Cytokine Profile in Plasma Extracellular Vesicles of Parkinson’s Disease and the Association with Cognitive Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Assessments
2.3. Plasma EV Isolation and Validation
2.4. Western Blot Analysis of Plasma EV Cytokine
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Rossi, A.; Berger, K.; Chen, H.; Leslie, D.; Mailman, R.B.; Huang, X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov. Disord. 2018, 33, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Harada, M.; Riederer, P.; Narabayashi, H.; Fujita, K.; Nagatsu, T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994, 165, 208–210. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Narabayashi, H.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996, 211, 13–16. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E.C. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Couch, Y.; Richardson, J.; Cooper, J.M.; Wood, M.J. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci. Res. 2011, 69, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Brudek, T. Inflammatory bowel diseases and Parkinson’s disease. J. Parkinsons Dis. 2019, 9, S331–S344. [Google Scholar] [CrossRef] [PubMed]
- Samii, A.; Etminan, M.; Wiens, M.O.; Jafari, S. NSAID use and the risk of Parkinson’s disease: Systematic review and meta-analysis of observational studies. Drugs Aging 2009, 26, 769–779. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Bhunia, A. Gut-Brain axis in Parkinson’s disease etiology: The role of lipopolysaccharide. Chem. Phys. Lipids 2020, 235, 105029. [Google Scholar]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-Related diseases: Role of Inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A Systematic review and meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.T.; Iqbal, A.J.; Norling, L.V. The Role and impact of extracellular vesicles in the modulation and delivery of cytokines during autoimmunity. Int. J. Mol. Sci. 2020, 21, 7096. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Malik, M.A.; Rahman, S.; Yeo, H.R.; Lee, E.J.; Abdullah, T.S.; Choi, I. Perspective Insights of Exosomes in Neurodegenerative Diseases: A Critical Appraisal. Front. Aging Neurosci. 2017, 9, 317. [Google Scholar] [CrossRef]
- Hezel, M.E.v.; Nieuwland, R.; Bruggen, R.v.; Juffermans, N.P. The ability of extracellular vesicles to induce a pro-inflammatory host response. Int. J. Mol. Sci. 2017, 18, 1285. [Google Scholar]
- Aiello, A.; Giannessi, F.; Percario, Z.A.; Affabris, E. An emerging interplay between extracellular vesicles and cytokines. Cytokine Growth Factor. Rev. 2020, 51, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Baek, J.; Kwon, W.S.; Rha, S.Y.; Hwang, K.W.; Kim, U.; Min, H. The comparison of exosome and exosomal cytokines between young and old individuals with or without gastric cancer. Int. J. Gerontol. 2018, 12, 233–238. [Google Scholar] [CrossRef]
- Konadu, K.A.; Chu, J.; Huang, M.B.; Amancha, P.K.; Armstrong, W.; Powell, M.D.; Villinger, F.; Bond, V.C. Association of Cytokines with exosomes in the plasma of HIV-1-Seropositive individuals. J. Infect. Dis. 2015, 211, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Chung, C.C.; Huang, P.H.; Chan, L.; Chen, J.H.; Chien, L.N.; Hong, C.T. Plasma exosomal brain-derived neurotrophic factor correlated with the postural instability and gait disturbance-related motor symptoms in patients with Parkinson’s disease. Diagnostics 2020, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.C.; Hong, C.T.; Huang, Y.H.; Su, E.C.; Chan, L.; Hu, C.J.; Chiu, H.W. Predicting major neurologic improvement and long-term outcome after thrombolysis using artificial neural networks. J. Neurol. Sci. 2020, 410, 116667. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of cytokines encapsulated in extracellular vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef] [PubMed]
- Holder, B.; Jones, T.; Shimizu, V.S.; Rice, T.F.; Donaldson, B.; Bouqueau, M.; Forbes, K.; Kampmann, B. Macrophage Exosomes induce placental inflammatory cytokines: A Novel mode of maternal–placental messaging. Traffic 2016, 17, 168–178. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Chen, X.; Zhang, Y.; Xu, M.; Yang, Z. The regulation of cancer cell migration by lung cancer cell-derived exosomes through TGF-β and IL-10. Oncol. Lett. 2016, 11, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-T.; Wang, B.; Wu, M.; Li, Z.-L.; Feng, Y.; Cao, J.-Y.; Yin, D.; Liu, H.; Tang, R.-N.; Crowley, S.D.; et al. Extracellular vesicle–encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 2020, 6, eaaz0748. [Google Scholar] [CrossRef]
- Santarlasci, V.; Cosmi, L.; Maggi, L.; Liotta, F.; Annunziato, F. IL-1 and T helper immune responses. Front. Immunol. 2013, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid Secretion of interleukin-1β by microvesicle shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Kumar, A.; Abbas, W.; Herbein, G. TNF and TNF Receptor superfamily members in HIV infection: New cellular targets for therapy? Mediat. Inflamm. 2013, 2013, 484378. [Google Scholar] [CrossRef]
- Lee, J.H.; Wittki, S.; Bräu, T.; Dreyer, F.S.; Krätzel, K.; Dindorf, J.; Johnston, I.C.; Gross, S.; Kremmer, E.; Zeidler, R.; et al. HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol. Cell 2013, 49, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-C.; Chan, L.; Bamodu, O.A.; Hong, C.-T.; Chiu, H.-W. Artificial neural network based prediction of postthrombolysis intracerebral hemorrhage and death. Sci. Rep. 2020, 10, 20501. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, R.; Leenders, K.L.; van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Junichi, M.; Tessandra, S.; William, A.B.; Jing, Z. The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar]
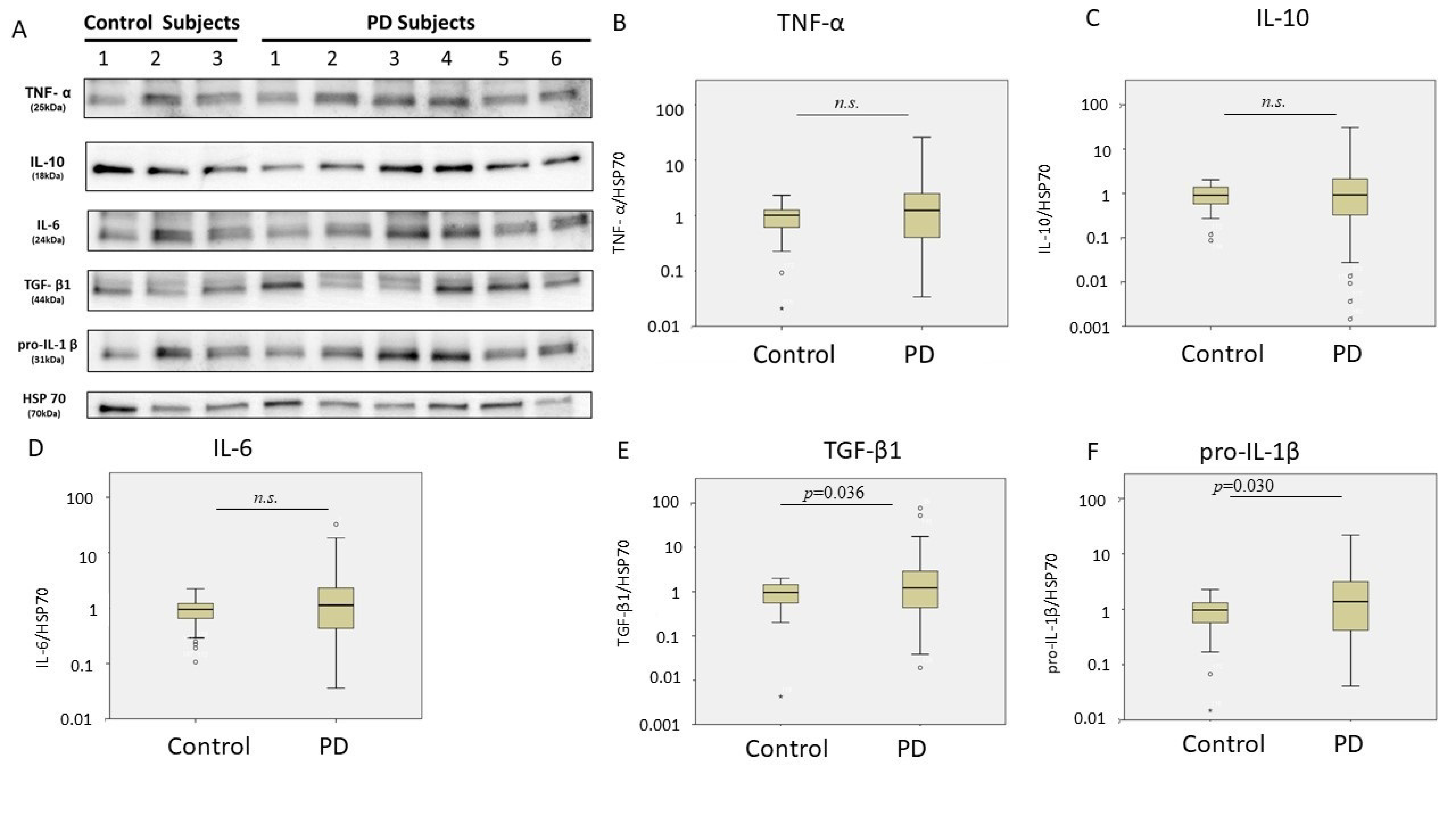
| Control | PD | p Value | |
|---|---|---|---|
| Number of patients | 48 | 113 | - |
| Age (years) | 67.94 ± 7.50 | 69.66 ± 8.41 | 0.214 |
| Female | 39 | 27 | 0.124 |
| Disease duration (years) | - | 2.71 ± 2.48 | - |
| MMSE | 27.36 ± 2.77 | 24.18 ± 6.36 | <0.001 |
| MoCA | 23.25 ± 3.89 | 20.43 ± 6.02 | 0.003 |
| UPDRS-I | - | 2.48 ± 2.00 | - |
| UPDRS-II | - | 7.92 ± 5.82 | - |
| UPDRS-III | - | 22.48 ± 9.85 | - |
| Std. β | p Value | 95% CI | R2 of the Model | |
|---|---|---|---|---|
| IL-6 | 0.150 | 0.056 | −0.03~2.26 | 0.067 |
| pro-IL-1β | 0.206 | 0.008 | 0.32~2.20 | 0.087 |
| TNF-α | 0.175 | 0.025 | 0.16~2.40 | 0.071 |
| TGF-β1 | 0.120 | 0.133 | −0.62~4.72 | 0.075 |
| IL-10 | 0.147 | 0.061 | −0.06~2.67 | 0.087 |
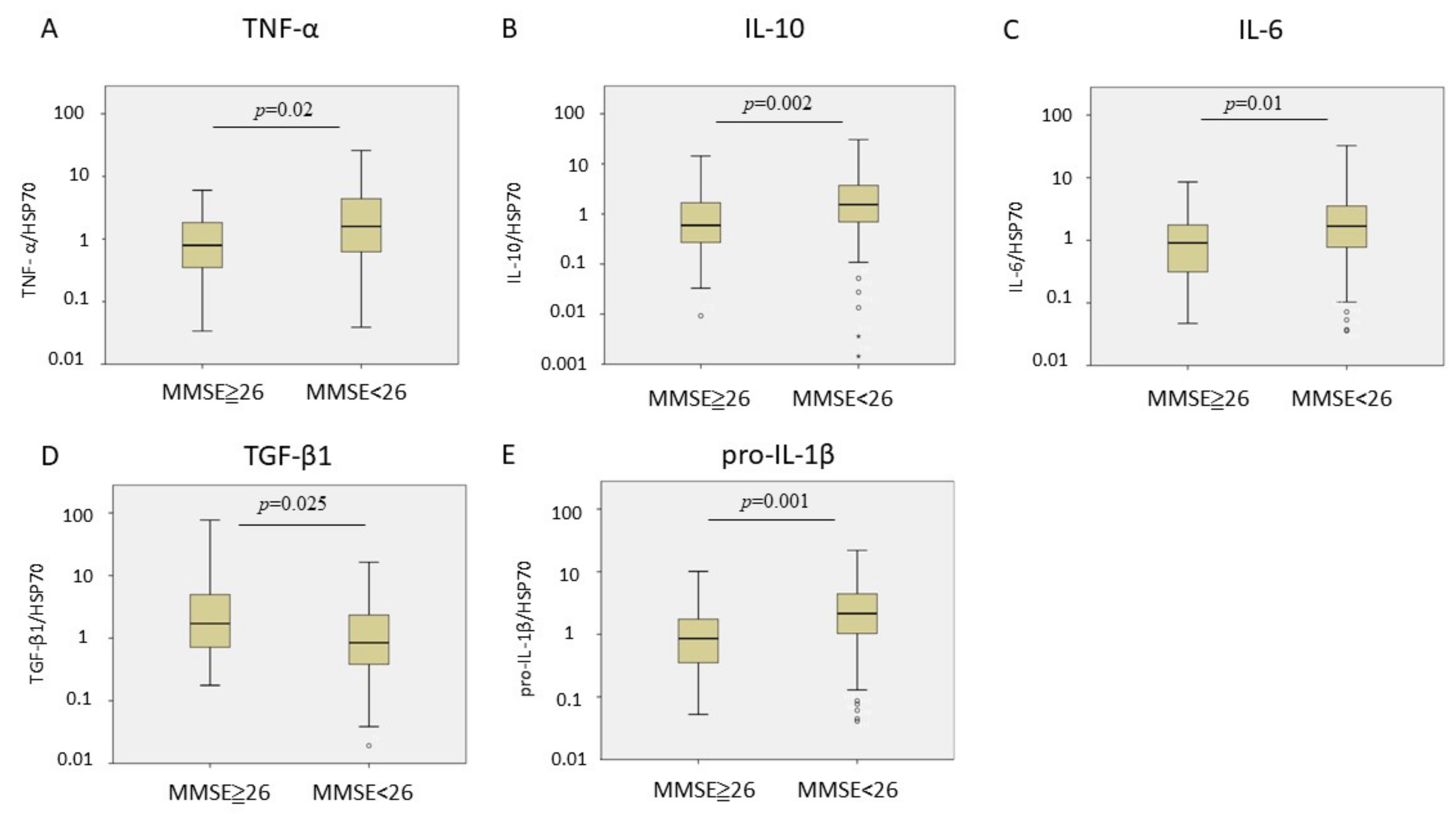
| MMSE | MoCA | |||||||
|---|---|---|---|---|---|---|---|---|
| Std. β | p Value | 95% CI | R2 of the Model | Std. β | p Value | 95% CI | R2 of the Model | |
| IL-6 | −0.217 | 0.024 | −0.27~−0.02 | 0.095 | −0.286 | 0.004 | −0.29~−0.06 | 0.133 |
| Pro-IL-1β | −0.284 | 0.003 | −0.25~−0.05 | 0.123 | −0.308 | 0.002 | −0.26~−0.06 | 0.133 |
| TNF-α | −0.227 | 0.017 | −0.26~−0.03 | 0.123 | −0.300 | 0.002 | −0.30~−0.07 | 0.168 |
| TGF-β1 | 0.121 | 0.215 | −0.07~0.30 | 0.054 | 0.153 | 0.132 | −0.04~0.34 | 0.061 |
| IL-10 | −0.239 | 0.012 | −0.33~−0.04 | 0.116 | −0.237 | 0.017 | −0.33~−0.03 | 0.111 |
| Std. β | p Value | 95% CI | R2 of the Model | |
|---|---|---|---|---|
| IL-6 | 0.209 | 0.031 | 0.15~3.13 | 0.090 |
| pro-IL-1β | 0.260 | 0.007 | 0.47~2.90 | 0.110 |
| TNF-α | 0.225 | 0.019 | 0.29~3.20 | 0.114 |
| TGF-β1 | −0.198 | 0.039 | −7.04~−0.18 | 0.083 |
| IL-10 | 0.240 | 0.013 | 0.50~4.07 | 0.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, L.; Chung, C.-C.; Chen, J.-H.; Yu, R.-C.; Hong, C.-T. Cytokine Profile in Plasma Extracellular Vesicles of Parkinson’s Disease and the Association with Cognitive Function. Cells 2021, 10, 604. https://doi.org/10.3390/cells10030604
Chan L, Chung C-C, Chen J-H, Yu R-C, Hong C-T. Cytokine Profile in Plasma Extracellular Vesicles of Parkinson’s Disease and the Association with Cognitive Function. Cells. 2021; 10(3):604. https://doi.org/10.3390/cells10030604
Chicago/Turabian StyleChan, Lung, Chen-Chih Chung, Jia-Hung Chen, Ruan-Ching Yu, and Chien-Tai Hong. 2021. "Cytokine Profile in Plasma Extracellular Vesicles of Parkinson’s Disease and the Association with Cognitive Function" Cells 10, no. 3: 604. https://doi.org/10.3390/cells10030604
APA StyleChan, L., Chung, C.-C., Chen, J.-H., Yu, R.-C., & Hong, C.-T. (2021). Cytokine Profile in Plasma Extracellular Vesicles of Parkinson’s Disease and the Association with Cognitive Function. Cells, 10(3), 604. https://doi.org/10.3390/cells10030604






