DNA or Protein Methylation-Dependent Regulation of Activator Protein-1 Function
Abstract
1. Introduction
2. DNA Methylation and AP-1 Signaling
2.1. DNA Methylation and Cancer
2.2. DNA Methylation and Osteoporosis
2.3. DNA Methylation and Inflammation
2.4. DNA Methylation and Autoimmune Disease
3. Histone Methylation and AP-1 Signaling
3.1. Histone Methylation and Cancer
3.2. Histone Methylation and Inflammation
3.3. Histone Methylation and Autoimmune Disease
4. Protein Methylation
4.1. PRMT1 and Cancer/Inflammation
4.2. PRMT5 and Cancer
4.3. PRMT5 and Arthritis
4.4. PRMT6 and Cancer
5. Conclusion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-aza-dC | 5-aza-2’deoxycytidine |
| ADMA | asymmetric dimethyl arginine |
| ALA | 5-aminolevulinic acid |
| Adox | adenosine dialdehyde |
| CCND1 | cyclin D1 |
| CRE | cAMP-responsive element |
| DNMTs | DNA methyltransferases |
| EDC | epidermal differentiation complex |
| ER | estrogen receptors |
| EREs | estrogen response elements |
| ERK | extracellular signal-regulated kinase |
| EZH2 | enhancer of zeste homolog 2 |
| HBV | hepatitis B virus |
| HBx | protein HBV X protein |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HIF | hypoxia-inducible factor |
| IGF-1 | insulin-like growth factor-1 |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| MAPKs | mitogen-activated protein kinases |
| MEK | MAPK/ERK kinase |
| MMA | monomethyl arginine |
| MMP | matrix metalloproteinase |
| MMSET | multiple myeloma SET domain |
| MT2A | metallothionein 2A |
| PDT | photodynamic therapy |
| PKMTs | protein lysine methyltransferases |
| PRMTs | protein arginine methyltransferases |
| PTMs | post-translational modifications |
| RACO-1 | RING domain AP-1 coactivator-1 |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| SDMA | symmetric dimethylarginine |
| SMYD3 | SET- and MYND-domain containing protein 3 |
| SRC | steroid-receptor coactivator |
References
- Lopez-Bergami, P.; Lau, E.; Ronai, Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat. Rev. Cancer 2010, 10, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef]
- Zenz, R.; Eferl, R.; Scheinecker, C.; Redlich, K.; Smolen, J.; Schonthaler, H.B.; Kenner, L.; Tschachler, E.; Wagner, E.F. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res. Ther. 2007, 10. [Google Scholar] [CrossRef] [PubMed]
- Gungl, A.; Biasin, V.; Wilhelm, J.; Olschewski, A.; Kwapiszewska, G.; Marsh, L.M. Fra2 Overexpression in mice leads to non-allergic asthma development in an IL-13 dependent manner. Front. Immunol. 2018, 9, 2018. [Google Scholar] [CrossRef] [PubMed]
- Trop-Steinberg, S.; Azar, Y. AP-1 expression and its clinical relevance in immune disorders and cancer. Am. J. Med. Sci. 2017, 353, 474–483. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, J.H.; Jeong, D.; Hong, Y.H.; Park, S.H.; Yang, Y.; Jang, Y.-J.; Kim, J.-H.; Cho, J.Y. 3-Deazaadenosine, an S-adenosylhomocysteine hydrolase inhibitor, attenuates lipopolysaccharide-induced inflammatory responses via inhibition of AP-1 and NF-κB signaling. Biochem. Pharmacol. 2020, 182, 114264. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, H.G.; Lee, Y.; Yoon, K.; Kim, S.; Kim, J.H.; Cho, J.Y. Isoprenylcysteine carboxyl methyltransferase inhibitors exerts anti-inflammatory activity. Biochem. Pharmacol. 2020, 182, 114219. [Google Scholar] [CrossRef]
- Belguise, K.; Cherradi, S.; Sarr, A.; Boissière, F.; Boulle, N.; Simony-Lafontaine, J.; Choesmel-Cadamuro, V.; Wang, X.; Chalbos, D. PKCθ-induced phosphorylations control the ability of Fra-1 to stimulate gene expression and cancer cell migration. Cancer Lett. 2017, 385, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Talotta, F.; Mega, T.; Bossis, G.; Casalino, L.; Basbous, J.; Jariel-Encontre, I.; Piechaczyk, M.; Verde, P. Heterodimerization with Fra-1 cooperates with the ERK pathway to stabilize c-Jun in response to the RAS oncoprotein. Oncogene 2010, 29, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, E.; Kim, J.H.; Yoon, K.; Kim, S.; Lee, J.; Cho, J.Y. AKT1-targeted proapoptotic activity of compound K in human breast cancer cells. J. Ginseng Res. 2019, 43, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Kim, J.H.; Kim, J.-H.; Yi, Y.-S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small molecule inhibitors targeting activator protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Al-Kuhlani, M.; Johnston, S.C.; Ojcius, D.M.; Chou, J.; Dean, D. Transcription factor complex AP-1 mediates inflammation initiated by Chlamydia pneumoniae infection. Cell. Microbiol. 2013, 15, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, B.W.; Spence, H.J.; McGarry, L.C.; Hennigan, R.F. Transcription factors control invasion: AP-1 the first among equals. Oncogene 2006, 26, 1–10. [Google Scholar] [CrossRef]
- Benbow, U.; Brinckerhoff, C.E. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997, 15, 519–526. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Biggar, K.K.; Li, S.S. Non-histone protein methylation as a regulator of cellular signalling and function. Nat. Rev. Mol. Cell Biol. 2015, 16, 5–17. [Google Scholar] [CrossRef]
- Wurm, S.; Zhang, J.; Guinea-Viniegra, J.; García, F.; Muñoz, J.; Bakiri, L.; Ezhkova, E.; Wagner, E.F. Terminal epidermal differen-tiation is regulated by the interaction of Fra-2/AP-1 with Ezh2 and ERK1/2. Genes Dev. 2015, 29, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.-G.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Gazon, H.; Barbeau, B.; Mesnard, J.-M.; Peloponese Jr, J.-M. Hijacking of the AP-1 Signaling pathway during development of ATL. Front. Microbiol. 2018, 8, 2686. [Google Scholar] [CrossRef]
- Karin, M.; Marshall, C.J. The regulation of AP-1 activity by mitogen-activated protein kinases. Phil. Trans. R. Soc. Lond. B 1996, 351, 127–134. [Google Scholar] [CrossRef]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2017, 128, 366–375. [Google Scholar] [CrossRef]
- Whitmarsh, A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Agron, M.; Brekhman, V.; Morgenstern, D.; Lotan, T. Regulation of AP-1 by MAPK signaling in metal-stressed sea anemone. Cell. Physiol. Biochem. 2017, 42, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, Y.; Dolganiuc, A.; Norkina, O.; Romics, L.; Li, W.; Kodys, K.; Bach, F.H.; Mandrekar, P.; Szabo, G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J. Immunol. 2006, 177, 2592–2600. [Google Scholar] [CrossRef]
- Looby, E.; Abdel-Latif, M.M.; Athié-Morales, V.; Duggan, S.; Long, A.; Kelleher, D. Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer 2009, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, L.N.; Lattal, K.M. Histone-Mediated Epigenetics in Addiction, Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2014; pp. 51–87. [Google Scholar]
- Aletta, J.M.; Cimato, T.R.; Ettinger, M.J. Protein methylation: A signal event in post-translational modification. Trends Biochem. Sci. 1998, 23, 89–91. [Google Scholar] [CrossRef]
- Elshorbagy, A.; Jernerén, F.; Samocha-Bonet, D.; Refsum, H.; Heilbronn, L. Serum S-adenosylmethionine, but not methionine, increases in response to overfeeding in humans. Nutr. Diabetes 2016, 6, e192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoo, B.C.; Yang, W.S.; Kim, E.; Hong, S.; Cho, J.Y. The role of protein arginine methyltransferases in inflammatory responses. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA methylation, methyltransferases, and cancer. Oncogene 2001, 20, 3139–3155. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Du, Y.; Xie, S.; Yang, X.; Lian, F.; Zhou, Z.; Qian, C. Structural and mechanistic insights into UHRF1-mediated DNMT1 activation in the maintenance DNA methylation. Nucleic Acids Res. 2018, 46, 3218–3231. [Google Scholar] [CrossRef]
- Urulangodi, M.; Mohanty, A. DNA damage response and repair pathway modulation by non-histone protein methylation: Implications in neurodegeneration. J. Cell Commun. Signal. 2020, 14, 31–45. [Google Scholar] [CrossRef]
- Lee, D.Y.; Teyssier, C.; Strahl, B.D.; Stallcup, M.R. Role of protein methylation in regulation of transcription. Endocr. Rev. 2005, 26, 147–170. [Google Scholar] [CrossRef]
- Hamamoto, R.; Saloura, V.; Nakamura, Y. Critical roles of non-histone protein lysine methylation in human tumorigenesis. Nat. Rev. Cancer 2015, 15, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Blanc, R.S.; Richard, S. Arginine methylation: The coming of age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Barnicle, A.; Seoighe, C.; Greally, J.M.; Golden, A.; Egan, L.J. Inflammation-associated DNA methylation patterns in epithelium of ulcerative colitis. Epigenetics 2017, 12, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Juttermann, R.; Li, E.; Jaenisch, R. Toxicity of 5-aza-2’-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc. Natl. Acad. Sci. USA 1994, 91, 11797–11801. [Google Scholar] [CrossRef]
- Lu, R.; Wang, X.; Chen, Z.-F.; Sun, D.-F.; Tian, X.-Q.; Fang, J.-Y. Inhibition of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway decreases dna methylation in colon cancer cells. J. Biol. Chem. 2007, 282, 12249–12259. [Google Scholar] [CrossRef]
- Pradhan, N.; Parbin, S.; Kar, S.; Das, L.; Kirtana, R.; Seshadri, G.S.; Sengupta, D.; Deb, M.; Kausar, C.; Patra, S.K. Epigenetic silencing of genes enhanced by collective role of reactive oxygen species and MAPK signaling downstream ERK/Snail axis: Ectopic application of hydrogen peroxide repress CDH1 gene by enhanced DNA methyltransferase activity in human breast cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1651–1665. [Google Scholar] [CrossRef]
- Jin, T.; Hao, J.; Fan, D. Nicotine induces aberrant hypermethylation of tumor suppressor genes in pancreatic epithelial ductal cells. Biochem. Biophys. Res. Commun. 2018, 499, 934–940. [Google Scholar] [CrossRef]
- Dunn, G.P.; Rinne, M.L.; Wykosky, J.; Genovese, G.; Quayle, S.N.; Dunn, I.F.; Agarwalla, P.K.; Chheda, M.G.; Campos, B.; Wang, A.; et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012, 26, 756–784. [Google Scholar] [CrossRef] [PubMed]
- Heiland, D.H.; Ferrarese, R.; Claus, R.; Dai, F.; Masilamani, A.P.; Kling, E.; Weyerbrock, A.; Kling, T.; Nelander, S.; Carro, M.S. c-Jun-N-terminal phosphorylation regulates DNMT1 expression and genome wide methylation in gliomas. Oncotarget 2016, 8, 6940–6954. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Li, H.-P.; Lu, Y.-J.; Hsueh, C.; Liang, Y.; Chen, C.-L.; Tsao, S.W.; Tse, K.-P.; Yu, J.-S.; Chang, Y.-S. Activation of DNA Methyltransferase 1 by EBV LMP1 Involves c-Jun NH2-Terminal Kinase Signaling. Cancer Res. 2006, 66, 11668–11676. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, P.; Mariano, M.C.; Geng, H.; Martin, T.G.; Wolf, J.L.; Wong, S.W.; Shah, N.; Wiita, A.P. DNA methyltransferase inhibitors upregulate CD38 protein expression and enhance daratumumab efficacy in multiple myeloma. Leukemia 2020, 34, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Isakova, A.; Groux, R.; Imbeault, M.; Rainer, P.; Alpern, D.; Dainese, R.; Ambrosini, G.; Trono, D.; Bucher, P.; Deplancke, B. SMiLE-seq identifies binding motifs of single and dimeric transcription factors. Nat. Methods 2017, 14, 316–322. [Google Scholar] [CrossRef]
- Gustems, M.; Woellmer, A.; Rothbauer, U.; Eck, S.H.; Wieland, T.; Lutter, D.; Hammerschmidt, W. c-Jun/c-Fos heterodimers regulate cellular genes via a newly identified class of methylated DNA sequence motifs. Nucleic Acids Res. 2014, 42, 3059–3072. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Ma, J.-X.; Li, G.-M.; Zhang, Y.; Xing, G.-S.; Liu, J.; Ma, X.-L. Overexpression of DNMT1 leads to hypermethylation of H19 promoter and inhibition of Erk signaling pathway in disuse osteoporosis. Bone 2018, 111, 82–91. [Google Scholar] [CrossRef]
- De La Rica, L.; Rodríguez-Ubreva, J.; García, M.; Islam, A.B.M.M.K.; Urquiza, J.M.; Hernando, H.; Christensen, J.; Helin, K.; Gómez-Vaquero, C.; Ballestar, E. PU.1 target genes undergo Tet2-coupled demethylation and DNMT3b-mediated methylation in monocyte-to-osteoclast differentiation. Genome Biol. 2013, 14, R99. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhan, M.; Li, Q.; Li, D.; Xu, Q. DNA methyltransferase DNMT1 inhibits lipopolysaccharide-induced inflammatory response in human dental pulp cells involving the methylation changes of IL-6 and TRAF6. Mol. Med. Rep. 2019, 21, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Li, D.; Feng, Z.; Xu, Q. MyD88 hypermethylation mediated by DNMT1 is associated with LTA-induced inflammatory response in human odontoblast-like cells. Cell Tissue Res. 2019, 376, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, B.; Reich, N.O. The early expressed HIV-1 genes regulate DNMT1 expression. Epigenetics 2008, 3, 149–156. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Q. DNA methylation in T cells from idiopathic lupus and drug-induced lupus patients. Autoimmun. Rev. 2008, 7, 376–383. [Google Scholar] [CrossRef]
- Scheinbart, L.S.; Johnson, M.A.; Gross, L.A.; Edelstein, S.R.; Richardson, B.C. Procainamide inhibits DNA methyltransferase in a human T cell line. J. Rheumatol. 1991, 18, 530–534. [Google Scholar] [PubMed]
- Klinman, D.M.; Mushinski, J.F.; Honda, M.; Ishigatsubo, Y.; Mountz, J.D.; Raveche, E.S.; Steinberg, A.D. Oncogene expression in autoimmune and normal peripheral blood mononuclear cells. J. Exp. Med. 1986, 163, 1292–1307. [Google Scholar] [CrossRef]
- Sunahori, K.; Nagpal, K.; Hedrich, C.M.; Mizui, M.; Fitzgerald, L.M.; Tsokos, G.C. The Catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA Hypomethylation by suppressing the phosphorylated mitogen-activated protein kinase/Extracellular Signal-regulated Kinase (ERK) Kinase (MEK)/Phosphorylated ERK/DNMT1 Protein pathway in t-cells from controls and systemic lupus erythematosus patients. J. Biol. Chem. 2013, 288, 21936–21944. [Google Scholar] [CrossRef]
- Deng, C.; Lu, Q.; Zhang, Z.; Rao, T.; Attwood, J.; Yung, R.; Richardson, B. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum. 2003, 48, 746–756. [Google Scholar] [CrossRef]
- Zhao, Q.; Wirka, R.; Nguyen, T.; Nagao, M.; Cheng, P.; Miller, C.L.; Kim, J.B.; Pjanic, M.; Quertermous, T. TCF21 and AP-1 interact through epigenetic modifications to regulate coronary artery disease gene expression. Genome Med. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Jaeger, M.; Ghisleni, E.C.; Cardoso, P.S.; Siniglaglia, M.; Falcon, T.; Brunetto, A.T.; Brunetto, A.L.; De Farias, C.B.; Taylor, M.D.; Nör, C.; et al. HDAC and MAPK/ERK Inhibitors cooperate to reduce viability and stemness in medulloblastoma. J. Mol. Neurosci. 2020, 70, 981–992. [Google Scholar] [CrossRef]
- Emmons, M.F.; Faião-Flores, F.; Sharma, R.; Thapa, R.; Messina, J.L.; Becker, J.C.; Schadendorf, D.; Seto, E.; Sondak, V.K.; Koomen, J.M.; et al. HDAC8 regulates a stress response pathway in melanoma to mediate escape from BRAF inhibitor therapy. Cancer Res. 2019, 79, 2947–2961. [Google Scholar] [CrossRef]
- Louveau, B.; Jouenne, F.; De Moura, C.R.; Sadoux, A.; Baroudjian, B.; Delyon, J.; Herms, F.; De Masson, A.; Da Meda, L.; Battistella, M.; et al. Baseline Genomic Features in BRAFV600-mutated metastatic melanoma patients treated with BRAF inhibitor + MEK inhibitor in routine care. Cancers 2019, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Wang, H.; Fu, C.; Du, J.; Wang, H.; He, R.; Yin, X.; Li, H.; Li, X.; Wang, H.; Li, K.; et al. Enhanced histone H3 acetylation of the PD-L1 promoter via the COP1/c-Jun/HDAC3 axis is required for PD-L1 expression in drug-resistant cancer cells. J. Exp. Clin. Cancer Res. 2020, 39, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.-Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef]
- Wagner, K.W.; Alam, H.; Dhar, S.S.; Giri, U.; Li, N.; Wei, Y.; Giri, D.; Cascone, T.; Kim, J.-H.; Ye, Y.; et al. KDM2A promotes lung tumorigenesis by epigenetically enhancing ERK1/2 signaling. J. Clin. Investig. 2013, 123, 5231–5246. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Yue, S.; Wang, Y.; Lu, F. Histone deacetylase inhibitors in cancer therapy. Curr. Top. Med. Chem. 2019, 18, 2420–2428. [Google Scholar] [CrossRef]
- Liu, W.-J.; Chen, Y.-J.; Chen, D.-N.; Wu, Y.-P.; Gao, Y.-J.; Li, J.; Zhong, W.-J.; Jiang, L. A new pair of enantiomeric lignans from the fruits of Morinda citrifolia and their absolute configuration. Nat. Prod. Res. 2017, 32, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Nakamura, Y.; Takemura, M.; Hisaoka-Nakashima, K.; Morioka, N. TLR4-TAK1-p38 MAPK pathway and HDAC6 regulate the expression of sigma-1 receptors in rat primary cultured microglia. J. Pharmacol. Sci. 2020, 144, 23–29. [Google Scholar] [CrossRef]
- Fang, W.-F.; Chen, Y.-M.; Lin, C.-Y.; Huang, H.-L.; Yeh, H.; Chang, Y.-T.; Huang, K.-T.; Lin, M.-C. Histone deacetylase 2 (HDAC2) attenuates lipopolysaccharide (LPS)-induced inflammation by regulating PAI-1 expression. J. Inflamm. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Tochiki, K.K.; Cunningham, J.; Hunt, S.P.; Géranton, S.M. The expression of spinal methyl-CpG-binding protein 2, DNA Methyltransferases and histone deacetylases is modulated in persistent pain states. Mol. Pain 2012, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Galeotti, N. The HDAC1/c-JUN complex is essential in the promotion of nerve injury-induced neuropathic pain through JNK signaling. Eur. J. Pharmacol. 2018, 825, 99–106. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, Y.; Tang, X.; Xia, Y.; He, G.; Min, Z.; Li, C.; Xiong, S.; Shi, Z.; Lu, Y.; et al. HDAC inhibitors suppress c-Jun/Fra-1-mediated proliferation through transcriptionally downregulating MKK7 and Raf1 in neuroblastoma cells. Oncotarget 2015, 7, 6727–6747. [Google Scholar] [CrossRef]
- Wu, C.; Li, A.; Hu, J.; Kang, J. Histone deacetylase 2 is essential for LPS-induced inflammatory responses in macrophages. Immunol. Cell Biol. 2018, 97, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Harman, J.L.; Dobnikar, L.; Chappell, J.; Stokell, B.G.; Dalby, A.; Foote, K.; Finigan, A.; Freire-Pritchett, P.; Taylor, A.L.; Worssam, M.D.; et al. Epigenetic Regulation of vascular smooth muscle cells by Histone H3 Lysine 9 dimethylation attenuates target gene-induction by inflammatory signaling. Arter. Thromb. Vasc. Biol. 2019, 39, 2289–2302. [Google Scholar] [CrossRef]
- De Oliveira, S.; Boudinot, P.; Calado, Â.; Mulero, V. Duox1-Derived H2O2 Modulates Cxcl8 Expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J. Immunol. 2015, 194, 1523–1533. [Google Scholar] [CrossRef]
- Renthal, W.; Nestler, E.J. Epigenetic mechanisms in drug addiction. Trends Mol. Med. 2008, 14, 341–350. [Google Scholar] [CrossRef]
- Seidman, J.S.; Troutman, T.D.; Sakai, M.; Gola, A.; Spann, N.J.; Bennett, H.; Bruni, C.M.; Ouyang, Z.; Li, R.Z.; Sun, X.; et al. niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity 2020, 52, 1057–1074.e7. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Lee, M.G.; Winter, B.; Droz, N.M.; Eissenberg, J.C.; Shiekhattar, R.; Shilatifard, A. Drosophila UTX Is a Histone H3 Lys27 demethylase that colocalizes with the elongating form of RNA polymerase II. Mol. Cell. Biol. 2007, 28, 1041–1046. [Google Scholar] [CrossRef]
- Cheon, C.K.; Sohn, Y.B.; Ko, J.M.; Lee, Y.J.; Song, J.S.; Moon, J.W.; Yang, B.K.; Ha, I.S.; Bae, E.J.; Jin, H.-S.; et al. Identification of KMT2D and KDM6A mutations by exome sequencing in Korean patients with Kabuki syndrome. J. Hum. Genet. 2014, 59, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Mulvey, B.; Salie, M.; Yang, X.; Matsui, Y.; Nityanandam, A.; Fan, Y.; Peng, J.C. UTX/KDM6A suppresses AP-1 and a gliogenesis program during neural differentiation of human pluripotent stem cells. Epigenetics Chromatin 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, Y.; Liu, Y.; Sun, Y.; Zhou, Y.; Gu, M.; Chen, Y.; Xia, R.; Chen, S.; Deng, A.; et al. β-Arrestin 1 Modulates functions of autoimmune t cells from primary biliary cirrhosis patients. J. Clin. Immunol. 2011, 31, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.A.; Johnston, D.R.; Kaur, S.; Lubin, F.D. Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. Sci. Signal. 2019, 12, eaaw9277. [Google Scholar] [CrossRef]
- Shaulian, E. AP-1—The jun proteins: Oncogenes or tumor suppressors in disguise? Cell. Signal. 2010, 22, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.C.; Chakraborty, A.; Diefenbacher, M.E.; Skehel, M.; Behrens, A. Arginine methylation of the c-Jun coactivator RACO-1 is required for c-Jun/AP-1 activation. EMBO J. 2013, 32, 1556–1567. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Liu, M.; Hua, W.; Chiou, Y.; Chen, C.; Yao, C.; Lai, Y.; Lin, C.; Lin, W. Calcium-dependent methylation by PRMT1 promotes erythroid differentiation through the p38α MAPK pathway. FEBS Lett. 2020, 594, 301–316. [Google Scholar] [CrossRef]
- Kim, E.; Jang, J.; Park, J.G.; Kim, K.-H.; Yoon, K.; Yoo, B.C.; Cho, J.Y. Protein arginine methyltransferase 1 (PRMT1) Selective Inhibitor, TC-E 5003, has anti-inflammatory properties in TLR4 signaling. Int. J. Mol. Sci. 2020, 21, 3058. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Chen, C.-T.; Chou, C.-K.; Kuo, H.-P.; Li, L.-Y.; Lin, C.-Y.; Lee, H.-J.; Wang, Y.-N.; Liu, M.; Liao, H.-W.; et al. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat. Cell Biol. 2011, 13, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Pérez, P.; Esteve-Puig, R.; De Torre-Minguela, C.; López-Fauqued, M.; Bech-Serra, J.J.; Tenbaum, S.; García-Trevijano, E.R.; Canals, F.; Merlino, G.; Ávila, M.A.; et al. Protein arginine methyltransferase 5 Regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci. Signal. 2011, 4, ra58. [Google Scholar] [CrossRef] [PubMed]
- Banasavadi-Siddegowda, Y.K.; Russell, L.; Frair, E.; Karkhanis, V.A.; Relation, T.; Yoo, J.Y.; Zhang, J.; Sif, S.; Imitola, J.; Baiocchi, R.; et al. PRMT5–PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells. Oncogene 2017, 36, 263–274. [Google Scholar] [CrossRef]
- Majumder, S.; Alinari, L.; Roy, S.; Miller, T.; Datta, J.; Sif, S.; Baiocchi, R.; Jacob, S.T. Methylation of histone H3 and H4 by PRMT5 regulates ribosomal RNA gene transcription. J. Cell. Biochem. 2009, 109, 553–563. [Google Scholar] [CrossRef]
- Chatterjee, B.; Ghosh, K.; Suresh, L.; Kanade, S.R. Curcumin ameliorates PRMT5-MEP50 arginine methyltransferase expression by decreasing the Sp1 and NF-YA transcription factors in the A549 and MCF-7 cells. Mol. Cell. Biochem. 2019, 455, 73–90. [Google Scholar] [CrossRef]
- Calabretta, S.; Vogel, G.; Yu, Z.; Choquet, K.; Darbelli, L.; Nicholson, T.B.; Kleinman, C.L.; Richard, S. Loss of PRMT5 promotes PDGFRα degradation during oligodendrocyte differentiation and myelination. Dev. Cell 2018, 46, 426–440.e5. [Google Scholar] [CrossRef]
- Hunter, D.J.; Schofield, D.J.; Callander, E.J. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Yang, Y.; Huang, J.; Dai, Z.; Zheng, W.; Li, Z.; Yao, Z.; Zhang, H.; Zheng, J. PRMT5 inhibition attenuates cartilage degradation by reducing MAPK and NF-κB signaling. Arthritis Res. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Limm, K.; Ott, C.; Wallner, S.; Mueller, D.W.; Oefner, P.; Hellerbrand, C.; Bosserhoff, A.-K. Deregulation of protein methylation in melanoma. Eur. J. Cancer 2013, 49, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Rios, D.; Graça, I.; Vieira, F.Q.; Ramalho-Carvalho, J.; Pereira-Silva, E.; Martins, A.T.; Oliveira, J.; Gonçalves, C.S.; Costa, B.M.; Henrique, R.; et al. Histone methyltransferase PRMT6 plays an oncogenic role of in prostate cancer. Oncotarget 2016, 7, 53018–53028. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.H.; Zhou, L.; Ng, K.Y.; Wong, T.L.; Lee, T.K.; Sharma, R.; Loong, J.H.; Ching, Y.P.; Yuan, Y.-F.; Xie, D.; et al. PRMT6 Regulates RAS/RAF Binding and MEK/ERK-mediated cancer stemness activities in hepatocellular carcinoma through CRAF methylation. Cell Rep. 2018, 25, 690–701.e8. [Google Scholar] [CrossRef] [PubMed]
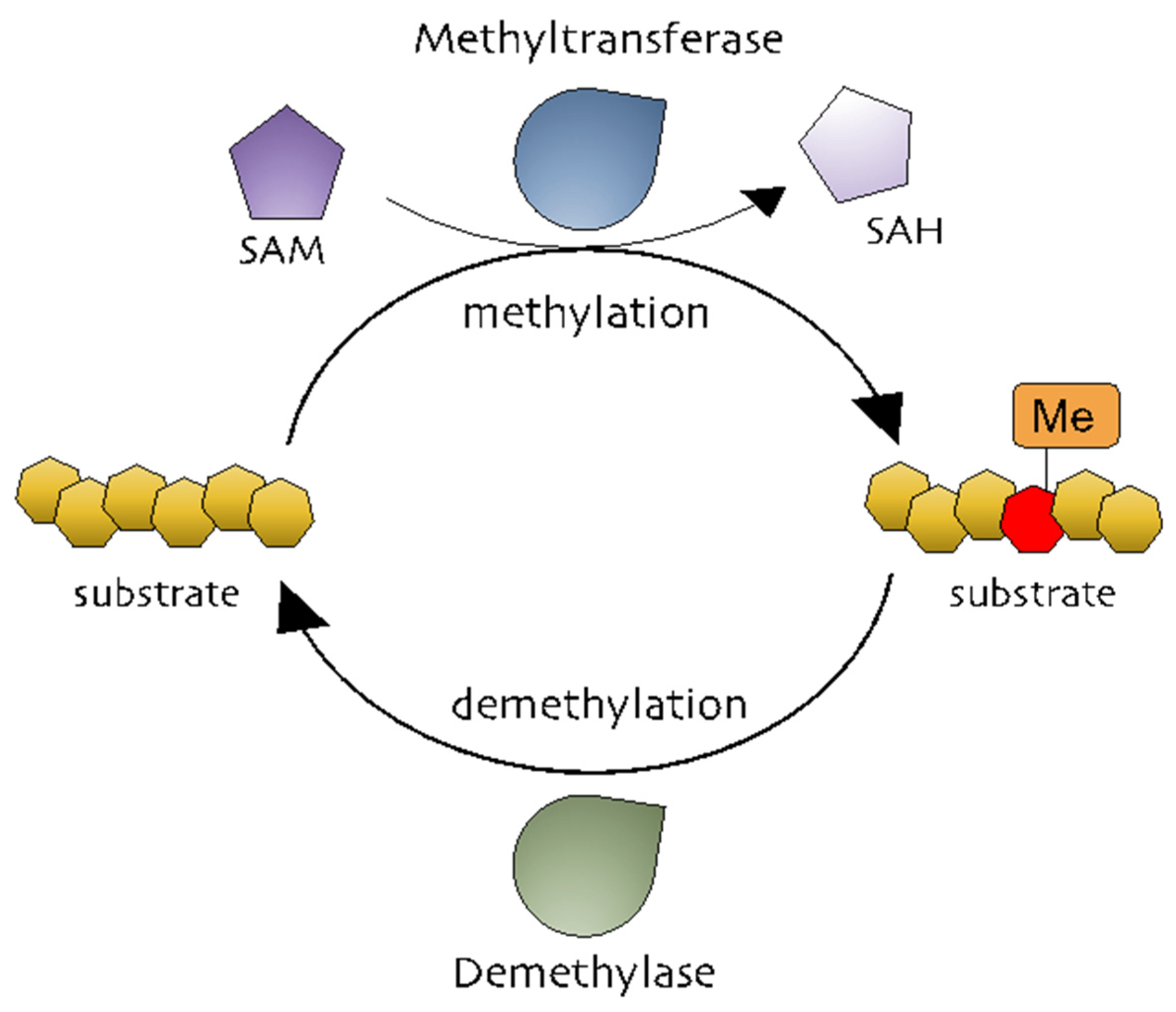
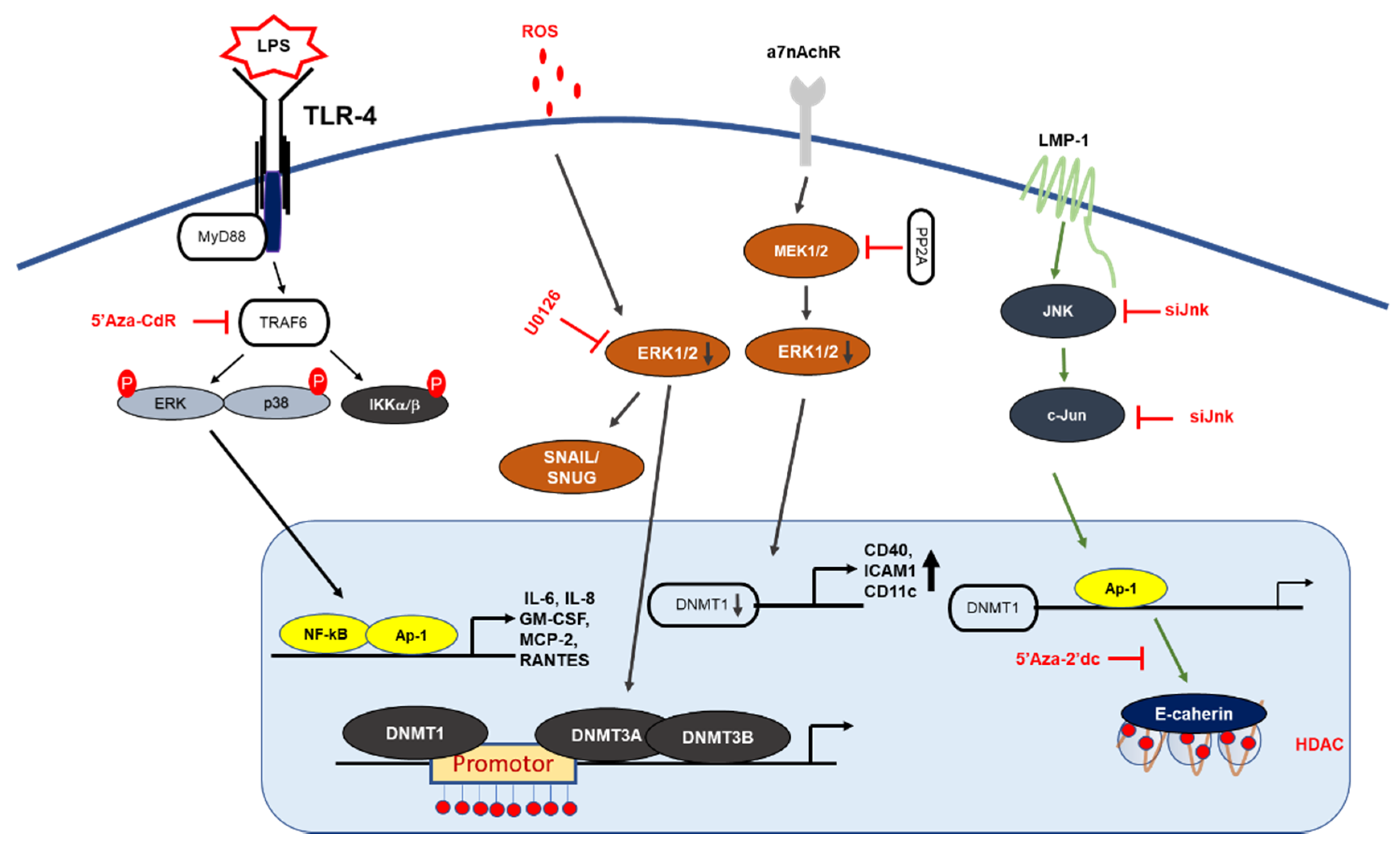



| Biological Function | Genes | Ref. |
|---|---|---|
| Inflammation | IL-1, -2, -6, -8, -15, TLR3, Cyclooxygenase 2, TNF-α, CXCL-1, -2 | [14] |
| Migration and invasion | MMPs (1, 3, and 9), ARP2/3, autotoxin, cathepsin L, CD44, Krp-1, Ezrin, Mts-1 | [15,16] |
| Proliferation, apoptosis, and cell cycle | TGF-α, -β, Cyclin D1, p16, FasL | [17] |
| Compound | Target | Disease |
|---|---|---|
| Azacytidine (5-Aza) | DNMT | Myeloid splastic syndrome Multiple myeloma |
| Decitabine (5-aza-2’-deoxycytidine) | DNMT | Myeloid splastic syndrome |
| Guadecitabine (SGI-110) | DNMT | Myeloid splastic syndrome |
| Inhibitor | Target | Ref. | |
|---|---|---|---|
| MAPK | |||
| Rottlerin | ERK | Demethylation of p16INK4A and p21WAF1 | [44] |
| U0126 | ERK | Reduction of DNMT-1 gene expression | [45] |
| U0126 and SB203580 | ERK, p38 | DNMT3A and DNMT3B downregulation | [46] |
| SP600125 | JNK | Decreased protein level of c-Jun, JNK and DNMT-1 | [48] |
| Curcumin, FR180204 and SB203580 | ERK1/2, p38 | Downregulation of PRMT5, NF-YA and Sp1 protein expression | [97] |
| Dabrafenib and trametinib | BRAF, MEK | Increased KIT expression | [66] |
| Methyltransferase | |||
| Procainamide | DNMT | ERK pathway inhibition | [59] |
| Hydralazine | DNMT-1 | ERK pathway inhibition | [62] |
| TC-E 5003 | PRMT1 | Downregulation of AP-1 activity | [92] |
| 5’-methylthioadensine | PRMT5 | Activation of RAS-ERK1/2 activity | [94] |
| Curcumin | PRMT5 | Increased protein of ERK1/2 and p38 | [96] |
| Deacetylase | |||
| JNJ-26481585 (quisinostat) | HDAC | Phosphorylation of JNK and c-Jun | [77] |
| LG325 | HDAC1 | Suppression of c-Jun activation | [76] |
| Panobinostat, PCI-30451 | HDAC | Enhancement of BRAF inhibitors | [65] |
| Co-treatment | |||
| U0126 and NaB | ERK, HDAC | Decreased gene expression level of CD133 and BMI1 | [64] |
| TAK, SB239063 and tubastatin | TAK, p38, HDAC6 | SigR1 expression | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Ahuja, A.; Kim, M.-Y.; Cho, J.Y. DNA or Protein Methylation-Dependent Regulation of Activator Protein-1 Function. Cells 2021, 10, 461. https://doi.org/10.3390/cells10020461
Kim E, Ahuja A, Kim M-Y, Cho JY. DNA or Protein Methylation-Dependent Regulation of Activator Protein-1 Function. Cells. 2021; 10(2):461. https://doi.org/10.3390/cells10020461
Chicago/Turabian StyleKim, Eunji, Akash Ahuja, Mi-Yeon Kim, and Jae Youl Cho. 2021. "DNA or Protein Methylation-Dependent Regulation of Activator Protein-1 Function" Cells 10, no. 2: 461. https://doi.org/10.3390/cells10020461
APA StyleKim, E., Ahuja, A., Kim, M.-Y., & Cho, J. Y. (2021). DNA or Protein Methylation-Dependent Regulation of Activator Protein-1 Function. Cells, 10(2), 461. https://doi.org/10.3390/cells10020461







