Choline Glycerophospholipid-Derived Prostaglandins Attenuate TNFα Gene Expression in Macrophages via a cPLA2α/COX-1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Stimulation Conditions
2.3. Liquid Chromatography/Mass Spectrometry (LC/MS) Analyses of Prostaglandins
2.4. Liquid Chromatography/Mass Spectrometry (LC/MS) Analyses of Phospholipids
2.5. Gas chromatography/Mass Spectrometry (GC/MS) Analyses
2.6. iPLA2β Antisense Inhibition Studies
2.7. Quantitative PCR
2.8. Data Analysis
3. Results
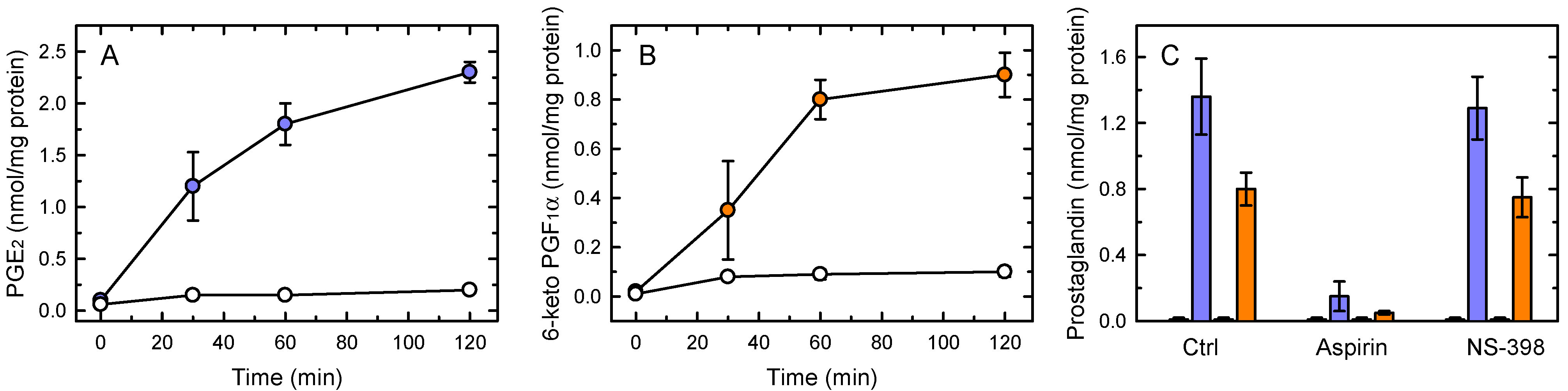
3.1. Immediate Synthesis of Prostaglandins by Macrophages
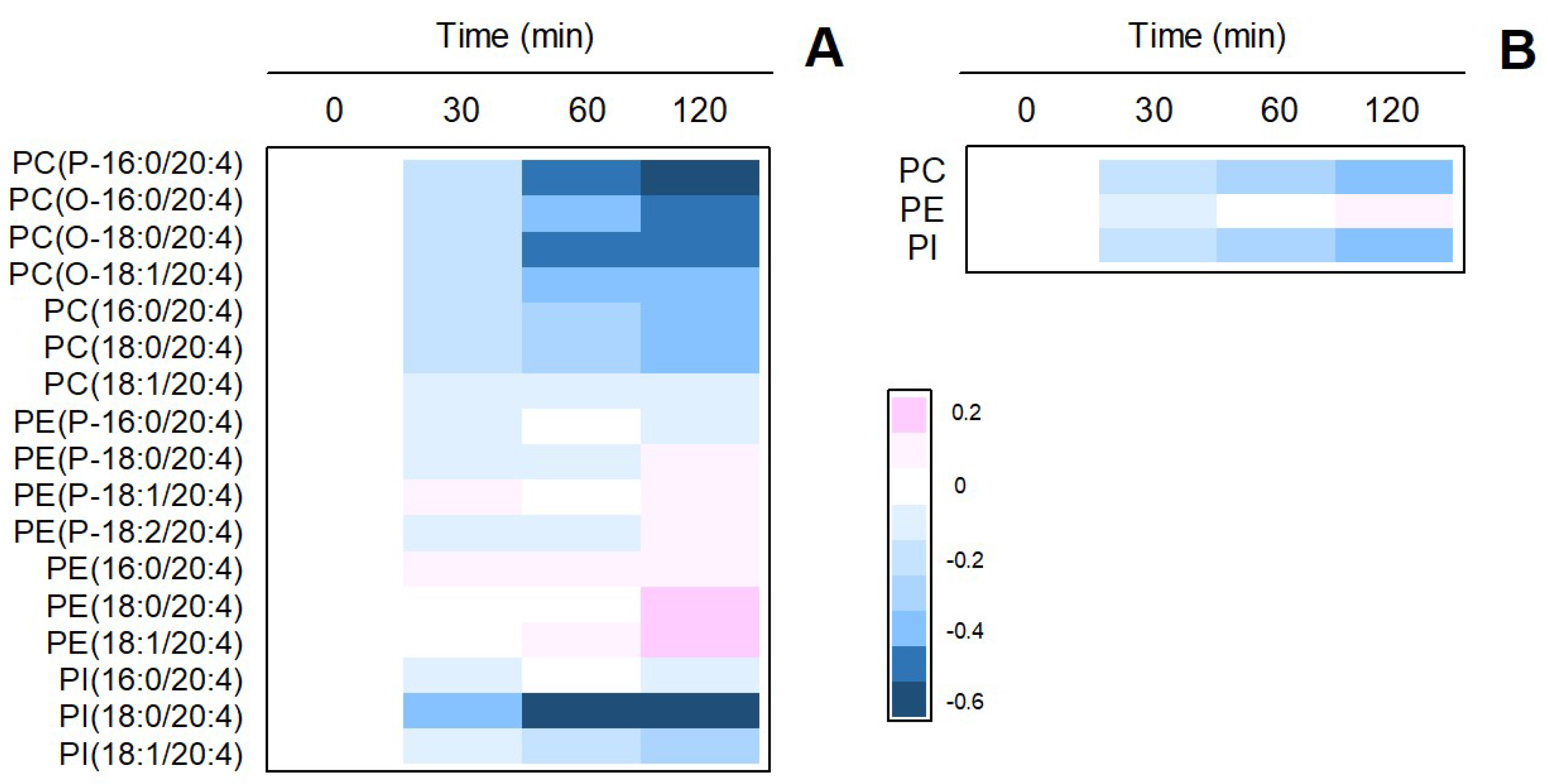
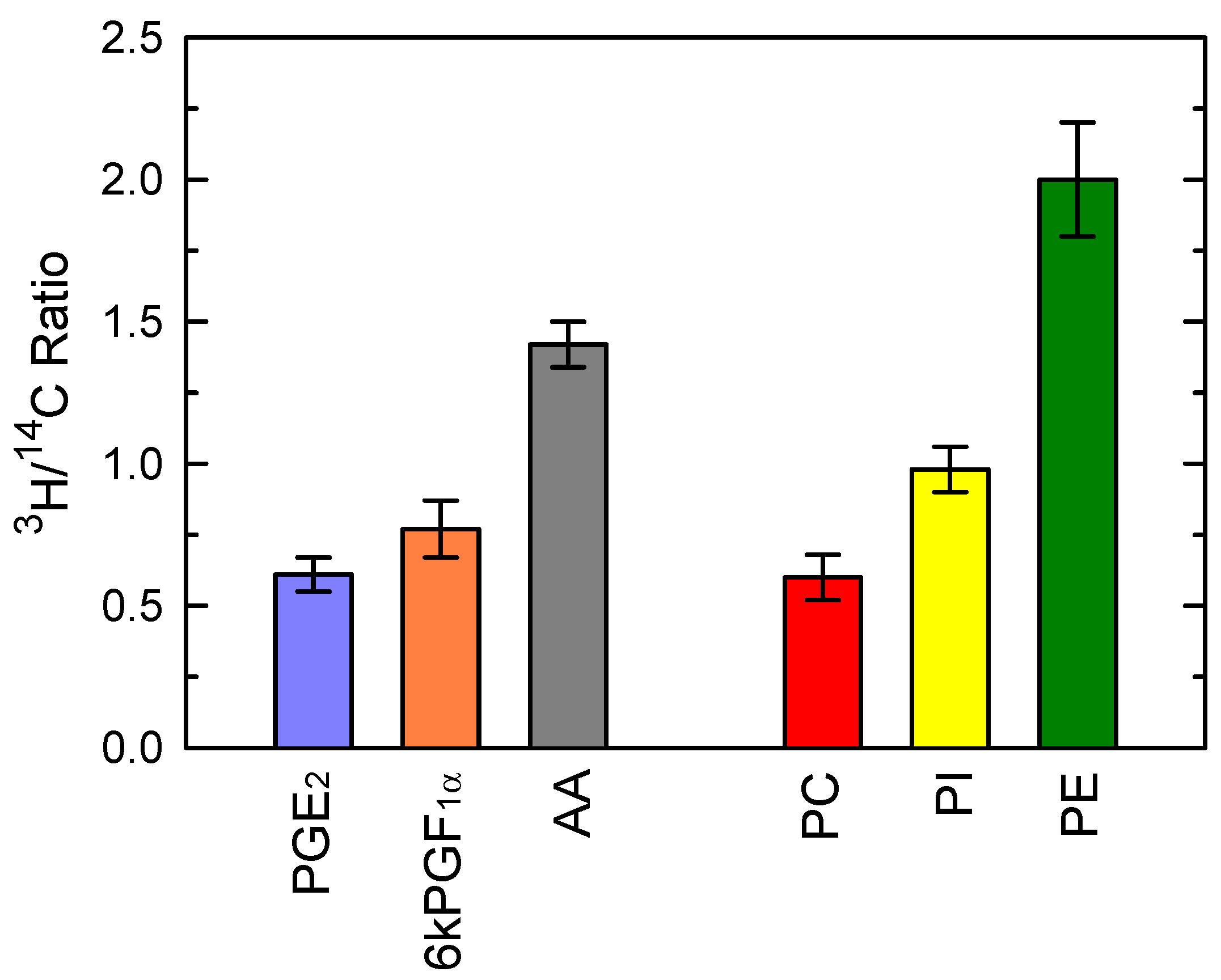
3.2. Analysis of the AA Mobilization Response
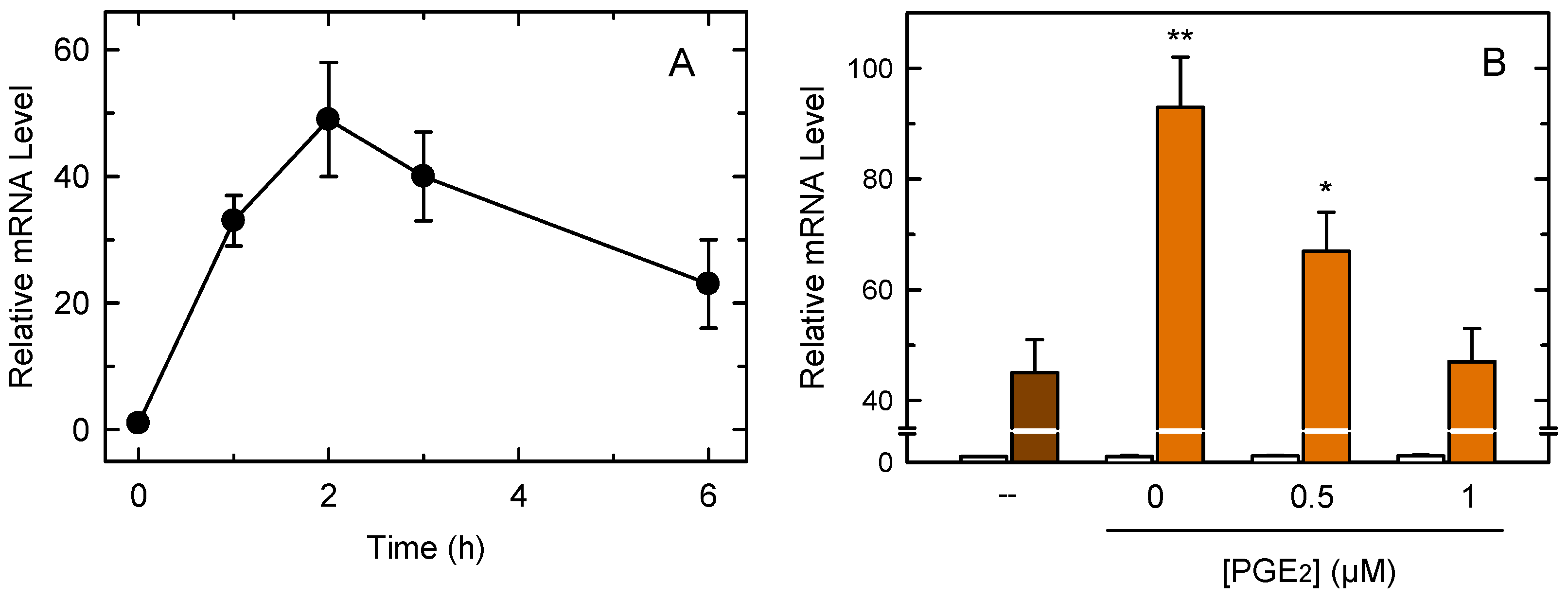
3.3. COX-1-Mediated Prostaglandin Production Regulates TNFα Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| COX | cyclooxygenase |
| GC/MS | gas chromatography coupled to mass spectrometry |
| LC/MS | liquid chromatography coupled to mass spectrometry |
| cPLA2α | group IVA cytosolic phospholipase A2 |
| iPLA2β | group VIA calcium-independent phospholipase A2 |
| PC | choline-containing glycerophospholipids |
| PE | ethanolamine-containing glycerophospholipids |
| PI | phosphatidylinositol |
| PG | prostaglandin |
| TNFα | tumor necrosis factor-α |
References
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Stumpo, R.; Kauer, M.; Martin, S.; Kolb, H. Alternative activation of macrophages by IL-10. Pathobiology 1999, 67, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochim. Biophys. Acta 2012, 1821, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Rodríguez, J.P.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipase A2 regulation of lipid droplet formation. Biochim. Biophys. Acta 2014, 1841, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Underhill, D.M. Macrophage recognition of zymosan particles. J. Endotoxin Res. 2003, 9, 176–180. [Google Scholar] [CrossRef]
- Taylor, P.R.; Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Gordon, S. The Role of SIGNR1 and the β-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J. Immunol. 2004, 172, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Underhill, D.M.; Touret, N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 2012, 13, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.H.; Magee, A.S.; Danielson, M.E.; et al. Activation of the innate immune receptor dectin-1 upon formation of a “phagocytic synapse”. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef]
- Walachowski, S.; Tabouret, G.; Foucras, G. Triggering dectin-1-pathway alone is not sufficient to induce cytokine production by murine macrophages. PLoS One 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Takahara, K.; Tokieda, S.; Nagaoka, K.; Inaba, K. Efficient capture of Candida albicans and zymosan by SIGNR1 augments TLR2-dependent TNF-α production. Int. Immunol. 2012, 24, 89–96. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Ivanova, P.T.; Byrne, M.O.; Milne, S.B.; Brown, H.A.; Marnett, L.J. Lipid profiling reveals glycerophospholipid remodeling in zymosan-stimulated macrophages. Biochemistry 2007, 46, 6026–6042. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-Gómez, L.; Astudillo, A.M.; Guijas, C.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages. J. Immunol. 2014, 192, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-Gómez, L.; Astudillo, A.M.; Meana, C.; Rubio, J.M.; Guijas, C.; Balboa, M.A.; Balsinde, J. A phosphatidylinositol species acutely generated by activated macrophages regulates innate immune responses. J. Immunol. 2013, 190, 5169–5177. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.M.; Astudillo, A.M.; Casas, J.; Balboa, M.A.; Balsinde, J. Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Front. Immunol. 2018, 9, 1723. [Google Scholar] [CrossRef] [PubMed]
- Monge, P.; Garrido, A.; Rubio, J.M.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. The contribution of cytosolic group IVA and calcium-independent group VIA phospholipase A2s to adrenic acid mobilization in murine macrophages. Biomolecules 2020, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Meana, C.; Bermúdez, M.A.; Pérez-Encabo, A.; Balboa, M.A.; Balsinde, J. Release of anti-inflammatory palmitoleic acid and its positional isomers by mouse peritoneal macrophages. Biomedicines 2020, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-Gómez, L.; Astudillo, A.M.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Essential role for ethanolamine plasmalogen hydrolysis in bacterial lipopolysaccharide priming of macrophages for enhanced arachidonic acid release. Front. Immunol. 2017, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-Gómez, L.; Monge, P.; Rodríguez, J.P.; Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Phospholipid arachidonic acid remodeling during phagocytosis in mouse peritoneal macrophages. Biomedicines 2020, 8, 274. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Pérez-Chacón, G.; Meana, C.; Balgoma, D.; Pol, A.; del Pozo, M.A.; Balboa, M.A.; Balsinde, J. Altered arachidonate distribution in macrophages from caveolin-1 null mice leading to reduced eicosanoid synthesis. J. Biol. Chem. 2011, 286, 35299–35307. [Google Scholar] [CrossRef] [PubMed]
- Balsinde, J.; Fernández, B.; Diez, E. Regulation of arachidonic acid release in mouse peritoneal macrophages. The role of extracellular calcium and protein kinase C. J. Immunol. 1990, 144, 4298–4304. [Google Scholar] [PubMed]
- Ruipérez, V.; Astudillo, M.A.; Balboa, M.A.; Balsinde, J. Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s. J. Immunol. 2009, 182, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Pindado, J.; Balsinde, J.; Balboa, M.A. TLR3-dependent induction of nitric oxide synthase in RAW 264.7 macrophage-like cells via a cytosolic phospholipase A2/cyclooxygenase-2 pathway. J. Immunol. 2007, 179, 4821–4828. [Google Scholar] [CrossRef]
- Balsinde, J.; Balboa, M.A.; Dennis, E.A. Identification of a third pathway for arachidonic acid mobilization and prostaglandin production in activated P388D1 macrophage-like cells. J. Biol. Chem. 2000, 275, 22544–22549. [Google Scholar] [CrossRef]
- Balboa, M.A.; Pérez, R.; Balsinde, J. Amplification mechanisms of inflammation: Paracrine stimulation of arachidonic acid mobilization by secreted phospholipase A2 is regulated by cytosolic phospholipase A2-derived hydroperoxyeicosatetraenoic acid. J. Immunol. 2003, 171, 989–994. [Google Scholar] [CrossRef]
- Balsinde, J.; Balboa, M.A.; Insel, P.A.; Dennis, E.A. Differential regulation of phospholipase D and phospholipase A2 by protein kinase C in P388D1 macrophages. Biochem. J. 1997, 321, 805–809. [Google Scholar] [CrossRef]
- Balboa, M.A.; Balsinde, J.; Dennis, E.A. Involvement of phosphatidate phosphohydrolase in arachidonic acid mobilization in human amnionic WISH cells. J. Biol. Chem. 1998, 273, 7684–7690. [Google Scholar] [CrossRef] [PubMed]
- Balboa, M.A.; Balsinde, J.; Dillon, D.A.; Carman, G.M.; Dennis, E.A. Proinflammatory macrophage-activating properties of the novel phospholipid diacylglycerol pyrophosphate. J. Biol. Chem. 1999, 274, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Ross, R.M.; Ayers, C.R.; Wills, M.R.; Savory, J. Rapid separation of prostaglandins by linear high performance thin layer chromatography. J. Liquid. Chromatogr. 1983, 6, 1265–1272. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Fine, J.B.; Sprecher, H. Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J. Lipid Res. 1982, 23, 660–663. [Google Scholar] [CrossRef]
- Rubio, J.M.; Rodríguez, J.P.; Gil-de-Gómez, L.; Guijas, C.; Balboa, M.A.; Balsinde, J. Group V secreted phospholipase A2 is up-regulated by interleukin-4 in human macrophages and mediates phagocytosis via hydrolysis of ethanolamine phospholipids. J. Immunol. 2015, 194, 3327–3339. [Google Scholar] [CrossRef]
- Balgoma, D.; Astudillo, A.M.; Pérez-Chacón, G.; Montero, O.; Balboa, M.A.; Balsinde, J. Markers of monocyte activation revealed by lipidomic profiling of arachidonic acid-containing phospholipids. J. Immunol. 2010, 184, 3857–3865. [Google Scholar] [CrossRef]
- Diez, E.; Balsinde, J.; Aracil, M.; Schüller, A. Ethanol induces release of arachidonic acid but not synthesis of eicosanoids in mouse peritoneal macrophages. Biochim. Biophys. Acta 1987, 921, 82–89. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Pérez-Chacón, G.; Balgoma, D.; Gil-de-Gómez, L.; Ruipérez, V.; Guijas, C.; Balboa, M.A.; Balsinde, J. Influence of cellular arachidonic acid levels on phospholipid remodeling and CoA-independent transacylase activity in human monocytes and U937 cells. Biochim. Biophys. Acta 2011, 1811, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Esquinas, E.; Meana, C.; Gil-de-Gómez, L.; Guijas, C.; Balsinde, J.; Balboa, M.A. Subcellular localization and role of lipin-1 in human macrophages. J. Immunol. 2011, 186, 6004–6013. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Pérez-Chacón, G.; Astudillo, A.M.; Rubio, J.M.; Gil-de-Gómez, L.; Balboa, M.A.; Balsinde, J. Simultaneous activation of p38 and JNK by arachidonic acid stimulates the cytosolic phospholipase A2-dependent synthesis of lipid droplets in human monocytes. J. Lipid Res. 2012, 53, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Meana, C.; Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Foamy monocytes are enriched in cis-7-hexadecenoic fatty acid (16:1n-9), a possible biomarker for early detection of cardiovascular disease. Cell Chem. Biol. 2016, 23, 689–699. [Google Scholar] [CrossRef]
- Balboa, M.A.; Balsinde, J. Involvement of calcium-independent phospholipase A2 in hydrogen peroxide-induced accumulation of free fatty acids in human U937 cells. J. Biol. Chem. 2002, 277, 40384–40389. [Google Scholar] [CrossRef]
- Balboa, M.A.; Sáez, Y.; Balsinde, J. Calcium-independent phospholipase A2 is required for lysozyme secretion in U937 promonocytes. J. Immunol. 2003, 170, 5276–5280. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Melero, R.; Balboa, M.A.; Balsinde, J. Role of group VIA calcium-independent phospholipase A2 in arachidonic acid release, phospholipid fatty acid incorporation, and apoptosis in U937 cells responding to hydrogen peroxide. J. Biol. Chem. 2004, 279, 40385–40391. [Google Scholar] [CrossRef]
- Pérez, R.; Balboa, M.A.; Balsinde, J. Involvement of group VIA calcium-independent phospholipase A2 in macrophage engulfment of hydrogen peroxide-treated U937 cells. J. Immunol. 2006, 176, 2555–2561. [Google Scholar] [CrossRef]
- Valdearcos, M.; Esquinas, E.; Meana, C.; Peña, L.; Gil-de-Gómez, L.; Balsinde, J.; Balboa, M.A. Lipin-2 reduces proinflammatory signaling induced by saturated fatty acids in macrophages. J. Biol. Chem. 2012, 287, 10894–10904. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Kingsley, P.J.; Wang, H.; Zhang, H.; Morrow, J.D.; Dey, S.K.; Marnett, L.J. Cyclooxygenase-1-dependent prostaglandin synthesis modulates tumor necrosis factor-α secretion in lipopolysaccharide-challenged murine resident peritoneal macrophages. J. Biol. Chem. 2004, 279, 34256–34268. [Google Scholar] [CrossRef]
- Suram, S.; Silveira, L.J.; Mahaffey, S.; Brown, G.D.; Bonventre, J.V.; Williams, D.L.; Gow, N.A.R.; Bratton, D.L.; Murphy, R.C.; Leslie, C.C. Cytosolic phospholipase A2α and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation. PLoS ONE 2013, 8, e69002. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Yamada, K.; Chikazawa, Y.; Ueno, M.; Nakamoto, S.; Okuno, T.; Seno, K. Characterization of a novel inhibitor of cytosolic phospholipase A2α, pyrrophenone. Biochem. J. 2002, 363, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Balsinde, J.; Dennis, E.A. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 1996, 271, 6758–6765. [Google Scholar] [CrossRef]
- Shirai, Y.; Balsinde, J.; Dennis, E.A. Localization and functional interrelationships among cytosolic group IV, secreted group V, and Ca2+-independent group VI phospholipase A2s in P388D1 macrophages using GFP/RFP constructs. Biochim. Biophys. Acta 2005, 1735, 119–129. [Google Scholar] [CrossRef]
- Balsinde, J.; Balboa, M.A. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell. Signal. 2005, 17, 1052–1062. [Google Scholar] [CrossRef]
- Bone, R.N.; Gai, Y.; Magrioti, V.; Kokotou, M.G.; Ali, T.; Lei, X.; Tse, H.M.; Kokotos, G.; Ramanadham, S. Inhibition of Ca2+-independent phospholipase A2β (iPLA2β) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes 2015, 64, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Balsinde, J.; Balboa, M.A.; Dennis, E.A. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 1997, 272, 29317–29321. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, K.A.; Cathcart, M.K. Calcium-independent phospholipase A2 is required for human monocyte chemotaxis to monocyte chemoattractant protein 1. J. Immunol. 2001, 167, 3414–3421. [Google Scholar] [CrossRef] [PubMed]
- Smani, T.; Zakharov, S.I.; Leno, E.; Csutora, P.; Trepakova, E.S.; Bolotina, V.M. Ca2+-independent phospholipase A2 is a novel determinant of store-operated Ca2+ entry. J. Biol. Chem. 2003, 278, 11909–11915. [Google Scholar] [CrossRef] [PubMed]
- Chilton, F.H.; Fonteh, A.N.; Surette, M.E.; Triggiani, M.; Winkler, J.D. Control of arachidonate levels within inflammatory cells. Biochim. Biophys. Acta 1996, 1299, 1–15. [Google Scholar] [CrossRef]
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 2014, 53, 18–81. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Balsinde, J.; Fernández, B.; Solís-Herruzo, J.A. Increased incorporation of arachidonic acid into phospholipids in zymosan-stimulated mouse peritoneal macrophages. Eur. J. Biochem. 1994, 221, 1013–1018. [Google Scholar] [CrossRef]
- Neuschäfer-Rube, F.; Pathe-Neuschäfer-Rube, A.; Hippenstiel, S.; Püschel, G.P. PGE2 enhanced TNFα-mediated IL-8 induction in monocytic cell lines and PBMC. Cytokine 2019, 113, 105–116. [Google Scholar] [CrossRef]
- Samiea, A.; Yoon, J.S.J.; Cheung, S.T.; Chamberlain, T.C.; Mui, A.L.F. Interleukin-10 contributes to PGE2 signalling through upregulation of EP4 via SHIP1 and STAT3. PLoS ONE 2020, 15, 1–18. [Google Scholar] [CrossRef]
- Leslie, C.C. Cytosolic phospholipase A₂: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim. Biophys. Acta 2019, 1864, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chacón, G.; Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. 2009. Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta 2009, 1791, 1103–1113. [Google Scholar] [CrossRef]
- Humes, J.L.; Bonney, R.J.; Pelus, L.; Dahlgren, M.E.; Sadowski, S.J.; Kuehl, F.A.; Davies, P. Macrophages synthesise and release prostaglandins in response to inflammatory stimuli. Nature 1977, 269, 149–151. [Google Scholar] [CrossRef]
- Scott, W.A.; Zrike, J.M.; Hamill, A.L.; Kempe, J.; Cohn, Z.A. Regulation of arachidonic acid metabolites in macrophages. J. Exp. Med. 1980, 152, 324–335. [Google Scholar] [CrossRef]
- Emilsson, A.; Sundler, R. Evidence for a catalytic role of phospholipase A in phorbol diester- and zymosan-induced mobilization of arachidonic acid in mouse peritoneal macrophages. Biochim. Biophys. Acta 1986, 876, 533–542. [Google Scholar] [CrossRef]
- Balsinde, J.; Fernández, B.; Solís-Herruzo, J.A.; Diez, E. Pathways for arachidonic acid mobilization in zymosan-stimulated mouse peritoneal macrophages. Biochim. Biophys. Acta 1992, 1136, 75–82. [Google Scholar] [CrossRef]
- Qiu, Z.H.; Leslie, C.C. Protein kinase C-dependent and -independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase A2. J. Biol Chem. 1994, 269, 19480–19487. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Ivanova, P.T.; Byrne, M.O.; Milne, S.B.; Marnett, L.J.; Brown, H.A. Lipid profiling reveals arachidonate deficiency in RAW264.7 cells: Structural and functional implications. Biochemistry 2006, 45, 14795–14808. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrero, R.; Balboa, M.A.; Balsinde, J. Occurrence and biological activity of palmitoleic acid isomers in phagocytic cells. J. Lipid Res. 2018, 59, 237–249. [Google Scholar] [CrossRef]
- Sugiura, T.; Nakajima, M.; Sekiguchi, N.; Nakagawa, Y.; Waku, K. Different fatty chain compositions of alkenylacyl, alkylacyl and diacyl phospholipids in rabbit alveolar macrophages: High amounts of arachidonic acid in ether phospholipids. Lipids 1983, 18, 125–129. [Google Scholar] [CrossRef]
- MacDonald, J.I.S.; Sprecher, H. Phospholipid fatty acid remodeling in mammalian cells. Biochim. Biophys. Acta 1991, 1084, 105–121. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Dennis, E.A. Phospholipase A2 catalysis and lipid mediator lipidomics. Biochim. Biophys. Acta 2019, 1864, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Chilton, F.H. Rapid remodeling of arachidonate from phosphatidylcholine to phosphatidylethanolamine pools during mast cell activation. J. Immunol. 1992, 148, 1784–1791. [Google Scholar]
- Fonteh, A.N.; Chilton, F.H. Mobilization of different arachidonate pools and their roles in the generation of leukotrienes and free arachidonic acid during immunologic activation of mast cells. J. Immunol. 1993, 150, 563–570. [Google Scholar] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Chilton, F.H. Potential phospholipid source(s) of arachidonate used for the synthesis of leukotrienes by the human neutrophil. Biochem. J. 1989, 258, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; Murphy, R.C. Working towards an exegesis for lipids in biology. Nat. Chem. Biol. 2009, 5, 602–606. [Google Scholar] [CrossRef]
- Lebrero, P.; Astudillo, A.M.; Rubio, J.M.; Fernández-Caballero, J.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cellular plasmalogen content does not influence arachidonic acid levels or distribution in macrophages: A role for cytosolic phospholipase A2γ in phospholipid remodeling. Cells 2019, 8, 799. [Google Scholar] [CrossRef]
- Guijas, C.; Bermúdez, M.A.; Meana, C.; Astudillo, A.M.; Pereira, L.; Fernández-Caballero, L.; Balboa, M.A.; Balsinde, J. Neutral lipids are not a source of arachidonic acid for lipid mediator signaling in human foamy monocytes. Cells 2019, 8, 941. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Koivuniemi, A. The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett. 2017, 591, 2700–2713. [Google Scholar] [CrossRef]
- Honsho, M.; Fujiki, Y. Plasmalogen homeostasis. Regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 2017, 591, 2720–2729. [Google Scholar] [CrossRef]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; Croix, C.M.S.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Forss-Petter, S.; Wimmer, I.; Berger, J. Plasmalogens, platelet-activating factor and beyond. Ether lipids in signaling and neurodegeneration. Neurobiol. Dis. 2020, 145, 105061. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Sugimoto, Y.; Tanaka, S.; Ichikawa, A. Prostaglandin E2 receptors EP2 and EP4 differentially modulate TNF-α and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem. Biophys. Res. Commun. 2000, 278, 224–228. [Google Scholar] [CrossRef]
- Treffkorn, L.; Scheibe, R.; Maruyama, T.; Dieter, P. PGE2 exerts its effect on the LPS-induced release of TNF-α, ET-1, IL-lα, IL-6 and IL-10 via the EP2 and EP4 receptor in rat liver macrophages. Prostaglandins Other Lipid Mediat. 2004, 74, 113–123. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo, A.M.; Rodríguez, J.P.; Guijas, C.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Choline Glycerophospholipid-Derived Prostaglandins Attenuate TNFα Gene Expression in Macrophages via a cPLA2α/COX-1 Pathway. Cells 2021, 10, 447. https://doi.org/10.3390/cells10020447
Astudillo AM, Rodríguez JP, Guijas C, Rubio JM, Balboa MA, Balsinde J. Choline Glycerophospholipid-Derived Prostaglandins Attenuate TNFα Gene Expression in Macrophages via a cPLA2α/COX-1 Pathway. Cells. 2021; 10(2):447. https://doi.org/10.3390/cells10020447
Chicago/Turabian StyleAstudillo, Alma M., Juan P. Rodríguez, Carlos Guijas, Julio M. Rubio, María A. Balboa, and Jesús Balsinde. 2021. "Choline Glycerophospholipid-Derived Prostaglandins Attenuate TNFα Gene Expression in Macrophages via a cPLA2α/COX-1 Pathway" Cells 10, no. 2: 447. https://doi.org/10.3390/cells10020447
APA StyleAstudillo, A. M., Rodríguez, J. P., Guijas, C., Rubio, J. M., Balboa, M. A., & Balsinde, J. (2021). Choline Glycerophospholipid-Derived Prostaglandins Attenuate TNFα Gene Expression in Macrophages via a cPLA2α/COX-1 Pathway. Cells, 10(2), 447. https://doi.org/10.3390/cells10020447







