Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue
Abstract
1. Background
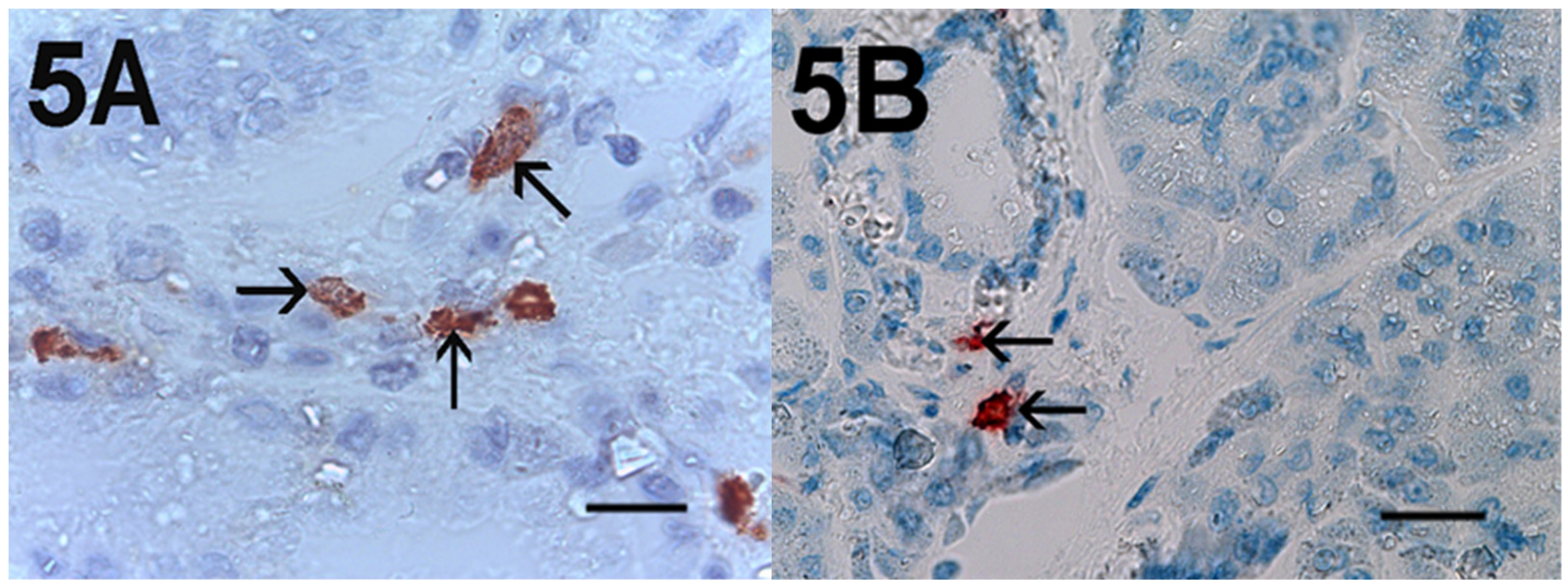
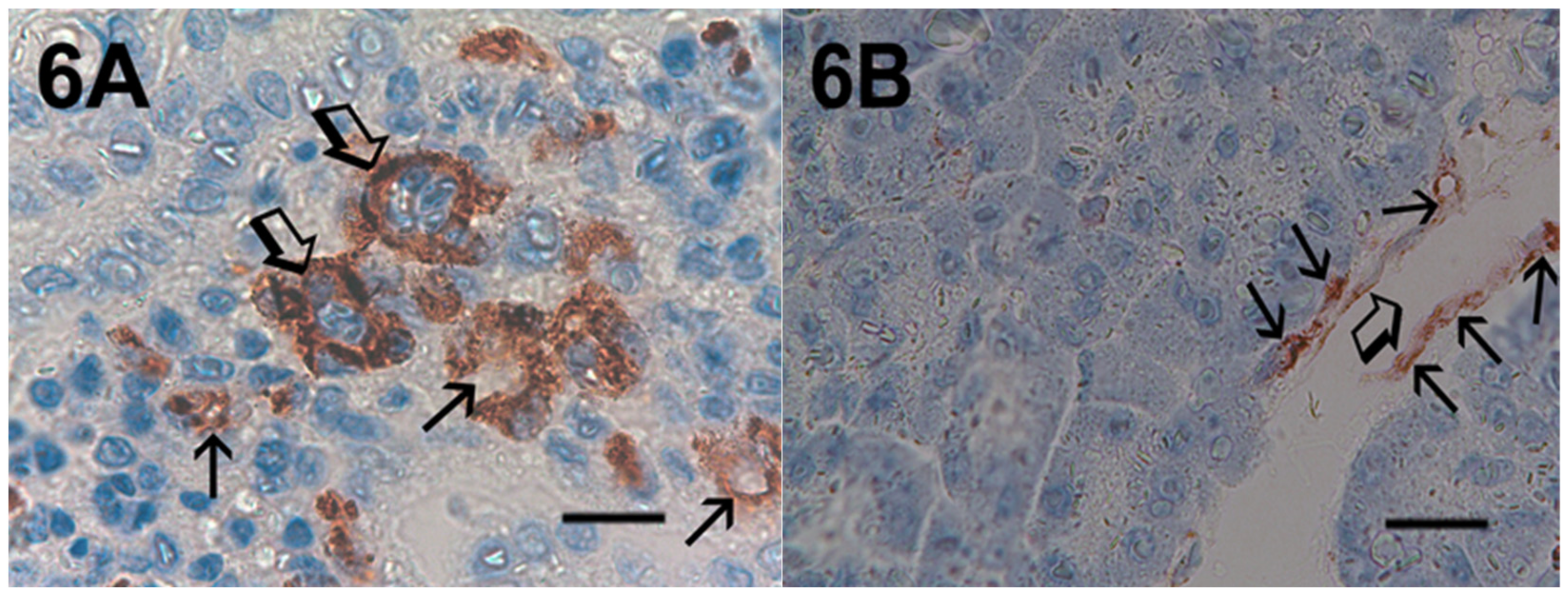
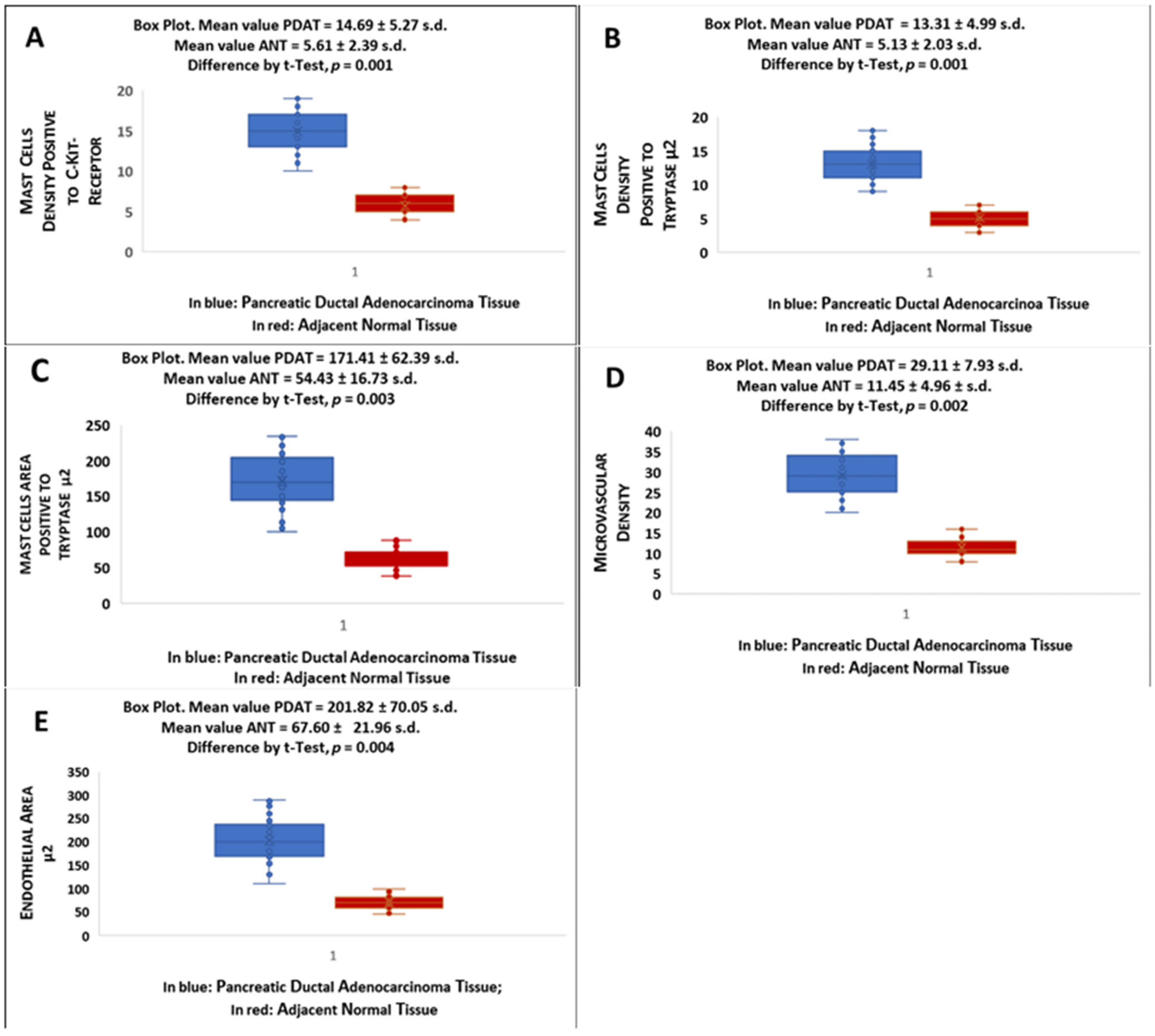
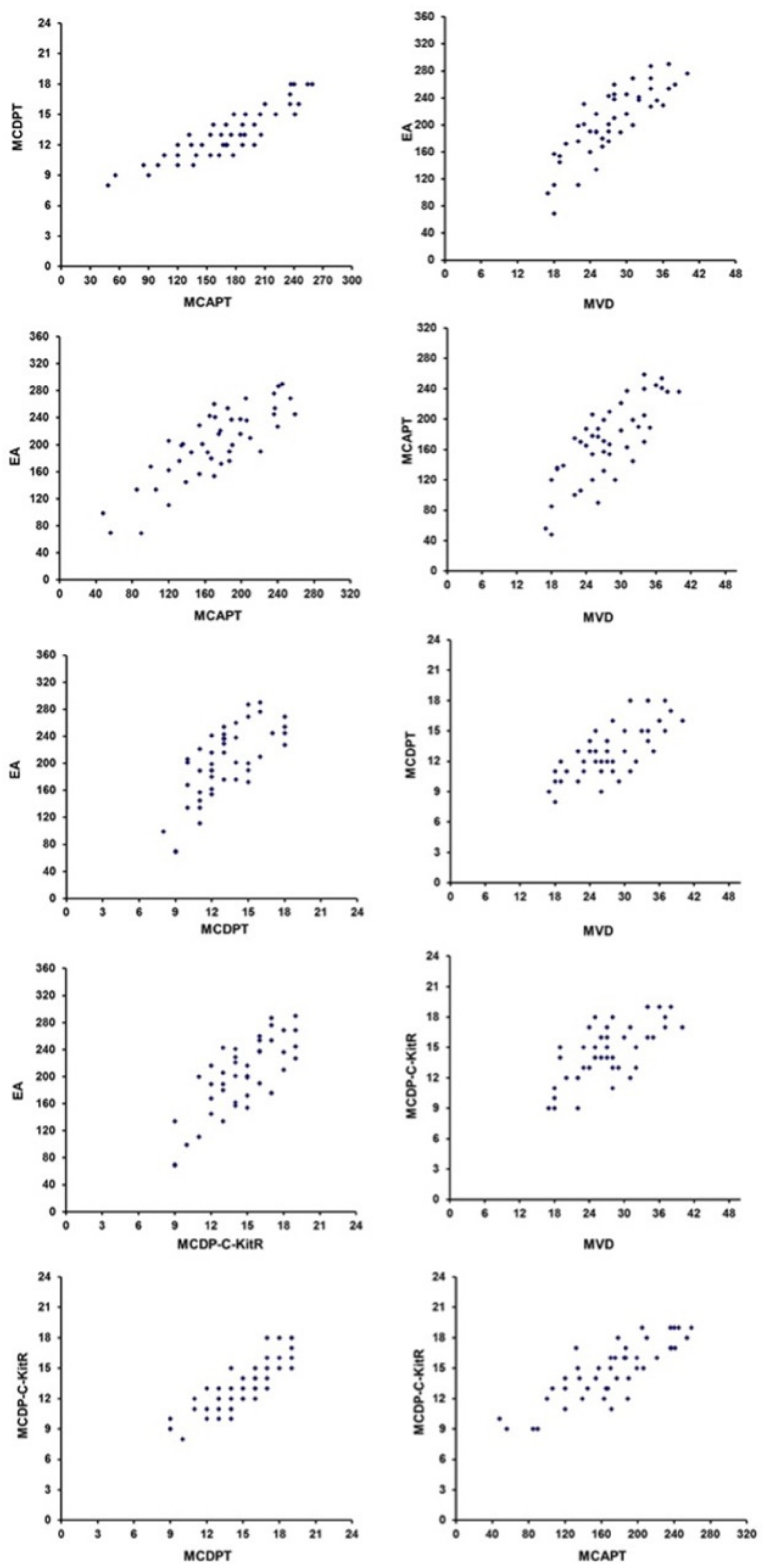
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. Immunohistochemistry
5.3. Morphometrical Assay
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Bronstein, Y.L.; Loyer, E.M.; Kaur, H.; Choi, H.; David, C.; Dubrow, R.A.; Broemeling, L.D.; Cleary, K.R.; Charnsangavej, C. Detection of Small Pancreatic Tumors with Multiphasic Helical CT. Am. J. Roentgenol. 2004, 182, 619–623. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Luposella, M.; Patruno, R.; Gadaleta, C.D.; De Sarro, G.; Ranieri, G. Mast Cell-Targeted Strategies in Cancer Therapy. Transfus. Med. Hemotherapy 2016, 43, 109–113. [Google Scholar] [CrossRef]
- Kim, W.-J.; Cha, H.-S.; Lee, M.-H.; Kim, S.-Y.; Kim, S.H.; Kim, T.-J. Effects ofCymbidiumRoot Ethanol Extract on Atopic Dermatitis. Evid. -Based Complement. Altern. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patruno, R.; Marech, I.; Zizzo, N.; Ammendola, M.; Nardulli, P.; Gadaleta, C.; Introna, M.; Capriuolo, G.; Rubini, R.A.; Ribatti, D.; et al. C-Kit Expression, Angiogenesis, and Grading in Canine Mast Cell Tumour: A Unique Model to Study C-Kit Driven Human Malignancies. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- González-De-Olano, D.; Álvarez-Twose, I. Mast Cells as Key Players in Allergy and Inflammation. J. Investig. Allergol. Clin. Immunol. 2018, 28, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Wasiuk, A.; De Vries, V.C.; Hartmann, K.; Roers, A.; Noelle, R.J. Mast cells as regulators of adaptive immunity to tumours. Clin. Exp. Immunol. 2009, 155, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Patruno, R.; Arpaia, N.; Gadaleta, C.D.; Passantino, L.; Zizzo, N.; Misino, A.; Lucarelli, N.M.; Catino, A.; Valerio, P.; Ribatti, D.; et al. VEGF concentration from plasma-activated platelets rich correlates with microvascular density and grading in canine mast cell tumour spontaneous model. J. Cell. Mol. Med. 2009, 13, 555–561. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Varricchi, G.; Loffredo, S.; Granata, F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2016, 778, 146–151. [Google Scholar] [CrossRef]
- Ferrara, A.L.; Piscitelli, F.; Petraroli, A.; Parente, R.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Triggiani, M.; Di Marzo, V.; Loffredo, S. Altered Metabolism of Phospholipases, Diacylglycerols, Endocannabinoids, and N-Acylethanolamines in Patients with Mastocytosis. J. Immunol. Res. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Schwartz, L.B. Active monomers of human β-tryptase have expanded substrate specificities. Int. Immunopharmacol. 2007, 7, 1900–1908. [Google Scholar] [CrossRef]
- Blair, R.J.; Meng, H.; Marchese, M.J.; Ren, S.; Schwartz, L.B.; Tonnesen, M.G.; Gruber, B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Investig. 1997, 99, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, M.; Ranieri, G.; Nico, B.; Benagiano, V.; Crivellato, E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int. J. Dev. Biol. 2011, 55, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sendo, T.; Oishi, R. Physiology and Pathophysiology of Proteinase-Activated Receptors (PARs): Role of Tryptase/PAR-2 in Vascular Endothelial Barrier Function. J. Pharmacol. Sci. 2005, 97, 14–19. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.-W.; Ni, P.-F.; Chen, Y.-N.; Dong, H.-Q.; Qian, Y.-N. Effect of tryptase on mouse brain microvascular endothelial cells via protease-activated receptor 2. J. Neuroinflammation 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Ranieri, G.; Sacco, R.; Sammarco, G.; Piardi, T.; Zuccalà, V.; Patruno, R.; Zullo, A.; Zizzo, N.; Nardo, B.; Marech, I.; et al. Mast cells positive to tryptase, endothelial cells positive to protease-activated receptor-2, and microvascular density correlate among themselves in hepatocellular carcinoma patients who have undergone surgery. OncoTargets Ther. 2016, 9, 4465–4471. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Vescio, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Marech, I.; Ruggieri, R.; et al. Tryptase mast cell density, protease-activated receptor-2 microvascular density, and classical microvascular density evaluation in gastric cancer patients undergoing surgery: Possible translational relevance. Ther. Adv. Gastroenterol. 2017, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Marech, I.; Montemurro, S.; Zizzo, N.; et al. Mast Cells Density Positive to Tryptase Correlates with Angiogenesis in Pancreatic Ductal Adenocarcinoma Patients Having Undergone Surgery. Gastroenterol. Res. Pr. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Giordano, M.; Ruggieri, E.; Zizzo, N.; et al. Microvascular density and endothelial area correlate with Ki-67 proliferative index in surgically-treated pancreatic ductal adenocarcinoma patients. Oncol. Lett. 2015, 10, 967–971. [Google Scholar] [CrossRef]
- Ammendola, M.; Gadaleta, C.D.; Frampton, A.E.; Piardi, T.; Memeo, R.; Zuccalà, V.; Luposella, M.; Patruno, R.; Zizzo, N.; Gadaleta, P.; et al. The density of mast cells c-Kit+ and tryptase+ correlates with each other and with angiogenesis in pancreatic cancer patients. Oncotarget 2017, 8, 70463–70471. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Tamma, R.; Brunetti, O.; Pisconti, S.; Argentiero, A.; Silvestris, N.; Ribatti, D. Mast cells and angiogenesis in pancreatic ductal adenocarcinoma. Clin. Exp. Med. 2018, 18, 319–323. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, B.; Tao, M.; Liu, J.; Li, W. The Role of Stromal Components in Pancreatic Cancer Progression. Anti-Cancer Agents Med. Chem. 2016, 16, 1117–1124. [Google Scholar] [CrossRef]
- Theoharides, T.C. Mast Cells and Pancreatic Cancer. N. Engl. J. Med. 2008, 358, 1860–1861. [Google Scholar] [CrossRef]
- Soucek, L.; Lawlor, E.R.; Soto, D.; Shchors, K.; Swigart, L.B.I.; Evan, G. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat. Med. 2007, 13, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Karamitopoulou, E.; Shoni, M.; Theoharides, T. Increased Number of Non-Degranulated Mast Cells in Pancreatic Ductal Adenocarcinoma but Not in Acute Pancreatitis. Int. J. Immunopathol. Pharmacol. 2014, 27, 213–220. [Google Scholar] [CrossRef]
- Esposito, I.; Menicagli, M.; Funel, N.; Bergmann, F.; Boggi, U.; Mosca, F.; Bevilacqua, G.; Campani, D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J. Clin. Pathol. 2004, 57, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Strouch, M.J.; Cheon, E.C.; Salabat, M.R.; Krantz, S.B.; Gounaris, E.; Melstrom, L.G.; Dangi-Garimella, S.; Wang, E.; Munshi, H.G.; Khazaie, K.; et al. Crosstalk between Mast Cells and Pancreatic Cancer Cells Contributes to Pancreatic Tumor Progression. Clin. Cancer Res. 2010, 16, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Z.; Ma, Y.; Ji, B.; Wang, H.; Deng, D.; Liu, Y.; Abbruzzese, J.L.; Liu, Y.-J.; Logsdon, C.D.; Hwu, P. Mast Cells in Tumor Microenvironment Promotes the In Vivo Growth of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2011, 17, 7015–7023. [Google Scholar] [CrossRef]
- Zhan, H.-X.; Zhou, B.; Cheng, Y.-G.; Xu, J.-W.; Wang, L.; Zhang, G.-Y.; Hu, S.-Y. Crosstalk between stromal cells and cancer cells in pancreatic cancer: New insights into stromal biology. Cancer Lett. 2017, 392, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhai, L.; Xue, R.; Shi, J.; Zeng, Q.; Gao, C. Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1. Int. J. Mol. Sci. 2016, 17, 834. [Google Scholar] [CrossRef] [PubMed]
- Haqq, J.; Howells, L.M.; Garcea, G.; Metcalfe, M.S.; Steward, W.P.; Dennison, A.R. Pancreatic stellate cells and pancreas cancer: Current perspectives and future strategies. Eur. J. Cancer 2014, 50, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Passantino, L.; Passantino, G.; Cianciotta, A.; Ribaud, M.R.; Presti, G.L.; Ranieri, G.; Perillo, A. Expression of Proto-Oncogene C-Kit and Correlation with Morphological Evaluations in Canine Cutaneous Mast Cell Tumors. Immunopharmacol. Immunotoxicol. 2008, 30, 609–621. [Google Scholar] [CrossRef]
- Ranieri, G.; Passantino, L.; Patruno, R.; Passantino, G.; Jirillo, F.; Catino, A.; Mattioli, V.; Gadaleta, C.; Ribatti, M. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol. Rep. 2003, 10, 1189–1193. [Google Scholar] [CrossRef]
- Patruno, R.; Passantino, G.; Laface, C.; Tinelli, A.; Zito, A.; Ruggieri, R.; Luposella, F.; Gadaleta, P.; Laforgia, M.; Lacitignola, L.; et al. Microvascular density, endothelial area, and Ki-67 proliferative index correlate each other in car post-injection fibrosarcoma. Cells 2021, 10, 31. [Google Scholar] [CrossRef]
- Goffredo, V.; Gadaleta, C.D.; Laterza, A.; Vacca, A.; Ranieri, G. Tryptase serum levels in patients suffering from hepatocellular carcinoma undergoing intra-arterial chemoembolization: Possible predictive role of response to treatment. Mol. Clin. Oncol. 2013, 1, 385–389. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Montemurro, S.; Ruggieri, E.; Patruno, R.; Marech, I.; Cariello, M.; Vacca, A.; et al. Correlation between Serum Tryptase, Mast Cells Positive to Tryptase and Microvascular Density in Colo-Rectal Cancer Patients: Possible Biological-Clinical Significance. PLoS ONE 2014, 9, e99512. [Google Scholar] [CrossRef]
- Marech, I.; Ammendola, M.; Sacco, R.; Capriuolo, G.S.; Patruno, R.; Rubini, R.; Luposella, M.; Zuccalà, V.; Savino, E.; Gadaleta, C.D.; et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Donato, G.; Zuccalà, V.; Russo, E.; Luposella, M.; Vescio, G.; Rizzuto, A.; Patruno, R.; De Sarro, G.; et al. Mast Cell Positivity to Tryptase Correlates with Metastatic Lymph Nodes in Gastrointestinal Cancer Patients Treated Surgically. Oncology 2013, 85, 111–116. [Google Scholar] [CrossRef]
- Ranieri, G.; Ammendola, M.; Patruno, R.; Celano, G.; Zito, F.A.; Montemurro, S.; Rella, A.; Di Lecce, V.; Gadaleta, C.D.; De Sarro, G.B.; et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int. J. Oncol. 2009, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating Mast Cells Correlate with Angiogenesis in Bone Metastases from Gastric Cancer Patients. Int. J. Mol. Sci. 2015, 16, 3237–3250. [Google Scholar] [CrossRef]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis from Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.M.; Tamma, R.; Ruggieri, S.; Nico, B.; Toma, A.; Ribatti, D. Mast cells in breast cancer angiogenesis. Crit. Rev. Oncol. 2017, 115, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Vahidian, F.; Alihasanzadeh, M.; Mokhtarzadeh, A.; Nezhad, P.L.; Baradaran, B. Mast cells: A double-edged sword in cancer. Immunol. Lett. 2019, 209, 28–35. [Google Scholar] [CrossRef]
- Gudiseva, S.; Santosh, A.B.R.; Chitturi, R.; Anumula, V.; Poosarla, C.; Baddam, V.R.R. The role of mast cells in oral squamous cell carcinoma. Współczesna Onkol. 2017, 21, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Labriola, A.; Achille, G.; Florio, G.; Zito, A.F.; Grammatica, L.; Paradiso, A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int. J. Oncol 2002, 21, 1317–1323. [Google Scholar]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2019, 58, 313–325. [Google Scholar] [CrossRef]
- Leporini, C.; Ammendola, M.; Marech, I.; Sammarco, G.; Sacco, R.; Gadaleta, C.D.; Oakley, C.; Russo, E.; De Sarro, G.; Ranieri, G. Targeting mast cells in gastric cancer with special reference to bone metastases. World J. Gastroenterol. 2015, 21, 10493–10501. [Google Scholar] [CrossRef] [PubMed]
- Popovici, R.A.; Raica, M.; Ribatti, D. Mast cells in lymphomas. BioMed Res. Int. 2016, 101, 207–212. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Vacca, A. Mast Cells and Angiogenesis in Human Plasma Cell Malignancies. Int. J. Mol. Sci. 2019, 20, 481. [Google Scholar] [CrossRef]
- Gaje, P.N.; Ceausu, R.A.; Jitariu, A.; Stratul, S.I.; Rusu, L.-C.; Popovici, R.A.; Raica, M. Mast Cells: Key Players in the Shadow in Oral Inflammation and in Squamous Cell Carcinoma of the Oral Cavity. BioMed Res. Int. 2016, 9235080. [Google Scholar] [CrossRef] [PubMed]
- Visciano, C.; Prevete, N.; Liotti, F.; Marone, G. Tumor-Associated Mast Cells in Thyroid Cancer. Int. J. Endocrinol. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Laface, C.; Ammendola, M.; Zuccalà, V.; Luposella, F.; Zizzo, N.; Patruno, R.; Porcelli, M.; Gadaleta, C.D.; Tucci, M.; Ranieri, G. Mast cells positive to c-kit receptor and to tryptase in normal to cancer pancreatic tissue and the correlation with angiogenesis. J. Clin. Oncol. 2020, 38, e16502. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccalà, V.; Romano, R.; Luposella, M.; Patruno, R.; Vallicelli, C.; Verdecchia, G.M.; et al. Mast Cells Positive to Tryptase and c-Kit Receptor Expressing Cells Correlates with Angiogenesis in Gastric Cancer Patients Surgically Treated. Gastroenterol. Res. Pr. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Ntellas, P.; Dadouli, K.; Perivoliotis, K.; Sogka, E.; Pentheroudakis, G.; Ioannou, M.; Hadjichristodoulou, C.; Tepetes, K.; Mauri, D. Microvessel Density and Impact of Angiogenesis on Survival of Resected Pancreatic Cancer Patients. Pancreas 2019, 48, 233–241. [Google Scholar] [CrossRef]
- Rao, Q.; Chen, Y.; Yeh, C.-R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2015, 7, 7842–7855. [Google Scholar] [CrossRef]
- Zhong, B.; Li, Y.; Liu, X.; Wang, D. Association of mast cell infiltration with gastric cancer progression. Oncol. Lett. 2017, 15, 755–764. [Google Scholar] [CrossRef]
- Khan, M.W.; Keshavarzian, A.; Gounaris, E.; Melson, J.E.; Cheon, E.C.; Blatner, N.R.; Chen, Z.E.; Tsai, F.-N.; Lee, G.; Ryu, H.; et al. PI3K/AKT Signaling Is Essential for Communication between Tissue-Infiltrating Mast Cells, Macrophages, and Epithelial Cells in Colitis-Induced Cancer. Clin. Cancer Res. 2013, 19, 2342–2354. [Google Scholar] [CrossRef]
- Huang, B.; Lei, Z.; Zhang, G.-M.; Li, D.; Song, C.; Li, B.; Liu, Y.; Yuan, Y.; Unkeless, J.; Xiong, H.; et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood 2008, 112, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Qian, J.; Zeng, F.; Li, S.; Guo, W.; Chen, L.; Li, G.; Zhang, Z.; Wang, Q.J.; Deng, F. Protein kinase Ds promote tumor angiogenesis through mast cell recruitment and expression of angiogenic factors in prostate cancer microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, L.; Xia, Y.; Qi, Y.; Chen, Y.; Chen, L.; Zhang, P.; Kong, Y.; Qu, Y.; Wang, Z.; et al. Tumor infiltrating mast cells determine oncogenic HIF-2α-conferred immune evasion in clear cell renal cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 731–741. [Google Scholar] [CrossRef]
- Chataigner, L.M.P.; Leloup, N.; Janssen, B.J.C. Structural Perspectives on Extracellular Recognition and Conformational Changes of Several Type-I Transmembrane Receptors. Front. Mol. Biosci. 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Leporini, C.; Patruno, R.; Luposella, M.; Zizzo, N.; Passantino, G.; Sacco, R.; Farooqi, A.A.; Zuccalà, V.; et al. C-Kit receptor and tryptase expressing mast cells correlate with angiogenesis in breast cancer patients. Oncotarget 2017, 9, 7918–7927. [Google Scholar] [CrossRef]
- Marech, I.; Gadaleta, C.D.; Ranieri, G. Possible Prognostic and Therapeutic Significance of c-Kit Expression, Mast Cell Count and Microvessel Density in Renal Cell Carcinoma. Int. J. Mol. Sci. 2014, 15, 13060–13076. [Google Scholar] [CrossRef] [PubMed]
- Taracanova, A.; Tsilioni, I.; Conti, P.; Norwitz, E.R.; Leeman, S.E.; Theoharides, T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. 2018, 115, E9381–E9390. [Google Scholar] [CrossRef]
- McHale, C.; Mohammed, Z.; Deppen, J.; Gomez, G. Interleukin-6 potentiates FcεRI-induced PGD 2 biosynthesis and induces VEGF from human in situ -matured skin mast cells. Biochim. et Biophys. Acta (BBA) -Gen. Subj. 2018, 1862, 1069–1078. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Lee, J.-W.; Ryu, H.-C.; Wei, J.-D.; Seong, C.-M.; Kim, J.-H. Proinflammatory Cytokine IL-1β Stimulates IL-8 Synthesis in Mast Cells via a Leukotriene B4 Receptor 2-Linked Pathway, Contributing to Angiogenesis. J. Immunol. 2010, 184, 3946–3954. [Google Scholar] [CrossRef]
- Chumanevich, A.; Wedman, P.; Oskeritzian, C.A. Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis Can Promote Mouse and Human Primary Mast Cell Angiogenic Potential through Upregulation of Vascular Endothelial Growth Factor-A and Matrix Metalloproteinase-2. Mediat. Inflamm. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nat. Cell Biol. 1990, 346, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Indoh, I.; Jackson, N.; Wakefield, D.; McNeil, H.P.; Yan, W.; Geczy, C.; Arm, J.P.; Tedla, N. Human Mast Cell-Derived Gelatinase B (Matrix Metalloproteinase-9) Is Regulated by Inflammatory Cytokines: Role in Cell Migration. J. Immunol. 2006, 177, 2638–2650. [Google Scholar] [CrossRef]
- Fang, K.C.; Wolters, P.J.; Steinhoff, M.; Bidgol, A.; Blount, J.L.; Caughey, G.H. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J. Immunol. 1999, 162, 5528–5535. [Google Scholar]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef]
- McNeil, H.P.; Adachi, R.; Stevens, R.L. Mast Cell-restricted Tryptases: Structure and Function in Inflammation and Pathogen Defense. J. Biol. Chem. 2007, 282, 20785–20789. [Google Scholar] [CrossRef]
- Maun, H.R.; Liu, P.S.; Franke, Y.; Eigenbrot, C.; Forrest, W.F.; Schwartz, L.B.; Lazarus, R.A. Dual functionality of β-tryptase protomers as both proteases and cofactors in the active tetramer. J. Biol. Chem. 2018, 293, 9614–9628. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.; Portell, C.; Kell, P.J.; Vinson, S.M.; McHowat, J. Protease-activated receptor stimulation activates a Ca2+-independent phospholipase A2 in bladder microvascular endothelial cells. Am. J. Physiol. Physiol. 2005, 288, F714–F721. [Google Scholar] [CrossRef]
- Matej, R.; Mandáková, P.; Netíková, I.; Poucková, P.; Olejár, T. Proteinase-activated receptor-2 expression in breast cancer and the role of trypsin on growth and metabolism of breast cancer cell line MDA MB-231. Physiol. Res. 2007, 56, 475–484. [Google Scholar]
- Liu, Y.; Mueller, B.M. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem. Biophys. Res. Commun. 2006, 344, 1263–1270. [Google Scholar] [CrossRef]
- Ranieri, G.; Laface, C.; Laforgia, M.; De Summa, S.; Porcelli, M.; Macina, F.; Ammendola, M.; Molinari, P.; Lauletta, G.; Di Palo, A.; et al. Bevacizumab Plus FOLFOX-4 Combined with Deep Electro-Hyperthermia as First-line Therapy in Metastatic Colon Cancer: A Pilot Study. Front. Oncol. 2020, 10, 590707. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.E.; Ullrich, S. Intratumoral mast cells promote the growth of pancreatic cancer. OncoImmunology 2013, 2, e25964. [Google Scholar] [CrossRef]
- Ma, Y.; Hwang, R.F.; Logsdon, C.D.; Ullrich, S.E. Dynamic Mast Cell–Stromal Cell Interactions Promote Growth of Pancreatic Cancer. Cancer Res. 2013, 73, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Gorzalczany, Y.; Akiva, E.; Klein, O.; Merimsky, O.; Sagi-Eisenberg, R. Mast cells are directly activated by contact with cancer cells by a mechanism involving autocrine formation of adenosine and autocrine/paracrine signaling of the adenosine A3 receptor. Cancer Lett. 2017, 397, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G.; Basile, A.; Azzariti, A.; Paradiso, A.; Vacca, A. Tumor endothelial markers as a target in cancer. Expert Opin. Ther. Targets 2012, 16, 1215–1225. [Google Scholar] [CrossRef]
- Mori, S.; Itoh, Y.; Shinohata, R.; Sendo, T.; Oishi, R.; Nishibori, M. Nafamostat Mesilate Is an Extremely Potent Inhibitor of Human Tryptase. J. Pharmacol. Sci. 2003, 92, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; De Sarro, G.; Russo, E.; Ranieri, G. Targeting Mast Cells Tryptase in Tumor Microenvironment: A Potential Antiangiogenetic Strategy. BioMed Res. Int. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Ni, W.-W.; Cao, M.-D.; Huang, W.; Meng, L.; Wei, J.-F. Tryptase inhibitors: A patent review. Expert Opin. Ther. Patents 2017, 27, 919–928. [Google Scholar] [CrossRef]
- Liang, G.; Aldous, S.; Merriman, G.; Levell, J.; Pribish, J.; Cairns, J.; Chen, X.; Maignan, S.; Mathieu, M.; Tsay, J.; et al. Structure-based library design and the discovery of a potent and selective mast cell β-tryptase inhibitor as an oral therapeutic agent. Bioorganic Med. Chem. Lett. 2012, 22, 1049–1054. [Google Scholar] [CrossRef]
- Humbert, M.; Casteran, N.; Letard, S.; Hanssens, K.; Iovanna, J.; Finetti, P.; Bertucci, F.; Bader, T.; Mansfield, C.D.; Moussy, A.; et al. Masitinib Combined with Standard Gemcitabine Chemotherapy: In Vitro and In Vivo Studies in Human Pancreatic Tumour Cell Lines and Ectopic Mouse Model. PLoS ONE 2010, 5, e9430. [Google Scholar] [CrossRef]
- Marech, I.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Introna, M.; Zito, A.F.; Gadaleta, C.D.; Ranieri, G. Masitinib (AB1010), from canine tumor model to human clinical development: Where we are? Crit. Rev. Oncol. 2014, 91, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, M.; Calabrò, C.; Scattone, A.; Laface, C.; Porcelli, M.; Gadaleta, C.D.; Nardulli, P.; Ranieri, G. Pharmacotherapy in Mast Cell Leukemia. Expert Opin. Pharmacother. 2020, 21, 1059–1069. [Google Scholar] [CrossRef]
- Laforgia, M.; Marech, I.; Nardulli, P.; Calabrò, C.; Gadaleta, C.D.; Ranieri, G. An evaluation of masitinib for treating systemic mastocytosis. Expert Opin. Pharmacother. 2019, 20, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Purvey, S.; Saif, M.W. Masitinib in treatment of pancreatic cancer. Expert Opin. Pharmacother. 2018, 19, 759–764. [Google Scholar] [CrossRef]
- Marech, I.; Ammendola, M.; Sacco, R.; Sammarco, G.; Zuccalà, V.; Zizzo, N.; Leporini, C.; Luposella, M.; Patruno, R.; Filippelli, G.; et al. Tumour-associated macrophages correlate with microvascular bed extension in colorectal cancer patients. J. Cell. Mol. Med. 2016, 20, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
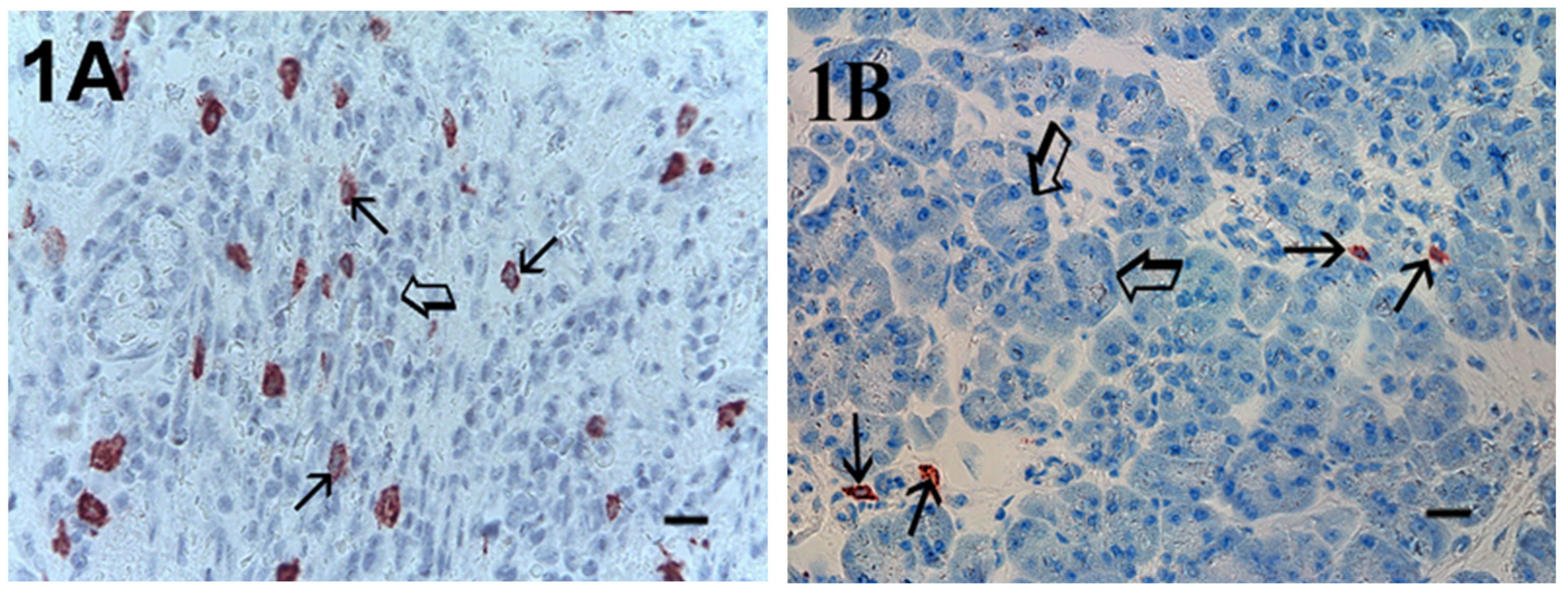
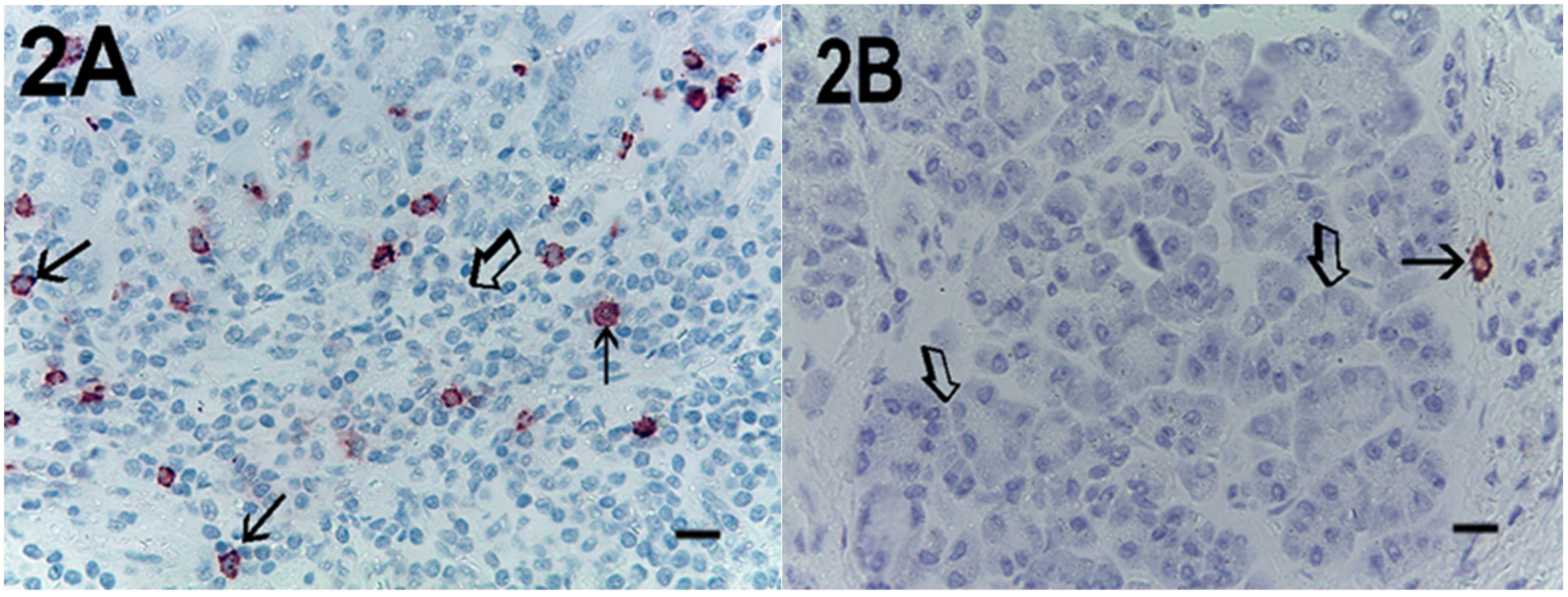
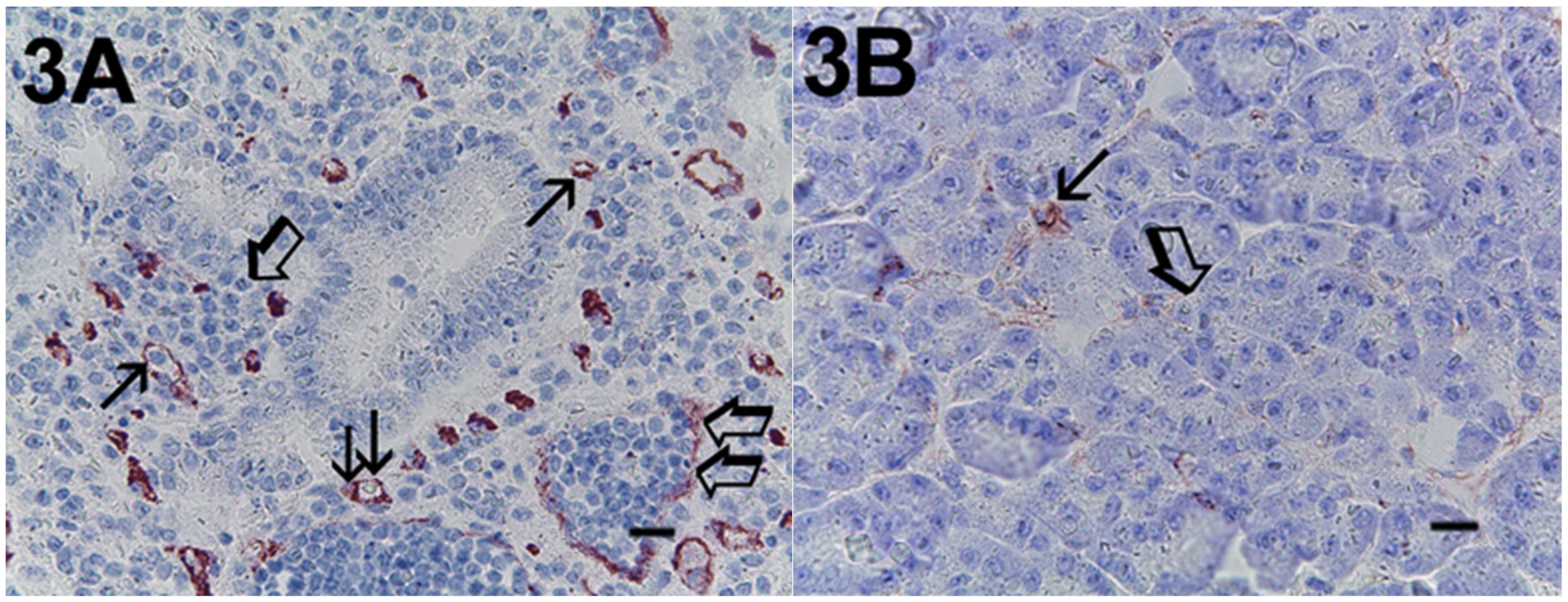
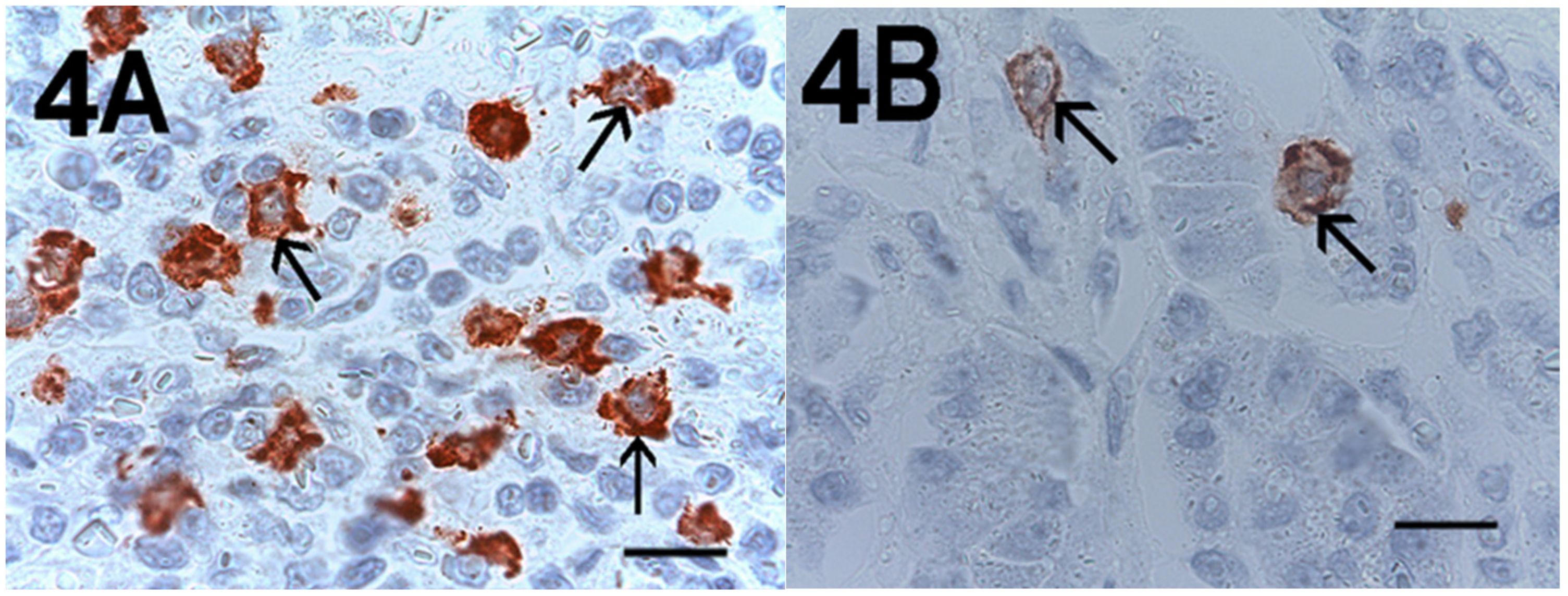
| Subgroup | No. of Patients |
|---|---|
| Age | |
| < 65 | 30 (67%) |
| > 65 | 15 (33%) |
| Gender: | |
| Female | 23 (51%) |
| Male | 22 (49%) |
| Tumour site: | |
| Head | 19 (42%) |
| Body-Tail | 26 (58%) |
| TNM by AJCC Stage | |
| T2N0–1M0 | 20 (44%) |
| T3N0–1M0 | 25 (56%) |
| Histologic type | |
| Ductal adenocarcinomas | 45 (100%) |
| Histologic grade | |
| G1–G2 | 34 (77%) |
| G3 | 11 (23%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammendola, M.; Currò, G.; Laface, C.; Zuccalà, V.; Memeo, R.; Luposella, F.; Laforgia, M.; Zizzo, N.; Zito, A.; Loisi, D.; et al. Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue. Cells 2021, 10, 444. https://doi.org/10.3390/cells10020444
Ammendola M, Currò G, Laface C, Zuccalà V, Memeo R, Luposella F, Laforgia M, Zizzo N, Zito A, Loisi D, et al. Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue. Cells. 2021; 10(2):444. https://doi.org/10.3390/cells10020444
Chicago/Turabian StyleAmmendola, Michele, Giuseppe Currò, Carmelo Laface, Valeria Zuccalà, Riccardo Memeo, Francesco Luposella, Mariarita Laforgia, Nicola Zizzo, Alfredo Zito, Donato Loisi, and et al. 2021. "Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue" Cells 10, no. 2: 444. https://doi.org/10.3390/cells10020444
APA StyleAmmendola, M., Currò, G., Laface, C., Zuccalà, V., Memeo, R., Luposella, F., Laforgia, M., Zizzo, N., Zito, A., Loisi, D., Patruno, R., Milella, L., Ugenti, I., Porcelli, M., Navarra, G., Gadaleta, C. D., & Ranieri, G. (2021). Mast Cells Positive for c-Kit Receptor and Tryptase Correlate with Angiogenesis in Cancerous and Adjacent Normal Pancreatic Tissue. Cells, 10(2), 444. https://doi.org/10.3390/cells10020444








