RNA Localization and Local Translation in Glia in Neurological and Neurodegenerative Diseases: Lessons from Neurons
Abstract
1. Introduction: What Is Local Protein Synthesis?
| Protein metabolic labeling | Technique | Label | Detection | References |
| BONCAT/ FUNCAT | Noncanonical aminoacids (azide or alkyne) | Covalent cycloaddition reaction with fluorescently tagged or biotinylated reactive group (alkyne or azide) | [18,19] | |
| SILAC/ pSILAC | Stable isotope | Mass spectrometry | [20,21] | |
| Puromycilation of nascent peptides | SUnSET/ PUNCH-P | tRNA analogue puromycin; biotinylated puromycin | Puromycin immunodetection; biotin-streptavidin conjugation; mass spectrometry following purification of tagged peptides | [22,23,24,25,26] |
| Translatomic approach | TRAP/ Ribo-Seq | Epitope-tagged ribosome | Epitope immunoprecipitation followed by mRNA purification and detection by RNA-Seq | [27,28] |
2. Neuronal RNA Localization, Local Translation and Nervous System Diseases
2.1. Brief Introduction to RNA Localization and Local Translation in Neuronal Processes
2.2. Neuronal Local Translation in NS Dysfunction
2.2.1. In Traumatic Nerve Injury
2.2.2. In Motor Neuron Diseases
2.2.3. In Alzheimer’s Disease and Dementia
2.2.4. In Movement Disorders
2.2.5. In Fragile X Syndrome, Autism Spectrum Disorders and Intellectual Disabilities
3. RNA Localization and Localized Translation in Glia
3.1. RNA Localization and Local Translation in Oligodendroglia
3.1.1. In Motor Neuron Diseases
3.1.2. In Demyelination:
3.1.3. In Alzheimer’s Disease and Dementias
3.1.4. In Fragile X Syndrome
3.2. RNA Localization and Local Translation in Astroglia
3.2.1. In Motor Neuron Diseases
3.2.2. In Alzheimer’s Disease and Dementia
3.2.3. In Movement Disorders
3.2.4. In Fragile X Syndrome
3.2.5. In Other Neurological Disorders
3.3. RNA Localization and Local Translation in Radial Glia
3.3.1. In Fragile X Syndrome, Autism Spectrum Disorders and Intellectual Disabilities
3.3.2. In Other Neurological Disorders
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberts, B.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Martin, K.C.; Ephrussi, A. mRNA localization: Gene expression in the spatial dimension. Cell 2009, 136, 719–730. [Google Scholar] [CrossRef]
- Lin, J.Q.; van Tartwijk, F.W.; Holt, C.E. Axonal mRNA translation in neurological disorders. RNA Biol. 2020, 1–26. [Google Scholar] [CrossRef]
- Holt, C.E.; Martin, K.C.; Schuman, E.M. Local translation in neurons: Visualization and function. Nat. Struct. Mol. Biol. 2019, 26, 557–566. [Google Scholar] [CrossRef]
- Jung, H.; Yoon, B.C.; Holt, C.E. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat. Rev. Neurosci. 2012, 13, 308–324. [Google Scholar] [CrossRef]
- Baleriola, J.; Hengst, U. Targeting axonal protein synthesis in neuroregeneration and degeneration. Neurotherapeutics 2015, 12, 57–65. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Kelly, T.K.; Chang, B.; Ryazantsev, S.; Rajasekaran, A.K.; Martin, K.C.; Twiss, J.L. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 9291–9303. [Google Scholar] [CrossRef]
- Coyne, A.N.; Siddegowda, B.B.; Estes, P.S.; Johannesmeyer, J.; Kovalik, T.; Daniel, S.G.; Pearson, A.; Bowser, R.; Zarnescu, D.C. Futsch/MAP1B mRNA is a translational target of TDP-43 and is neuroprotective in a Drosophila model of amyotrophic lateral sclerosis. J. Neurosci. 2014, 34, 15962–15974. [Google Scholar] [CrossRef]
- Lopez-Erauskin, J.; Tadokoro, T.; Baughn, M.W.; Myers, B.; McAlonis-Downes, M.; Chillon-Marinas, C.; Asiaban, J.N.; Artates, J.; Bui, A.T.; Vetto, A.P.; et al. ALS/FTD-Linked Mutation in FUS Suppresses Intra-axonal Protein Synthesis and Drives Disease Without Nuclear Loss-of-Function of FUS. Neuron 2018, 100, 816–830.e7. [Google Scholar] [CrossRef]
- Alami, N.H.; Smith, R.B.; Carrasco, M.A.; Williams, L.A.; Winborn, C.S.; Han, S.S.W.; Kiskinis, E.; Winborn, B.; Freibaum, B.D.; Kanagaraj, A.; et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 2014, 81, 536–543. [Google Scholar] [CrossRef]
- Rage, F.; Boulisfane, N.; Rihan, K.; Neel, H.; Gostan, T.; Bertrand, E.; Bordonne, R.; Soret, J. Genome-wide identification of mRNAs associated with the protein SMN whose depletion decreases their axonal localization. RNA 2013, 19, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Kye, M.J.; Niederst, E.D.; Wertz, M.H.; Goncalves Ido, C.; Akten, B.; Dover, K.Z.; Peters, M.; Riessland, M.; Neveu, P.; Wirth, B.; et al. SMN regulates axonal local translation via miR-183/mTOR pathway. Hum. Mol. Genet. 2014, 23, 6318–6331. [Google Scholar] [CrossRef]
- Khalil, B.; Morderer, D.; Price, P.L.; Liu, F.; Rossoll, W. mRNP assembly, axonal transport, and local translation in neurodegenerative diseases. Brain Res. 2018, 1693, 75–91. [Google Scholar] [CrossRef]
- Baleriola, J.; Walker, C.A.; Jean, Y.Y.; Crary, J.F.; Troy, C.M.; Nagy, P.L.; Hengst, U. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell 2014, 158, 1159–1172. [Google Scholar] [CrossRef]
- Walker, C.A.; Randolph, L.K.; Matute, C.; Alberdi, E.; Baleriola, J.; Hengst, U. Abeta1-42 triggers the generation of a retrograde signaling complex from sentinel mRNAs in axons. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tanaka, T.; Soeda, Y.; Almeida, O.F.X.; Takashima, A. Local Somatodendritic Translation and Hyperphosphorylation of Tau Protein Triggered by AMPA and NMDA Receptor Stimulation. EBioMedicine 2017, 20, 120–126. [Google Scholar] [CrossRef]
- Li, C.; Gotz, J. Somatodendritic accumulation of Tau in Alzheimer’s disease is promoted by Fyn-mediated local protein translation. EMBO J. 2017, 36, 3120–3138. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Link, A.J.; Graumann, J.; Tirrell, D.A.; Schuman, E.M. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl. Acad. Sci. USA 2006, 103, 9482–9487. [Google Scholar] [CrossRef]
- Dieterich, D.C.; Hodas, J.J.; Gouzer, G.; Shadrin, I.Y.; Ngo, J.T.; Triller, A.; Tirrell, D.A.; Schuman, E.M. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat. Neurosci. 2010, 13, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Cagnetta, R.; Frese, C.K.; Shigeoka, T.; Krijgsveld, J.; Holt, C.E. Rapid Cue-Specific Remodeling of the Nascent Axonal Proteome. Neuron 2018, 99, 29–46.e4. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 2009, 6, 275–277. [Google Scholar] [CrossRef]
- Gamarra, M.; Blanco-Urrejola, M.; Batista, A.F.R.; Imaz, J.; Baleriola, J. Object-Based Analyses in FIJI/ImageJ to Measure Local RNA Translation Sites in Neurites in Response to Abeta1-42 Oligomers. Front. Neurosci. 2020, 14, 547. [Google Scholar] [CrossRef]
- Hafner, A.S.; Donlin-Asp, P.G.; Leitch, B.; Herzog, E.; Schuman, E.M. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 2019, 364. [Google Scholar] [CrossRef] [PubMed]
- Terenzio, M.; Koley, S.; Samra, N.; Rishal, I.; Zhao, Q.; Sahoo, P.K.; Urisman, A.; Marvaldi, L.; Oses-Prieto, J.A.; Forester, C.; et al. Locally translated mTOR controls axonal local translation in nerve injury. Science 2018, 359, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Aviner, R.; Geiger, T.; Elroy-Stein, O. Novel proteomic approach (PUNCH-P) reveals cell cycle-specific fluctuations in mRNA translation. Genes Dev. 2013, 27, 1834–1844. [Google Scholar] [CrossRef]
- Shigeoka, T.; Jung, H.; Jung, J.; Turner-Bridger, B.; Ohk, J.; Lin, J.Q.; Amieux, P.S.; Holt, C.E. Dynamic Axonal Translation in Developing and Mature Visual Circuits. Cell 2016, 166, 181–192. [Google Scholar] [CrossRef]
- Zappulo, A.; van den Bruck, D.; Ciolli Mattioli, C.; Franke, V.; Imami, K.; McShane, E.; Moreno-Estelles, M.; Calviello, L.; Filipchyk, A.; Peguero-Sanchez, E.; et al. RNA localization is a key determinant of neurite-enriched proteome. Nat. Commun. 2017, 8, 583. [Google Scholar] [CrossRef]
- Bannister, N.J.; Larkman, A.U. Dendritic morphology of CA1 pyramidal neurones from the rat hippocampus: II. Spine distributions. J. Comp. Neurol. 1995, 360, 161–171. [Google Scholar] [CrossRef]
- Rangaraju, V.; Tom Dieck, S.; Schuman, E.M. Local translation in neuronal compartments: How local is local? EMBO Rep. 2017, 18, 693–711. [Google Scholar] [CrossRef]
- Yoon, B.C.; Jung, H.; Dwivedy, A.; O’Hare, C.M.; Zivraj, K.H.; Holt, C.E. Local translation of extranuclear lamin B promotes axon maintenance. Cell 2012, 148, 752–764. [Google Scholar] [CrossRef]
- Garner, C.C.; Tucker, R.P.; Matus, A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature 1988, 336, 674–677. [Google Scholar] [CrossRef]
- Burgin, K.E.; Waxham, M.N.; Rickling, S.; Westgate, S.A.; Mobley, W.C.; Kelly, P.T. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J. Neurosci. Off. J. Soc. Neurosci. 1990, 10, 1788–1798. [Google Scholar] [CrossRef]
- Landry, C.F.; Watson, J.B.; Kashima, T.; Campagnoni, A.T. Cellular influences on RNA sorting in neurons and glia: An in situ hybridization histochemical study. Brain Res. Mol. Brain Res. 1994, 27, 1–11. [Google Scholar] [CrossRef]
- Furuichi, T.; Simon-Chazottes, D.; Fujino, I.; Yamada, N.; Hasegawa, M.; Miyawaki, A.; Yoshikawa, S.; Guenet, J.L.; Mikoshiba, K. Widespread expression of inositol 1,4,5-trisphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Recept. Channels. 1993, 1, 11–24. [Google Scholar]
- Miyashiro, K.; Dichter, M.; Eberwine, J. On the nature and differential distribution of mRNAs in hippocampal neurites: Implications for neuronal functioning. Proc. Natl. Acad. Sci. USA 1994, 91, 10800–10804. [Google Scholar] [CrossRef]
- Racca, C.; Gardiol, A.; Triller, A. Dendritic and postsynaptic localizations of glycine receptor alpha subunit mRNAs. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 1691–1700. [Google Scholar] [CrossRef]
- Link, W.; Konietzko, U.; Kauselmann, G.; Krug, M.; Schwanke, B.; Frey, U.; Kuhl, D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA 1995, 92, 5734–5738. [Google Scholar] [CrossRef]
- Lyford, G.L.; Yamagata, K.; Kaufmann, W.E.; Barnes, C.A.; Sanders, L.K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Lanahan, A.A.; Worley, P.F. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 1995, 14, 433–445. [Google Scholar] [CrossRef]
- Tiruchinapalli, D.M.; Oleynikov, Y.; Kelic, S.; Shenoy, S.M.; Hartley, A.; Stanton, P.K.; Singer, R.H.; Bassell, G.J. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 3251–3261. [Google Scholar] [CrossRef]
- Tongiorgi, E.; Righi, M.; Cattaneo, A. Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 9492–9505. [Google Scholar] [CrossRef]
- Kang, H.; Schuman, E.M. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 1996, 273, 1402–1406. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Wu, B.; Buxbaum, A.R.; Das, S.; Tsai, A.; English, B.P.; Grimm, J.B.; Lavis, L.D.; Singer, R.H. Glutamate-induced RNA localization and translation in neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E6877–E6886. [Google Scholar] [CrossRef]
- Bassell, G.J.; Zhang, H.; Byrd, A.L.; Femino, A.M.; Singer, R.H.; Taneja, K.L.; Lifshitz, L.M.; Herman, I.M.; Kosik, K.S. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 1998, 18, 251–265. [Google Scholar] [CrossRef]
- Leung, K.M.; van Horck, F.P.; Lin, A.C.; Allison, R.; Standart, N.; Holt, C.E. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006, 9, 1247–1256. [Google Scholar] [CrossRef]
- Wong, H.H.; Lin, J.Q.; Strohl, F.; Roque, C.G.; Cioni, J.M.; Cagnetta, R.; Turner-Bridger, B.; Laine, R.F.; Harris, W.A.; Kaminski, C.F.; et al. RNA Docking and Local Translation Regulate Site-Specific Axon Remodeling In Vivo. Neuron 2017, 95, 852–868.e8. [Google Scholar] [CrossRef]
- Wu, K.Y.; Hengst, U.; Cox, L.J.; Macosko, E.Z.; Jeromin, A.; Urquhart, E.R.; Jaffrey, S.R. Local translation of RhoA regulates growth cone collapse. Nature 2005, 436, 1020–1024. [Google Scholar] [CrossRef]
- Hengst, U.; Deglincerti, A.; Kim, H.J.; Jeon, N.L.; Jaffrey, S.R. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat. Cell Biol. 2009, 11, 1024–1030. [Google Scholar] [CrossRef]
- Gracias, N.G.; Shirkey-Son, N.J.; Hengst, U. Local translation of TC10 is required for membrane expansion during axon outgrowth. Nat. Commun. 2014, 5, 3506. [Google Scholar] [CrossRef]
- Taylor, A.M.; Wu, J.; Tai, H.C.; Schuman, E.M. Axonal translation of beta-catenin regulates synaptic vesicle dynamics. J. Neurosci. 2013, 33, 5584–5589. [Google Scholar] [CrossRef]
- Batista, A.F.R.; Martinez, J.C.; Hengst, U. Intra-axonal Synthesis of SNAP25 Is Required for the Formation of Presynaptic Terminals. Cell Rep. 2017, 20, 3085–3098. [Google Scholar] [CrossRef]
- Cox, L.J.; Hengst, U.; Gurskaya, N.G.; Lukyanov, K.A.; Jaffrey, S.R. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat. Cell Biol. 2008, 10, 149–159. [Google Scholar] [CrossRef]
- Ji, S.J.; Jaffrey, S.R. Intra-axonal translation of SMAD1/5/8 mediates retrograde regulation of trigeminal ganglia subtype specification. Neuron 2012, 74, 95–107. [Google Scholar] [CrossRef]
- Ben-Yaakov, K.; Dagan, S.Y.; Segal-Ruder, Y.; Shalem, O.; Vuppalanchi, D.; Willis, D.E.; Yudin, D.; Rishal, I.; Rother, F.; Bader, M.; et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012, 31, 1350–1363. [Google Scholar] [CrossRef]
- Lezana, J.P.; Dagan, S.Y.; Robinson, A.; Goldstein, R.S.; Fainzilber, M.; Bronfman, F.C.; Bronfman, M. Axonal PPARgamma promotes neuronal regeneration after injury. Dev. Neurobiol. 2016, 76, 688–701. [Google Scholar] [CrossRef]
- Hanz, S.; Perlson, E.; Willis, D.; Zheng, J.Q.; Massarwa, R.; Huerta, J.J.; Koltzenburg, M.; Kohler, M.; van-Minnen, J.; Twiss, J.L.; et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 2003, 40, 1095–1104. [Google Scholar] [CrossRef]
- Pereiro, X.; Ruzafa, N.; Urcola, J.H.; Sharma, S.C.; Vecino, E. Differential Distribution of RBPMS in Pig, Rat, and Human Retina after Damage. Int. J. Mol. Sci. 2020, 21, 9330. [Google Scholar] [CrossRef]
- Scotter, E.L.; Chen, H.J.; Shaw, C.E. TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets. Neurotherapeutics 2015, 12, 352–363. [Google Scholar] [CrossRef]
- Swanger, S.A.; Bassell, G.J. Dendritic protein synthesis in the normal and diseased brain. Neuroscience 2013, 232, 106–127. [Google Scholar] [CrossRef]
- Rehorst, W.A.; Thelen, M.P.; Nolte, H.; Turk, C.; Cirak, S.; Peterson, J.M.; Wong, G.W.; Wirth, B.; Kruger, M.; Winter, D.; et al. Muscle regulates mTOR dependent axonal local translation in motor neurons via CTRP3 secretion: Implications for a neuromuscular disorder, spinal muscular atrophy. Acta Neuropathol. Commun. 2019, 7, 154. [Google Scholar] [CrossRef]
- Pearson, R.C.; Esiri, M.M.; Hiorns, R.W.; Wilcock, G.K.; Powell, T.P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1985, 82, 4531–4534. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Rabbito, A.; Dulewicz, M.; Kulczynska-Przybik, A.; Mroczko, B. Biochemical Markers in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1989. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Aronov, S.; Aranda, G.; Behar, L.; Ginzburg, I. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J. Neurosci. 2001, 21, 6577–6587. [Google Scholar] [CrossRef]
- Aronov, S.; Aranda, G.; Behar, L.; Ginzburg, I. Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J. Cell Sci. 2002, 115, 3817–3827. [Google Scholar] [CrossRef]
- Hoek, K.S.; Kidd, G.J.; Carson, J.H.; Smith, R. hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry 1998, 37, 7021–7029. [Google Scholar] [CrossRef]
- Munro, T.P.; Magee, R.J.; Kidd, G.J.; Carson, J.H.; Barbarese, E.; Smith, L.M.; Smith, R. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J. Biol. Chem. 1999, 274, 34389–34395. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Ghetti, B.; Spillantini, M.G. Frontotemporal dementia: Implications for understanding Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006254. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Block-Galarza, J.; Chase, K.O.; Sapp, E.; Vaughn, K.T.; Vallee, R.B.; DiFiglia, M.; Aronin, N. Fast transport and retrograde movement of huntingtin and HAP 1 in axons. Neuroreport 1997, 8, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Brunholz, S.; Sisodia, S.; Lorenzo, A.; Deyts, C.; Kins, S.; Morfini, G. Axonal transport of APP and the spatial regulation of APP cleavage and function in neuronal cells. Exp. Brain Res. 2012, 217, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Savas, J.N.; Ma, B.; Deinhardt, K.; Culver, B.P.; Restituito, S.; Wu, L.; Belasco, J.G.; Chao, M.V.; Tanese, N. A role for huntington disease protein in dendritic RNA granules. J. Biol. Chem. 2010, 285, 13142–13153. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Culver, B.P.; Baj, G.; Tongiorgi, E.; Chao, M.V.; Tanese, N. Localization of BDNF mRNA with the Huntington’s disease protein in rat brain. Mol. Neurodegener. 2010, 5, 22. [Google Scholar] [CrossRef]
- Ma, B.; Savas, J.N.; Yu, M.S.; Culver, B.P.; Chao, M.V.; Tanese, N. Huntingtin mediates dendritic transport of beta-actin mRNA in rat neurons. Sci. Rep. 2011, 1, 140. [Google Scholar] [CrossRef]
- Hernandez, I.H.; Cabrera, J.R.; Santos-Galindo, M.; Sanchez-Martin, M.; Dominguez, V.; Garcia-Escudero, R.; Perez-Alvarez, M.J.; Pintado, B.; Lucas, J.J. Pathogenic SREK1 decrease in Huntington’s disease lowers TAF1 mimicking X-linked dystonia parkinsonism. Brain 2020, 143, 2207–2219. [Google Scholar] [CrossRef]
- Creus-Muncunill, J.; Badillos-Rodriguez, R.; Garcia-Forn, M.; Masana, M.; Garcia-Diaz Barriga, G.; Guisado-Corcoll, A.; Alberch, J.; Malagelada, C.; Delgado-Garcia, J.M.; Gruart, A.; et al. Increased translation as a novel pathogenic mechanism in Huntington’s disease. Brain 2019, 142, 3158–3175. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Cabal-Herrera, A.M.; Punatar, R.H.; Clark, C.J.; Romney, C.A.; Hagerman, R.J. Overlapping Molecular Pathways Leading to Autism Spectrum Disorders, Fragile X Syndrome, and Targeted Treatments. Neurotherapeutics 2020, 19. [Google Scholar] [CrossRef]
- Banerjee, A.; Ifrim, M.F.; Valdez, A.N.; Raj, N.; Bassell, G.J. Aberrant RNA translation in fragile X syndrome: From FMRP mechanisms to emerging therapeutic strategies. Brain Res. 2018, 1693, 24–36. [Google Scholar] [CrossRef]
- Antar, L.N.; Afroz, R.; Dictenberg, J.B.; Carroll, R.C.; Bassell, G.J. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J. Neurosci. 2004, 24, 2648–2655. [Google Scholar] [CrossRef]
- Christie, S.B.; Akins, M.R.; Schwob, J.E.; Fallon, J.R. The FXG: A presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci. 2009, 29, 1514–1524. [Google Scholar] [CrossRef]
- Akins, M.R.; Berk-Rauch, H.E.; Fallon, J.R. Presynaptic translation: Stepping out of the postsynaptic shadow. Front. Neural Circuits 2009, 3, 17. [Google Scholar] [CrossRef]
- Akins, M.R.; Berk-Rauch, H.E.; Kwan, K.Y.; Mitchell, M.E.; Shepard, K.A.; Korsak, L.I.; Stackpole, E.E.; Warner-Schmidt, J.L.; Sestan, N.; Cameron, H.A.; et al. Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains. Hum. Mol. Genet. 2017, 26, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Berry-Kravis, E.; Hagerman, R.J. Fragile X: Leading the way for targeted treatments in autism. Neurotherapeutics 2010, 7, 264–274. [Google Scholar] [CrossRef]
- Hagerman, R.; Hoem, G.; Hagerman, P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol. Autism 2010, 1, 12. [Google Scholar] [CrossRef]
- Troca-Marin, J.A.; Alves-Sampaio, A.; Montesinos, M.L. An increase in basal BDNF provokes hyperactivation of the Akt-mammalian target of rapamycin pathway and deregulation of local dendritic translation in a mouse model of Down’s syndrome. J. Neurosci. 2011, 31, 9445–9455. [Google Scholar] [CrossRef]
- Alves-Sampaio, A.; Troca-Marin, J.A.; Montesinos, M.L. NMDA-mediated regulation of DSCAM dendritic local translation is lost in a mouse model of Down’s syndrome. J. Neurosci. 2010, 30, 13537–13548. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Richter, J.D. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb. Perspect. Biol. 2012, 4, a012344. [Google Scholar] [CrossRef]
- Colman, D.R.; Kreibich, G.; Frey, A.B.; Sabatini, D.D. Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol. 1982, 95, 598–608. [Google Scholar] [CrossRef]
- Berger, U.V.; DeSilva, T.M.; Chen, W.; Rosenberg, P.A. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J. Comp. Neurol. 2005, 492, 78–89. [Google Scholar] [CrossRef]
- Tsunekawa, Y.; Britto, J.M.; Takahashi, M.; Polleux, F.; Tan, S.S.; Osumi, N. Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. EMBO J. 2012, 31, 1879–1892. [Google Scholar] [CrossRef]
- Pfeiffer, S.E.; Warrington, A.E.; Bansal, R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993, 3, 191–197. [Google Scholar] [CrossRef]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef]
- Ghandour, M.S.; Skoff, R.P. Double-labeling in situ hybridization analysis of mRNAs for carbonic anhydrase II and myelin basic protein: Expression in developing cultured glial cells. Glia 1991, 4, 1–10. [Google Scholar] [CrossRef]
- LoPresti, P.; Szuchet, S.; Papasozomenos, S.C.; Zinkowski, R.P.; Binder, L.I. Functional implications for the microtubule-associated protein tau: Localization in oligodendrocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 10369–10373. [Google Scholar] [CrossRef]
- Holz, A.; Schaeren-Wiemers, N.; Schaefer, C.; Pott, U.; Colello, R.J.; Schwab, M.E. Molecular and developmental characterization of novel cDNAs of the myelin-associated/oligodendrocytic basic protein. J. Neurosci. 1996, 16, 467–477. [Google Scholar] [CrossRef]
- Garcia-Ladona, F.J.; Huss, Y.; Frey, P.; Ghandour, M.S. Oligodendrocytes express different isoforms of beta-amyloid precursor protein in chemically defined cell culture conditions: In situ hybridization and immunocytochemical detection. J. Neurosci. Res. 1997, 50, 50–61. [Google Scholar] [CrossRef]
- Thakurela, S.; Garding, A.; Jung, R.B.; Muller, C.; Goebbels, S.; White, R.; Werner, H.B.; Tiwari, V.K. The transcriptome of mouse central nervous system myelin. Sci. Rep. 2016, 6, 25828. [Google Scholar] [CrossRef]
- White, R.; Gonsior, C.; Kramer-Albers, E.M.; Stohr, N.; Huttelmaier, S.; Trotter, J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J. Cell Biol. 2008, 181, 579–586. [Google Scholar] [CrossRef]
- Laursen, L.S.; Chan, C.W.; Ffrench-Constant, C. Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K. J. Cell Biol. 2011, 192, 797–811. [Google Scholar] [CrossRef]
- Torvund-Jensen, J.; Steengaard, J.; Reimer, L.; Fihl, L.B.; Laursen, L.S. Transport and translation of MBP mRNA is regulated differently by distinct hnRNP proteins. J. Cell Sci. 2014, 127, 1550–1564. [Google Scholar] [CrossRef]
- Herbert, A.L.; Fu, M.M.; Drerup, C.M.; Gray, R.S.; Harty, B.L.; Ackerman, S.D.; O’Reilly-Pol, T.; Johnson, S.L.; Nechiporuk, A.V.; Barres, B.A.; et al. Dynein/dynactin is necessary for anterograde transport of Mbp mRNA in oligodendrocytes and for myelination in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E9153–E9162. [Google Scholar] [CrossRef] [PubMed]
- Lorente Pons, A.; Higginbottom, A.; Cooper-Knock, J.; Alrafiah, A.; Alofi, E.; Kirby, J.; Shaw, P.J.; Wood, J.D.; Highley, J.R. Oligodendrocyte pathology exceeds axonal pathology in white matter in human amyotrophic lateral sclerosis. J. Pathol. 2020, 251, 262–271. [Google Scholar] [CrossRef]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef]
- Highley, J.R.; Kirby, J.; Jansweijer, J.A.; Webb, P.S.; Hewamadduma, C.A.; Heath, P.R.; Higginbottom, A.; Raman, R.; Ferraiuolo, L.; Cooper-Knock, J.; et al. Loss of nuclear TDP-43 in amyotrophic lateral sclerosis (ALS) causes altered expression of splicing machinery and widespread dysregulation of RNA splicing in motor neurones. Neuropathol. Appl. Neurobiol. 2014, 40, 670–685. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.C.; Wang, Y.D.; Scarborough, E.A.; Moore, J.; Diaz, Z.; MacLea, K.S.; Freibaum, B.; Li, S.; Molliex, A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495, 467–473. [Google Scholar] [CrossRef]
- Wang, J.; Ho, W.Y.; Lim, K.; Feng, J.; Tucker-Kellogg, G.; Nave, K.A.; Ling, S.C. Cell-autonomous requirement of TDP-43, an ALS/FTD signature protein, for oligodendrocyte survival and myelination. Proc. Natl. Acad. Sci. USA 2018, 115, E10941–E10950. [Google Scholar] [CrossRef]
- Balendra, R.; Isaacs, A.M. C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat. Rev. Neurol. 2018, 14, 544–558. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, D.; Feng, Y. Destabilization and mislocalization of myelin basic protein mRNAs in quaking dysmyelination lacking the QKI RNA-binding proteins. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 4944–4953. [Google Scholar] [CrossRef]
- Larocque, D.; Pilotte, J.; Chen, T.; Cloutier, F.; Massie, B.; Pedraza, L.; Couture, R.; Lasko, P.; Almazan, G.; Richard, S. Nuclear retention of MBP mRNAs in the quaking viable mice. Neuron 2002, 36, 815–829. [Google Scholar] [CrossRef]
- Sakers, K.; Lake, A.M.; Khazanchi, R.; Ouwenga, R.; Vasek, M.J.; Dani, A.; Dougherty, J.D. Astrocytes locally translate transcripts in their peripheral processes. Proc. Natl. Acad. Sci. USA 2017, 114, E3830–E3838. [Google Scholar] [CrossRef]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Lopez, T.; Ortiz-Sanz, C.; Serrano-Regal, M.P.; Gaminde-Blasco, A.; Valero, J.; Baleriola, J.; Sanchez-Gomez, M.V.; Matute, C.; Alberdi, E. Abeta oligomers promote oligodendrocyte differentiation and maturation via integrin beta1 and Fyn kinase signaling. Cell Death Dis. 2019, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, Y.; Liu, Z.; Geng, Q.; Chen, Z.; Zhang, Y. Alterations of myelin morphology and oligodendrocyte development in early stage of Alzheimer’s disease mouse model. Neurosci. Lett. 2017, 642, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Pitman, K.A.; Wang, S.; Summers, B.S.; Bye, N.; Young, K.M.; Cullen, C.L. Amyloidosis is associated with thicker myelin and increased oligodendrogenesis in the adult mouse brain. J. Neurosci. Res. 2020, 98, 1905–1932. [Google Scholar] [CrossRef]
- Ahmed, Z.; Bigio, E.H.; Budka, H.; Dickson, D.W.; Ferrer, I.; Ghetti, B.; Giaccone, G.; Hatanpaa, K.J.; Holton, J.L.; Josephs, K.A.; et al. Globular glial tauopathies (GGT): Consensus recommendations. Acta Neuropathol. 2013, 126, 537–544. [Google Scholar] [CrossRef]
- Ferrer, I.; Andres-Benito, P.; Zelaya, M.V.; Aguirre, M.E.E.; Carmona, M.; Ausin, K.; Lachen-Montes, M.; Fernandez-Irigoyen, J.; Santamaria, E.; Del Rio, J.A. Familial globular glial tauopathy linked to MAPT mutations: Molecular neuropathology and seeding capacity of a prototypical mixed neuronal and glial tauopathy. Acta Neuropathol. 2020, 139, 735–771. [Google Scholar] [CrossRef]
- Seiberlich, V.; Bauer, N.G.; Schwarz, L.; Ffrench-Constant, C.; Goldbaum, O.; Richter-Landsberg, C. Downregulation of the microtubule associated protein tau impairs process outgrowth and myelin basic protein mRNA transport in oligodendrocytes. Glia 2015, 63, 1621–1635. [Google Scholar] [CrossRef]
- Pasciuto, E.; Bagni, C. SnapShot: FMRP mRNA targets and diseases. Cell 2014, 158, 1446–1446.e1. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Carson, J.H.; Barbarese, E. FMRP and myelin protein expression in oligodendrocytes. Mol. Cell Neurosci. 2013, 56, 333–341. [Google Scholar] [CrossRef]
- Wang, H.; Ku, L.; Osterhout, D.J.; Li, W.; Ahmadian, A.; Liang, Z.; Feng, Y. Developmentally-programmed FMRP expression in oligodendrocytes: A potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum. Mol. Genet. 2004, 13, 79–89. [Google Scholar] [CrossRef]
- Pacey, L.K.; Xuan, I.C.; Guan, S.; Sussman, D.; Henkelman, R.M.; Chen, Y.; Thomsen, C.; Hampson, D.R. Delayed myelination in a mouse model of fragile X syndrome. Hum. Mol. Genet. 2013, 22, 3920–3930. [Google Scholar] [CrossRef]
- Doll, C.A.; Yergert, K.M.; Appel, B.H. The RNA binding protein fragile X mental retardation protein promotes myelin sheath growth. Glia 2020, 68, 495–508. [Google Scholar] [CrossRef]
- Blackburn, D.; Sargsyan, S.; Monk, P.N.; Shaw, P.J. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 2009, 57, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci. 2019, 25, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Pallesen, J.; Daugaard, T.F.; Borglum, A.D.; Nielsen, A.L. Genome wide assessment of mRNA in astrocyte protrusions by direct RNA sequencing reveals mRNA localization for the intermediate filament protein nestin. Glia 2013, 61, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Daugaard, T.F.; Holm, I.E.; Nielsen, A.L. Alternative mRNA splicing from the glial fibrillary acidic protein (GFAP) gene generates isoforms with distinct subcellular mRNA localization patterns in astrocytes. PLoS ONE 2013, 8, e72110. [Google Scholar] [CrossRef]
- Ogata, K.; Kosaka, T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 2002, 113, 221–233. [Google Scholar] [CrossRef]
- Mazare, N.; Oudart, M.; Moulard, J.; Cheung, G.; Tortuyaux, R.; Mailly, P.; Mazaud, D.; Bemelmans, A.P.; Boulay, A.C.; Blugeon, C.; et al. Local Translation in Perisynaptic Astrocytic Processes Is Specific and Changes after Fear Conditioning. Cell Rep. 2020, 32, 108076. [Google Scholar] [CrossRef]
- Boulay, A.C.; Saubamea, B.; Adam, N.; Chasseigneaux, S.; Mazare, N.; Gilbert, A.; Bahin, M.; Bastianelli, L.; Blugeon, C.; Perrin, S.; et al. Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Discov. 2017, 3, 17005. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef]
- Tong, J.; Huang, C.; Bi, F.; Wu, Q.; Huang, B.; Liu, X.; Li, F.; Zhou, H.; Xia, X.G. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. EMBO J. 2013, 32, 1917–1926. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Van Kammen, M.; Levey, A.I.; Martin, L.J.; Kuncl, R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 1995, 38, 73–84. [Google Scholar] [CrossRef]
- Barton, S.K.; Gregory, J.M.; Chandran, S.; Turner, B.J. Could an Impairment in Local Translation of mRNAs in Glia be Contributing to Pathogenesis in ALS? Front. Mol. Neurosci. 2019, 12, 124. [Google Scholar] [CrossRef]
- Liedtke, W.; Edelmann, W.; Bieri, P.L.; Chiu, F.C.; Cowan, N.J.; Kucherlapati, R.; Raine, C.S. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 1996, 17, 607–615. [Google Scholar] [CrossRef]
- Kamphuis, W.; Middeldorp, J.; Kooijman, L.; Sluijs, J.A.; Kooi, E.J.; Moeton, M.; Freriks, M.; Mizee, M.R.; Hol, E.M. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 492–510. [Google Scholar] [CrossRef]
- Oudart, M.; Tortuyaux, R.; Mailly, P.; Mazare, N.; Boulay, A.C.; Cohen-Salmon, M. AstroDot—A new method for studying the spatial distribution of mRNA in astrocytes. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Farnsworth, B.; Peuckert, C.; Zimmermann, B.; Jazin, E.; Kettunen, P.; Emilsson, L.S. Gene Expression of Quaking in Sporadic Alzheimer’s Disease Patients is Both Upregulated and Related to Expression Levels of Genes Involved in Amyloid Plaque and Neurofibrillary Tangle Formation. J. Alzheimers Dis. 2016, 53, 209–219. [Google Scholar] [CrossRef]
- Vidal, R.; Ghetti, B.; Takao, M.; Brefel-Courbon, C.; Uro-Coste, E.; Glazier, B.S.; Siani, V.; Benson, M.D.; Calvas, P.; Miravalle, L.; et al. Intracellular ferritin accumulation in neural and extraneural tissue characterizes a neurodegenerative disease associated with a mutation in the ferritin light polypeptide gene. J. Neuropathol. Exp. Neurol. 2004, 63, 363–380. [Google Scholar] [CrossRef]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef]
- Higashimori, H.; Morel, L.; Huth, J.; Lindemann, L.; Dulla, C.; Taylor, A.; Freeman, M.; Yang, Y. Astroglial FMRP-dependent translational down-regulation of mGluR5 underlies glutamate transporter GLT1 dysregulation in the fragile X mouse. Hum. Mol. Genet. 2013, 22, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Higashimori, H.; Schin, C.S.; Chiang, M.S.; Morel, L.; Shoneye, T.A.; Nelson, D.L.; Yang, Y. Selective Deletion of Astroglial FMRP Dysregulates Glutamate Transporter GLT1 and Contributes to Fragile X Syndrome Phenotypes In Vivo. J. Neurosci. 2016, 36, 7079–7094. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Salmon, M.; Slaoui, L.; Mazare, N.; Gilbert, A.; Oudart, M.; Alvear-Perez, R.; Elorza-Vidal, X.; Chever, O.; Boulay, A.C. Astrocytes in the regulation of cerebrovascular functions. Glia 2020, 69, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yang, G.Y. Aquaporin-4: A Potential Therapeutic Target for Cerebral Edema. Int J. Mol. Sci. 2016, 17, 1413. [Google Scholar] [CrossRef]
- Zheng, L.; Cheng, W.; Wang, X.; Yang, Z.; Zhou, X.; Pan, C. Overexpression of MicroRNA-145 Ameliorates Astrocyte Injury by Targeting Aquaporin 4 in Cerebral Ischemic Stroke. Biomed. Res. Int. 2017, 2017, 9530951. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, L.; Chen, J.; Chen, Y.; Zhang, L.; Wang, L.; Li, X.; Li, Y.; Yu, H. Inhibition of Connexin43 hemichannels with Gap19 protects cerebral ischemia/reperfusion injury via the JAK2/STAT3 pathway in mice. Brain Res. Bull. 2019, 146, 124–135. [Google Scholar] [CrossRef]
- Eilam, R.; Segal, M.; Malach, R.; Sela, M.; Arnon, R.; Aharoni, R. Astrocyte disruption of neurovascular communication is linked to cortical damage in an animal model of multiple sclerosis. Glia 2018, 66, 1098–1117. [Google Scholar] [CrossRef] [PubMed]
- Alvestad, S.; Hammer, J.; Hoddevik, E.H.; Skare, O.; Sonnewald, U.; Amiry-Moghaddam, M.; Ottersen, O.P. Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res. 2013, 105, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gotz, M.; Barde, Y.A. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron 2005, 46, 369–372. [Google Scholar] [CrossRef]
- Yao, B.; Christian, K.M.; He, C.; Jin, P.; Ming, G.L.; Song, H. Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 2016, 17, 537–549. [Google Scholar] [CrossRef]
- Martinez-Cerdeno, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef]
- Komada, M.; Saitsu, H.; Kinboshi, M.; Miura, T.; Shiota, K.; Ishibashi, M. Hedgehog signaling is involved in development of the neocortex. Development 2008, 135, 2717–2727. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.R. The structural role of radial glial endfeet in confining spinal motor neuron somata is controlled by the Reelin and Notch pathways. Exp. Neurol. 2013, 249, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Pilaz, L.J.; Lennox, A.L.; Rouanet, J.P.; Silver, D.L. Dynamic mRNA Transport and Local Translation in Radial Glial Progenitors of the Developing Brain. Curr. Biol. 2016, 26, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, Y.; Kikkawa, T.; Osumi, N. Asymmetric inheritance of Cyclin D2 maintains proliferative neural stem/progenitor cells: A critical event in brain development and evolution. Dev. Growth Differ. 2014, 56, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Pilaz, L.J.; Silver, D.L. Moving messages in the developing brain-emerging roles for mRNA transport and local translation in neural stem cells. FEBS Lett. 2017, 591, 1526–1539. [Google Scholar] [CrossRef]
- Mirzaa, G.; Parry, D.A.; Fry, A.E.; Giamanco, K.A.; Schwartzentruber, J.; Vanstone, M.; Logan, C.V.; Roberts, N.; Johnson, C.A.; Singh, S.; et al. De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat. Genet. 2014, 46, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Vasek, M.J.; Deajon-Jackson, J.D.; Liu, Y.; Crosby, H.W.; Yi, J.; Dougherty, J.D. Microglia perform local protein synthesis at perisynaptic and phagocytic structures. bioRxiv 2021. [Google Scholar] [CrossRef]
- Berson, A.; Barbash, S.; Shaltiel, G.; Goll, Y.; Hanin, G.; Greenberg, D.S.; Ketzef, M.; Becker, A.J.; Friedman, A.; Soreq, H. Cholinergic-associated loss of hnRNP-A/B in Alzheimer’s disease impairs cortical splicing and cognitive function in mice. EMBO Mol. Med. 2012, 4, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Lavon, I.; Leykin, I.; Charbit, H.; Binyamin, O.; Brill, L.; Ovadia, H.; Vaknin-Dembinsky, A. QKI-V5 is downregulated in CNS inflammatory demyelinating diseases. Mult. Scler. Relat. Disord. 2019, 39, 101881. [Google Scholar] [CrossRef]
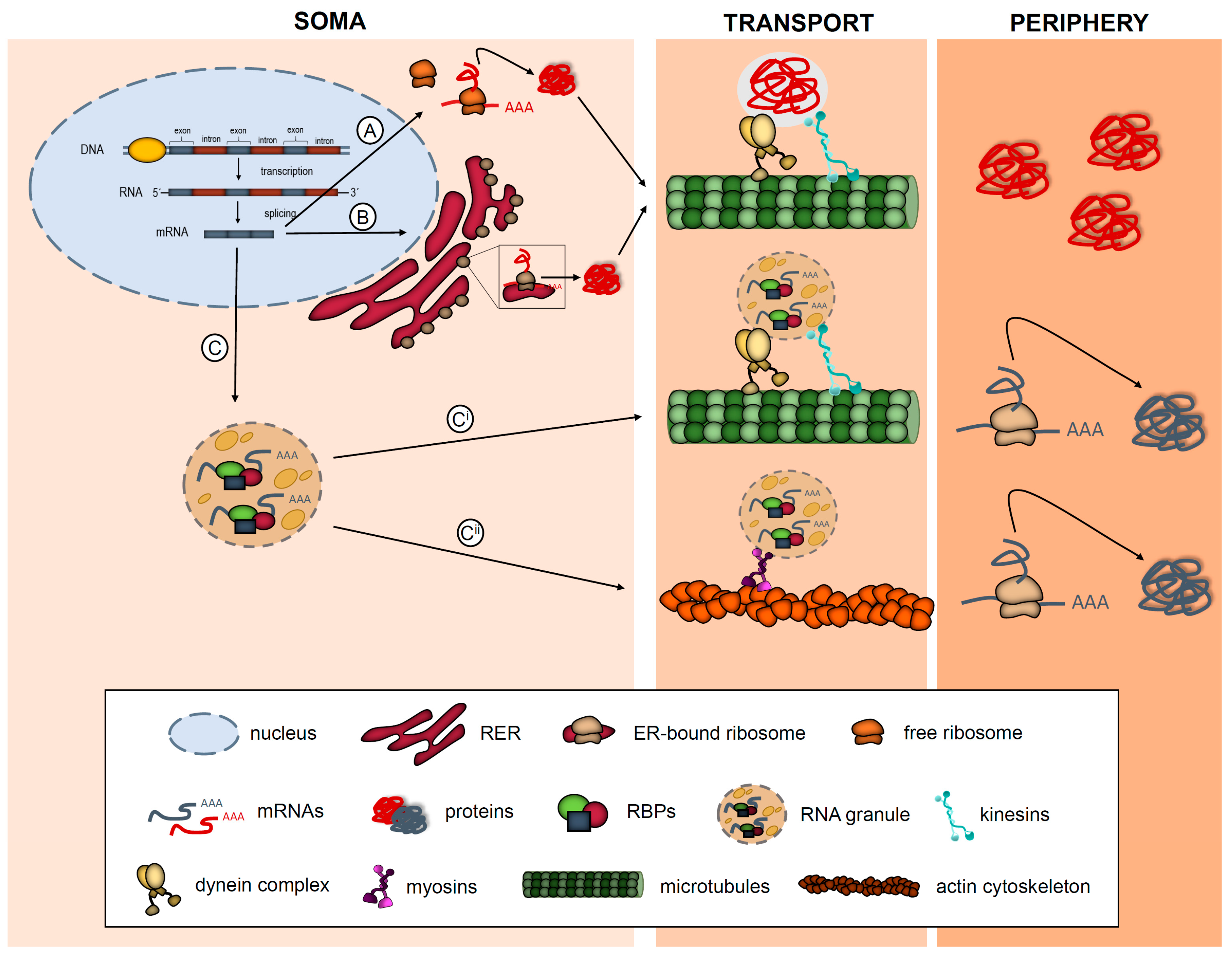

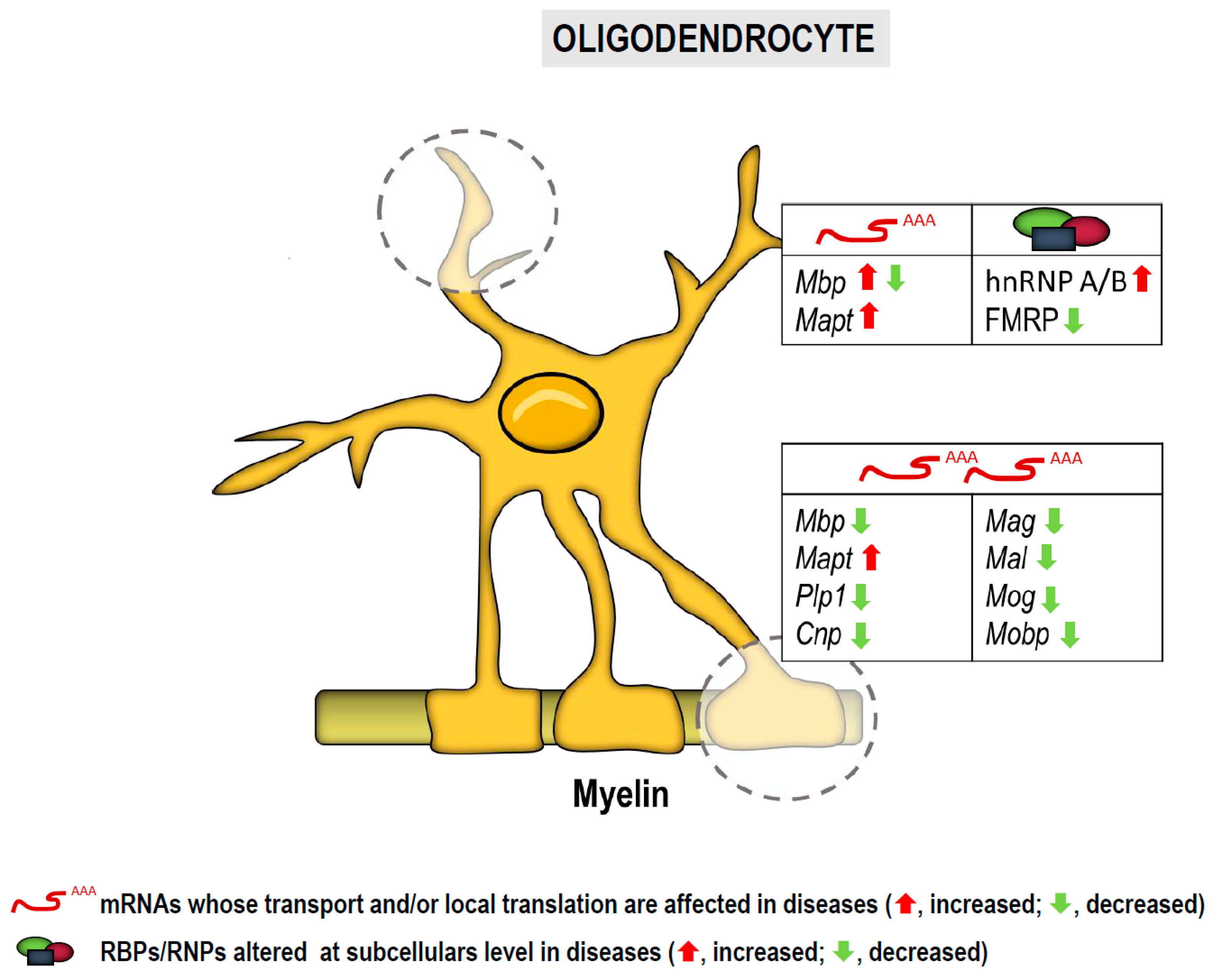
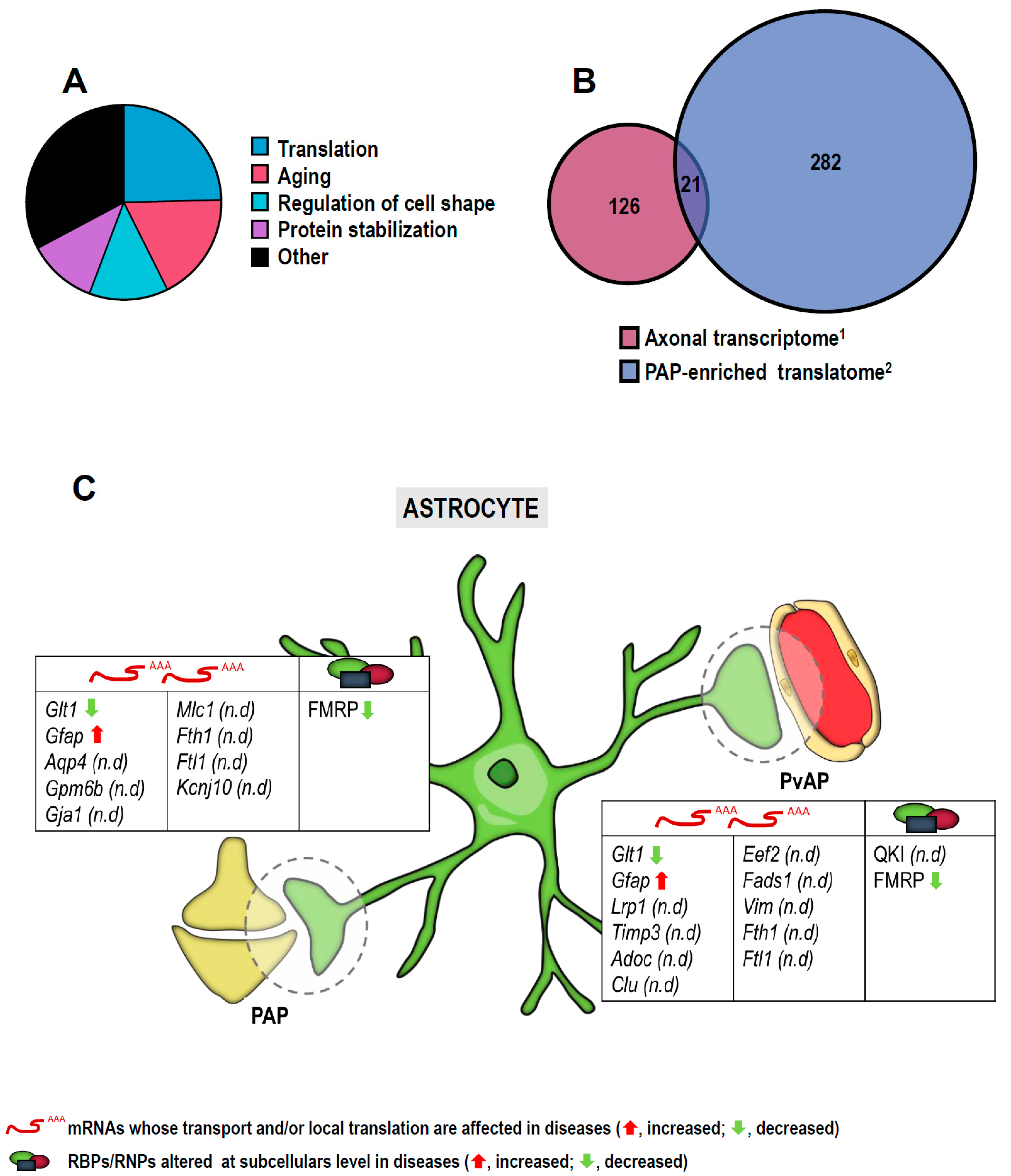
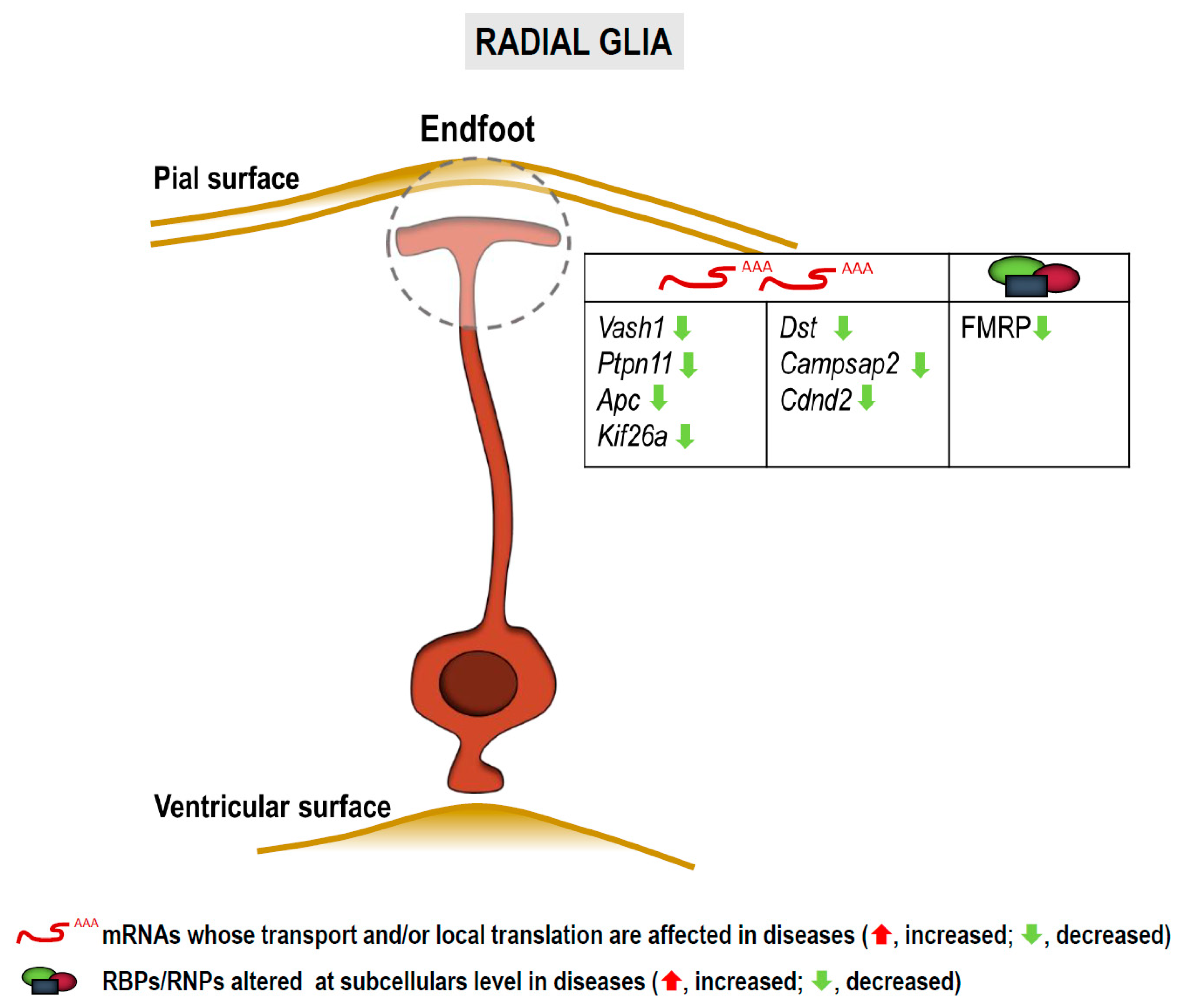
| List of mRNAs/microRNAs Localized to Subcellular Compartments in Neurons and Glia and Implicated in Diseases | |||
|---|---|---|---|
| Transcript | Disease | Cell Type | References |
| Actb | SMA, HTT | N | [13,73,75] |
| Aldoc | AD | N1, A | [14,111,130] |
| Anxa2 | SMA | N | [11] |
| Apc | FXS | R | [158] |
| ApoE | AD | N | [6,14] |
| App | AD | N | [6,14] |
| Aqp4 | AD, MS, other | N1, A | [14,131,145,150] |
| Arc | FXS | N | [79,80] |
| Atf4 | AD | N | [14] |
| Bdnf | HTT, DS, other | N | [59,86] |
| Bin1 | AD | N | [6,14] |
| Camk2a | FXS | N | [79,80] |
| Campsap2 | FXS | R | [156] |
| Ccdn2 | FXS, other | R | [156,159] |
| Celf1 | AD | N | [6,14] |
| Clu | AD | N, A | [6,14,111,130] |
| Cnp | GGT | O | [117] |
| Cox4i2 | SMA | N | [11] |
| Dlg4 | FXS | N | [79,80] |
| Dscam | DS | N | [59,87] |
| Dst | FXS | R | [156] |
| Eef2 | AD | N1, A | [14,111,130] |
| Fads1 | AD | N1, A | [14,111,130] |
| Fermt2 | AD | N | [6,14] |
| Fmrp (Fmr1) | FXS | N | [79,80] |
| Fth1 | other | A | [141] |
| Ftl1 | other | A | [141] |
| Gap43 | SMA | N | [13] |
| Gfap | AD | N1, A | [6,14,111,130,131,139] |
| Gja1 | AD, MS | N1, A | [14,131,145] |
| Glt1 | ALS, FXS | A | [124,143,144] |
| Gpm6b | AD | N1, A | [14,131] |
| Kpnb | TNI | N | [56] |
| Kcnj10 | HTT | A | [142] |
| Ki26a | FXS | R | [156] |
| Lrp1 | AD | N1, A | [14,111,130] |
| Mag | GGT | O | [117] |
| Mal | GGT | O | [117] |
| Mapb1 | ALS, FXS | N | [8,79,80] |
| Mapt | AD, GGT | N, O | [16,17,117] |
| Mbp | ALS, MS2, AD, GGT, FXS | O | [107,110,111,113,117,122,123] |
| miR-183 | SMA | N | [12] |
| Mlc1 | AD | N1, A | [14,131] |
| Mobp | GGT | O | [117] |
| Mog | GGT | O | [117] |
| mTor | TNI, SMA, ASD, DS | N | [12,25,84,85,86] |
| Nrn1 | SMA | N | [13] |
| Picalm | AD | N | [6,14] |
| Plp1 | GGT | O | [117] |
| Pparg | TNI | N | [55] |
| Ptk2 | AD | N | [6,14] |
| Ptpn11 | FXS | R | [156] |
| Sorl1 | AD | N | [6,14] |
| Stat3 | TNI | N | [54] |
| Timp3 | AD | N1, A | [14,111,130] |
| Vash1 | FXS | R | [156] |
| Vim | AD | N, A | [14,15,111,130] |
| Ago2 | HD | N | [73,74,75] |
| FMRP | FXS | N, O, A, R | [79,80,122,133,143,144,156] |
| FUS | FTD | N | [8,9,59] |
| hnRNP A/B | AD, ALS | N, O | [67,68,105,106,107,161] |
| HTT | HD | N | [73,74,75] |
| QKI | MS, ALS, AD | O, A | [110,111,136,140,162] |
| RBPMS | TNI | N | [57] |
| SMN | SMA | N | [13] |
| TDP-43 | ALS, FTD | N, A | [8,9,59,124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Urrejola, M.; Gaminde-Blasco, A.; Gamarra, M.; de la Cruz, A.; Vecino, E.; Alberdi, E.; Baleriola, J. RNA Localization and Local Translation in Glia in Neurological and Neurodegenerative Diseases: Lessons from Neurons. Cells 2021, 10, 632. https://doi.org/10.3390/cells10030632
Blanco-Urrejola M, Gaminde-Blasco A, Gamarra M, de la Cruz A, Vecino E, Alberdi E, Baleriola J. RNA Localization and Local Translation in Glia in Neurological and Neurodegenerative Diseases: Lessons from Neurons. Cells. 2021; 10(3):632. https://doi.org/10.3390/cells10030632
Chicago/Turabian StyleBlanco-Urrejola, Maite, Adhara Gaminde-Blasco, María Gamarra, Aida de la Cruz, Elena Vecino, Elena Alberdi, and Jimena Baleriola. 2021. "RNA Localization and Local Translation in Glia in Neurological and Neurodegenerative Diseases: Lessons from Neurons" Cells 10, no. 3: 632. https://doi.org/10.3390/cells10030632
APA StyleBlanco-Urrejola, M., Gaminde-Blasco, A., Gamarra, M., de la Cruz, A., Vecino, E., Alberdi, E., & Baleriola, J. (2021). RNA Localization and Local Translation in Glia in Neurological and Neurodegenerative Diseases: Lessons from Neurons. Cells, 10(3), 632. https://doi.org/10.3390/cells10030632







