 10 µL per 1 × 107 cells, and mixed with the cell suspension. |
| Incubation for 5 min in the dark at 2–8 °C. | Incubation for 15 min in the dark at 2–8 °C. | |
| PB buffer was added, 1 mL per 1 × 107 cells, followed by centrifugation at 300× g and 4 °C for 5 Min. The supernatant was aspirated completely. The cell pellet was resuspended in 80 µL PB buffer per 1 × 107 cells. | ||
| 3. Magnetic labeling of biotinylated cells, washing of astrocytes, and preparation of columns | Anti-Biotin MicroBeads (mouse; Miltenyi Biotec) were added, 20 µL per 1 × 107 cells, and mixed with the cell suspension. | Cells were washed by adding 1 mL AstroMACS Separation buffer per 1 × 107 cells followed by centrifugation at 300× g and 4 °C for 10 min. |
| Incubation for 10 min in the dark at 2–8 °C. | ||
| Meanwhile, one LS Column per 4 × 107 cells was placed with its column wings to the front in one of four gaps of a QuadroMACS Separator attached to a MultiStand. | ||
| Each column was equilibrated with 3 mL PB buffer. The flow-through was discarded. | Each column was equilibrated with 3 mL AstroMACS Separation buffer. The flow-through was discarded. | |
| 6. Magnetic separation—negative cell fraction | Upon termination of the centrifugation, the supernatant was aspirated completely. | |
| Up to 1 × 107 cells were resuspended in 500 µL PB buffer. | Up to 1 × 107 cells were resuspended in 500 µL AstroMACS Separation buffer. | |
| The cell suspension was distributed among the utilized number of columns per cell type. The flow-through containing the unlabeled cells was collected in a new 15 mL falcon placed on ice underneath each column. | ||
| The columns were washed twice with 1 mL PB buffer. | The columns were washed three times with 3 mL AstroMACS Separation buffer. | |
| The flow-through was pooled together with the unlabeled cells from the previous step in the 15 mL falcon. | ||
| This negative fraction contained all targeted cells (neurons or Biotin− cells). | This negative fraction contained all non-targeted cells (ACSA-2− cells). | |
| 7. Magnetic separation—positive cell fraction | The columns were removed from the magnetic field and placed on a new 15 mL falcon. | |
| PB buffer was added to each LS column (5 mL). | AstroMACS Separation buffer was added to each LS column (5 mL). | |
| The magnetically labeled cells were immediately flushed out by firmly pushing the plunger into the column. | ||
| This positive fraction contained all non-targeted cells (non-neuronal or Biotin+ cells). | This positive fraction contained all targeted cells (astrocytes or ACSA-2+ cells). | |
| 8. Counting of targeted cells | The number of isolated cells was determined with the help of the electronic cell counter and analyzer system CASY TT (Roche Innovatis AG, Bielefeld, Germany). | |
| Settings for counting of neurons: Capillary: 150 µm, sample vol: 400 µL, x-axis: 30 µm, cycles: 5, dilution 1.00 × 103, y-axis: auto. Eval. Cursor: 8.03–25.80 µm, norm. cursor: 8.03–30.00 µm. % Calculation: % via, debris: off. | ||
| Settings for counting of astrocytes: Capillary: 150 µm, sample vol: 400 µL, x-axis: 20 µm, cycles: 3, dilution 2.01 × 102, y-axis: auto. Eval. Cursor: 6.95–20.00 µm, norm. cursor: 4.90–20.00 µm. % Calculation: % via, debris: off. | ||
| Header | Microglia | Astrocytes | Oligodendrocytes | Neurons |
|---|---|---|---|---|
| Coating | Four- or 24-wells were coated with 0.5% poly-L-lysine hydrobromide (Merck KGaA) overnight at 4 °C. | Four- or 24-wells were coated with 50 µg/mL poly-D-lysine hydrobromide (Merck KGaA) overnight at 4 °C. | ||
| Dishes were washed once with ddH2O before plating of cells. | Dishes were washed three times with ddH2O followed by a second coating with 10 µg/mL laminin for two hours at 37 °C. Subsequently, dishes were washed once with ddH2O before plating of cells. | Dishes were washed once with ddH2O before plating of cells. | ||
| Medium | Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific) | MACS Neuro Medium (Miltenyi Biotec) | ||
| +10% fetal bovine serum | +2% MACS NeuroBrew-21 (Miltenyi Biotec) | |||
| +1% non-essential amino acids solution (Thermo Fisher Scientific) | +1% penicillin-streptomycin | |||
| +1% penicillin-streptomycin | +0.25% GlutaMAX supplement | |||
| +0.1% 2-mercaptoethanol | ||||
| +1% GlutaMAX supplement (Thermo Fisher Scientific) | ||||
| +0.2% AstroMACS supplement (Miltenyi Biotec) | +10 ng/mL human platelet derived growth factor AA (PeproTech, NJ, USA) | |||
| +10 ng/mL human fibroblast growth factor 2 (PeproTech) | ||||
| +5 ng/mL human neurotrophin-3 (PeproTech) | ||||
| Cultivation | Per well, 2 × 105 cells were seeded. | Per well, 3 × 105 cells were seeded. | ||
| The isolated cells were resuspended in warm medium and plated as a drop of 50 µL in the middle of each coated 24-well. After incubation for 45 min in a 37 °C incubator (5% CO2), allowing the cells to settle down, 450 µL of warm medium was added carefully to each well. | ||||
| On the next morning, the medium was changed to remove all cell debris caused by the MACS procedure. | The whole medium was replaced immediately to remove all non-attached and dead cells. | |||
| Continuous cultivation at 37 °C (5% CO2). No further medium changes were performed afterward. Cells were inspected daily to check for their confluence and morphology. | ||||
| Further processing | Microglia were processed on day 2 of cultivation. | The cultivated cells were processed on day 5 of cultivation. | Neurons were processed on day 7 of cultivation. | |
| Cell Type | Primary Antibody (Host, Dilution; Company) | Secondary Antibody (Host, Dilution; Company) |
|---|---|---|
| Microglia | Ionized calcium-binding adapter molecule 1 (Iba-1, rabbit, 1:2000; FUJIFILM Wako Chemicals GmbH, Neuss, Germany) | AF594 (goat, 1:500; Thermo Fisher Scientific) |
| CD11b (rat, 1:100; Bio-Rad Laboratories, Santa Rosa, CA, USA) | Cy3 (donkey, 1:500; Dianova, Hamburg, Germany) | |
| Oligodendrocytes | NOGO-A (rabbit, 1:200; Merck KGaA) | AF594 (goat, 1:500; Thermo Fisher Scientific) |
| Astrocytes | Glial fibrillary acidic protein (GFAP, rabbit, 1:1000; Abcam) | Cy3 (goat, 1:300; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) |
| Neurons | Microtubule-associated protein 2 (MAP2, rabbit, 1:200; Santa Cruz Biotechnology, Street Dallas, TX, USA) | Cy3 (donkey, 1:500; Dianova) |
| FMO | Microglia | CD45highCD11b+ | |
|---|---|---|---|
| Ly6C | 1,499.65 | 1,733.91 | 5,735.73 |
| Ly6G | 1,497.07 | 1,803.32 | 5,485.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeter, C.B.; Herrmann, A.M.; Bock, S.; Vogelsang, A.; Eichler, S.; Albrecht, P.; Meuth, S.G.; Ruck, T. One Brain—All Cells: A Comprehensive Protocol to Isolate All Principal CNS-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and EAE Mice. Cells 2021, 10, 651. https://doi.org/10.3390/cells10030651
Schroeter CB, Herrmann AM, Bock S, Vogelsang A, Eichler S, Albrecht P, Meuth SG, Ruck T. One Brain—All Cells: A Comprehensive Protocol to Isolate All Principal CNS-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and EAE Mice. Cells. 2021; 10(3):651. https://doi.org/10.3390/cells10030651
Chicago/Turabian StyleSchroeter, Christina B., Alexander M. Herrmann, Stefanie Bock, Anna Vogelsang, Susann Eichler, Philipp Albrecht, Sven G. Meuth, and Tobias Ruck. 2021. "One Brain—All Cells: A Comprehensive Protocol to Isolate All Principal CNS-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and EAE Mice" Cells 10, no. 3: 651. https://doi.org/10.3390/cells10030651
APA StyleSchroeter, C. B., Herrmann, A. M., Bock, S., Vogelsang, A., Eichler, S., Albrecht, P., Meuth, S. G., & Ruck, T. (2021). One Brain—All Cells: A Comprehensive Protocol to Isolate All Principal CNS-Resident Cell Types from Brain and Spinal Cord of Adult Healthy and EAE Mice. Cells, 10(3), 651. https://doi.org/10.3390/cells10030651






