Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options
Abstract
1. Introduction
1.1. Secondary Malignant Melanoma of the GI Tract
1.2. Primary Malignant Melanoma of the GI Tract
1.3. Symptoms
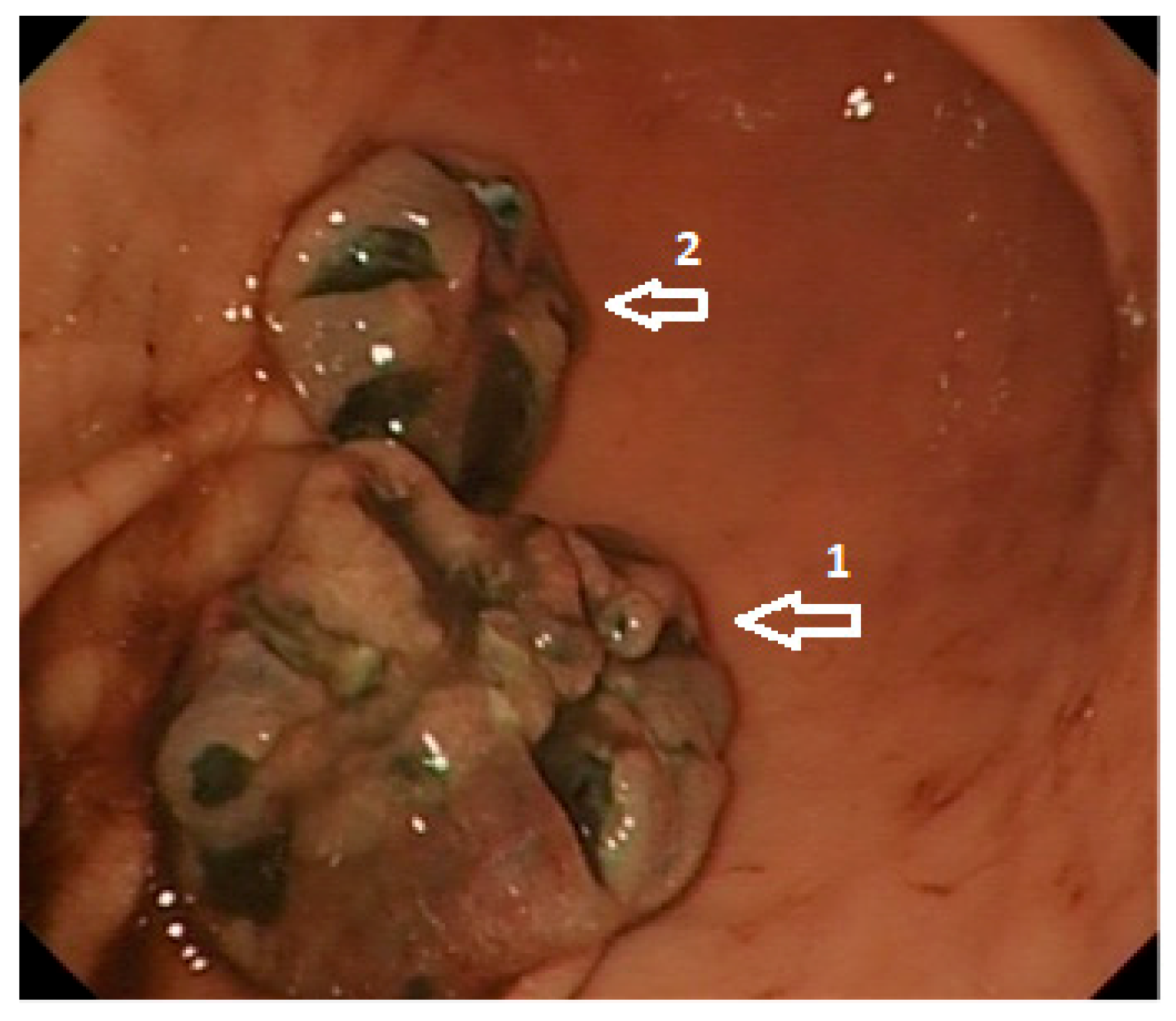
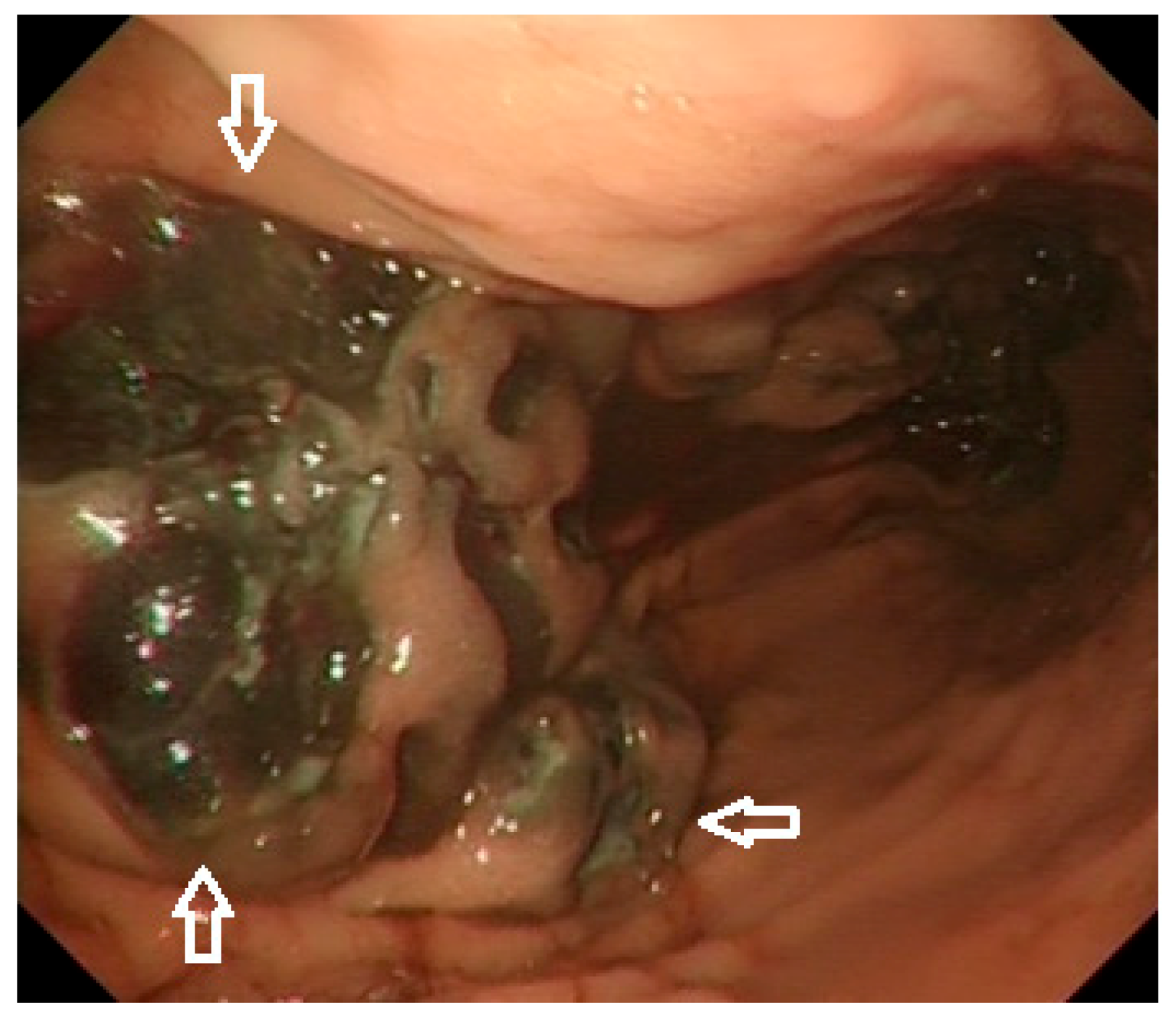
1.4. Diagnosis
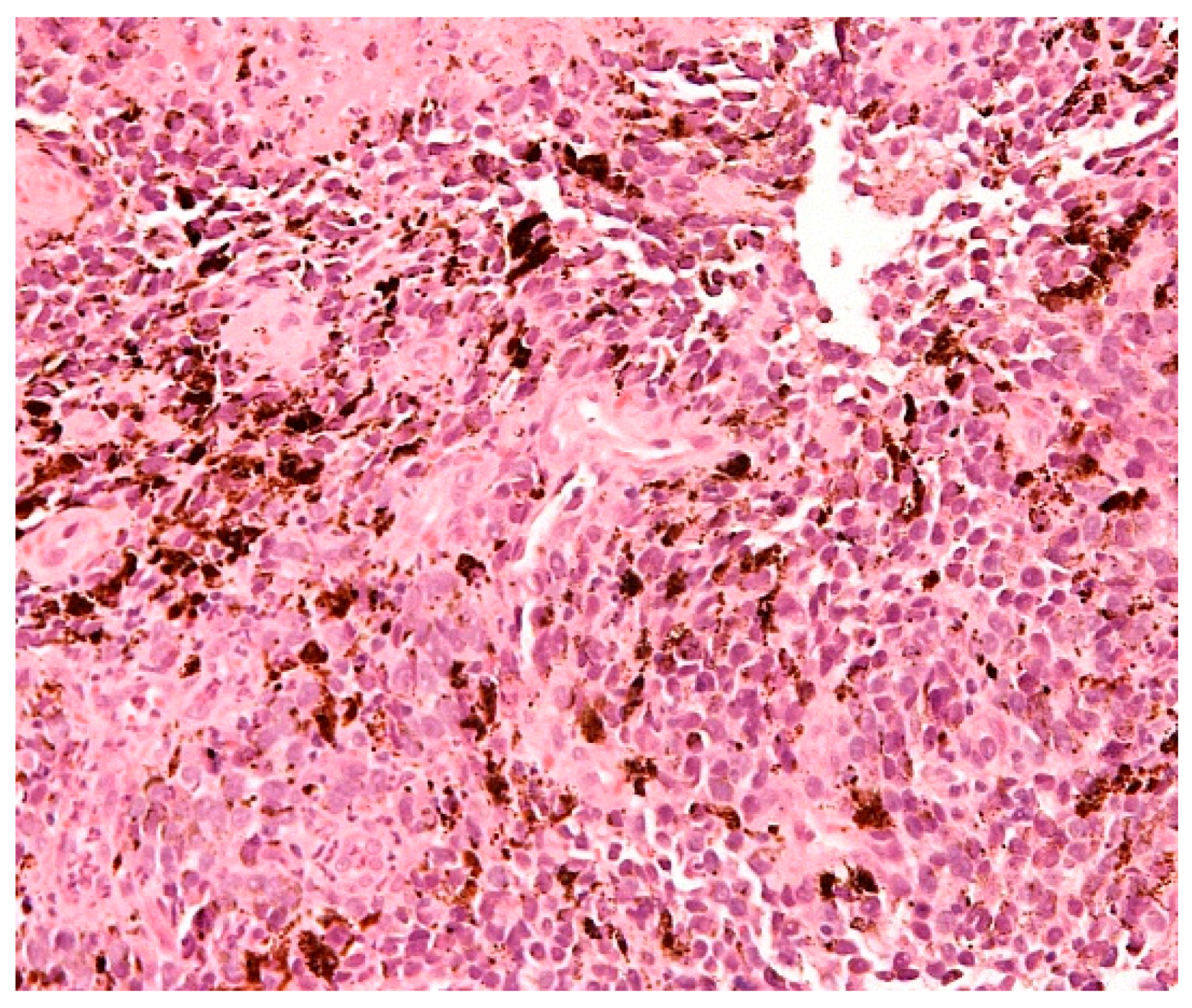
1.5. Molecular Characteristics
1.6. Treatment
1.6.1. Endoscopy
1.6.2. Surgery
1.6.3. Chemotherapy
1.6.4. Systemic Treatment in Advance Disease
1.6.5. Systemic Treatment in the Adjuvant Setting
1.6.6. Combination of Surgery and Systemic Treatment in Stage IV Melanoma
1.6.7. Side Effects of Systemic Therapies
2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ait Idir, B.; Riany, A.; Jahid, A.; Chad, B. Primary melanoma of the small bowel revealed by gastrointestinal bleeding: A case report. J. Med. Case Rep. 2016, 10, 335. [Google Scholar] [CrossRef][Green Version]
- Cancer Research UK Melanoma Skin Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer (accessed on 19 December 2020).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Langner, C. Secondary tumors of the gastrointestinal tract. Patholege 2012, 33, 45–52. (In German) [Google Scholar] [CrossRef]
- Asad-Ur-Rahman, F.N.U.; Abbass, A.; Majeed, U.; Navaneethan, U. Melanoma Metastasizing to the Small Intestine: A Case Report Illustrating Symptomatic and Asymptomatic Involvement. Cureus 2016, 8, e608. [Google Scholar] [CrossRef]
- López, R.; López, R.G.; Santomé, P.M.; Porto, E.I.; Moreiras, M.I.; Gómez, C.G.; Villanueva, J.M.; Canosa, M.A.; Veiga, O.R.; Gutiérrez, A.E.; et al. Intestinal perforation due to cutaneous malignant melanoma metastatic implants. Rev. Esp. Enferm. Dig. 2011, 103, 386–388. [Google Scholar] [CrossRef]
- Blecker, D.; Abraham, S.; Furth, E.E.; Kochman, M.L. Melanoma in the Gastrointestinal Tract. Am. J. Gastroenterol. 1999, 94, 3427–3433. [Google Scholar] [CrossRef]
- Hadjinicolaou, A.V.; Hadjittofi, C.; Athanasopoulos, P.G.; Shah, R.; Ala, A.A. Primary small bowel melanomas: Fact or myth? Ann. Transl. Med. 2016, 4, 113. [Google Scholar] [CrossRef]
- Silva, S.; Tenreiro, N.; Melo, A.; Lage, J.; Moreira, H.; Próspero, F.; Avelar, P. Metastatic melanoma: An unusual cause of gastrointestinal bleeding and intussusception-A case report. Int. J. Surg. Case Rep. 2018, 53, 144–146. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Amar, A.; Jougon, J.; Edouard, A.; Laban, P.; Marry, J.P.; Hillion, G. Primary malignant melanoma of the small intestine. Gastroenterol. Clin. Biol. 1992, 16, 365–367. (In French) [Google Scholar]
- Krausz, M.M.; Ariel, I.; Behar, A.J. Primary malignant melanoma of the small intestine and the APUD cell concept. J. Surg. Oncol. 1978, 10, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y. Melanocytic and nevocytic malignant melanomas.Cellular and subcellular differentiation. Cancer 1967, 20, 632–649. [Google Scholar] [CrossRef]
- Schuchter, L.M.; Green, R.; Fraker, D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr. Opin. Oncol. 2000, 12, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Gilg, M.M.; Gröchenig, H.-P.; Schlemmer, A.; Eherer, A.; Högenauer, C.; Langner, C. Secondary tumors of the GI tract: Origin, histology, and endoscopic findings. Gastrointest. Endosc. 2018, 88, 151–158. [Google Scholar] [CrossRef]
- Kouladouros, K.; Gärtner, D.; Münch, S.; Paul, M.; Schön, M.R. Recurrent intussusception as initial manifestation of primary intestinal melanoma: Case report and literature review. World J. Gastroenterol. 2015, 21, 3114–3120. [Google Scholar] [CrossRef]
- Raymond, A.R.; Rorat, E.; Goldstein, D.; Lubat, E.; Strutynsky, N.; Gelb, A. An unusual case of malignant melanoma of the small intestine. Am. J. Gastroenterol. 1984, 79, 689–692. [Google Scholar]
- Lens, M.; Bataille, V.; Krivokapic, Z. Melanoma of the small intestine. Lancet Oncol. 2009, 10, 516–521. [Google Scholar] [CrossRef]
- Tarantino, L.; Nocera, V.; Perrotta, M.; Balsamo, G.; Schiano, A.; Orabona, P.; Sordelli, I.F.; Ripa, C.; Parmeggiani, D.; Sperlongano, P. Primary small-bowel melanoma: Color Doppler ultrasonographic, computed tomographic, and radiologic findings with pathologic correlations. J. Ultrasound Med. 2007, 26, 121–127. [Google Scholar] [CrossRef]
- Atiq, O.; Khan, A.S.; Abrams, G.A. Metastatic Amelanotic Melanoma of the Jejunum Diagnosed on Capsule Endoscopy. Gastroenterol. Hepatol. 2012, 8, 691–693. [Google Scholar]
- Tatlidil, R.; Mandelkern, M. FDG-PET in the detection of gastrointestinal metastases in melanoma. Melanoma Res. 2001, 11, 297–301. [Google Scholar] [CrossRef]
- Othman, A.E.; Eigentler, T.K.; Bier, G.; Pfannenberg, C.; Bösmüller, H.; Thiel, C.; Garbe, C.; Nikolaou, K.; Klumpp, B. Imaging of gastrointestinal melanoma metastases: Correlation with surgery and histopathology of resected specimen. Eur. Radiol. 2017, 27, 2538–2545. [Google Scholar] [CrossRef]
- Oda, I.; Kondo, H.; Yamao, T.; Saito, D.; Ono, H.; Gotoda, T.; Yamaguchi, H.; Yoshida, S.; Shimoda, T. Metastatic tumors to the stomach: Analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy 2001, 33, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-C.; Su, W.-C.; Chang, M.-C.; Chang, Y.-T.; Wang, C.-Y.; Wong, J.-M. Incidence, endoscopic morphology and distribution of metastatic lesions in the gastrointestinal tract. J. Gastroenterol. Hepatol. 2006, 22, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Argyrides, J. Gastrointestinal Bleeding from Metastatic Melanoma. N. Engl. J. Med. 2020, 382, e7. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.; Basar, O.; Koklu, S.; Yuksel, O.; Purnak, T.; Sokmensuer, C. An Unusual Presentation of Malignant Melanoma: Amelanotic Gastric Metastasis. Am. J. Gastroenterol. 2015, 110, 476. [Google Scholar] [CrossRef]
- Albert, J.G.; Fechner, M.; Fiedler, E.; Voderholzer, W.; Lochs, H.; Trefzer, U.; Sterry, W.; Vay, S.; Stremmel, W.; Enk, A.; et al. Algorithm for detection of small-bowel metastasis in malignant melanoma of the skin. Endoscopy 2011, 43, 490–498. [Google Scholar] [CrossRef]
- Prakoso, E.; Fulham, M.; Thompson, J.F.; Selby, W. Capsule endoscopy versus positron emission tomography for detection of small-bowel metastatic melanoma: A pilot study. Gastrointest. Endosc. 2011, 73, 750–756. [Google Scholar] [CrossRef]
- Yamamoto, H.; Sekine, Y.; Sato, Y.; Higashizawa, T.; Miyata, T.; Iino, S.; Ido, K.; Sugano, K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest. Endosc. 2001, 53, 216–220. [Google Scholar] [CrossRef]
- Neuhaus, H.H.; Beyna, T.T.; Schneider, M.; Devière, J. Novel motorized spiral enteroscopy: First clinical case. VideoGIE 2016, 1, 32–33. [Google Scholar] [CrossRef]
- Schneider, M.; Höllerich, J.; Beyna, T. Device-assisted enteroscopy: A review of available techniques and upcoming new technologies. World J. Gastroenterol. 2019, 25, 3538–3545. [Google Scholar] [CrossRef]
- Lipka, S.; Rabbanifard, R.; Kumar, A.; Brady, P. Single versus double balloon enteroscopy for small bowel diagnostics: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2015, 49, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Primers 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.D.; Masone, S.; Rega, M.; Simeoli, I.; Donisi, M.; Addeo, P.; Iannone, L.; Pilone, V.; Persico, G. Metastatic tumors to the stomach: Clinical and endoscopic features. World J Gastroenterol. 2006, 12, 7326–7328. [Google Scholar] [CrossRef]
- Bargiggia, S.; Parente, F.; Ucci, G.; Tricomi, P.; Zerbi, P.; Vago, L. Bleeding gastric metastatic melanoma. Dig. Liver Dis. 2008, 40, 699. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Prueksapanich, P.; Rerknimitr, R. The efficacy of Hemospray in patients with upper gastrointestinal bleeding from tumor. Endosc. Int. Open 2016, 4, E933–E936. [Google Scholar] [CrossRef] [PubMed]
- Ollila, D.W.; Essner, R.; Wanek, L.A.; Morton, D.L. Surgical Resection for Melanoma Metastatic to the Gastrointestinal Tract. Arch. Surg. 1996, 131, 975–980. [Google Scholar]
- Sharpless, S.M.; Das Gupta, T.K. Surgery for metastatic melanoma. Semin. Surg. Oncol. 1998, 14, 311–318. [Google Scholar] [CrossRef]
- Panagiotou, I.; Brountzos, E.N.; Bafaloukos, D.; Stoupis, C.; Brestas, P.; Kelekis, D.A. Malignant melanoma metastatic to the gastrointestinal tract. Melanoma Res. 2002, 12, 169–173. [Google Scholar] [CrossRef]
- Berger, A.C.; Buell, J.F.; Venzon, D.; Baker, A.R.; Libutti, S.K. Management of Symptomatic Malignant Melanoma of the Gastrointestinal Tract. Ann. Surg. Oncol. 1999, 6, 155–160. [Google Scholar] [CrossRef]
- Essner, R. Surgical treatment of malignant melanoma. Surg. Clin. N. Am. 2003, 83, 109–156. [Google Scholar] [CrossRef]
- Ollila, D.W.; Caudle, A.S. Surgical Management of Distant Metastases. Surg. Oncol. Clin. N. Am. 2006, 15, 385–398. [Google Scholar] [CrossRef]
- Deutsch, G.B.; Flaherty, D.C.; Kirchoff, D.D.; Bailey, M.; Vitug, S.; Foshag, L.J.; Faries, M.B.; Bilchik, A.J. Association of Surgical Treatment, Systemic Therapy, and Survival in Patients with Abdominal Visceral Melanoma Metastases, 1965–2014. JAMA Surg. 2017, 152, 672–678. [Google Scholar] [CrossRef]
- Lui, P.; Cashin, R.; Machado, M.; Hemels, M.; Corey-Lisle, P.K.; Einarson, T.R. Treatments for metastatic melanoma: Synthesis of evidence from randomized trials. Cancer Treat. Rev. 2007, 33, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.L.; Liu, P.-Y.; Lee, S.J.; Chapman, J.-A.W.; Niedzwiecki, D.; Suman, V.J.; Moon, J.; Sondak, V.K.; Atkins, M.B.; Eisenhauer, E.A.; et al. Meta-Analysis of Phase II Cooperative Group Trials in Metastatic Stage IV Melanoma to Determine Progression-Free and Overall Survival Benchmarks for Future Phase II Trials. J. Clin. Oncol. 2008, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Dobry, A.S.; Zogg, C.K.; Hodi, F.S.; Smith, T.R.; Ott, P.A.; Iorgulescu, J.B. Management of metastatic melanoma: Improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol. Immunother. 2018, 67, 1833–1844. [Google Scholar] [CrossRef]
- Nobel Prize Organisation. Press Release: The Nobel Prize in Physiology or Medicine 2018. Available online: https://www.nobelprize.org/prizes/medicine/2018/press-release/ (accessed on 19 December 2020).
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 2019, 7, 405–418. [Google Scholar] [CrossRef]
- Cancer Research Institute. Available online: https://www.cancerresearch.org/news/2011/fda-approves-new-immunotherapy-for-melanoma (accessed on 19 December 2020).
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, D.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF V600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Dummer, R.; A Ascierto, P.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Weber, J.S.; Mandalà, M.; Del Vecchio, M.; Gogas, H.; Arance, A.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Rodas, I.M.; et al. Adjuvant therapy with nivolumab (NIVO) versus ipilimumab (IPI) after complete resection of stage III/IV melanoma: Updated results from a phase III trial (CheckMate 238). J. Clin. Oncol. 2018, 36, 9502. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Smith, M.; Smith, H.G.; Joshi, K.; Gore, M.; Strauss, D.; Hayes, A.; Larkin, J. The impact of effective systemic therapies on surgery for stage IV melanoma. Eur. J. Cancer 2018, 103, 24–31. [Google Scholar] [CrossRef]
- Hryniewicki, A.T.; Wang, C.; Shatsky, R.A.; Coyne, C.J. Management of Immune Checkpoint Inhibitor Toxicities: A Review and Clinical Guideline for Emergency Physicians. J. Emerg. Med. 2018, 55, 489–502. [Google Scholar] [CrossRef]
- Bajwa, R.; Cheema, A.; Khan, T.; Amirpour, A.; Paul, A.; Chaughtai, S.; Patel, S.; Patel, T.; Bramson, J.; Gupta, V.; et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019, 11, 225–236. [Google Scholar] [CrossRef]
- Ibraheim, H.; Perucha, E.; Powell, N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology 2019, 58, vii17–vii28. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.J.; Corrie, P. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther. Adv. Med. Oncol. 2015, 7, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Del Vecchio, M.; Ascierto, P.A.; Krajsova, I.; Schachter, J.; Neyns, B.; Espinosa, E.; Garbe, C.; Sileni, V.C.; Gogas, H.; et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: An open-label, multicentre, safety study. Lancet Oncol. 2014, 15, 436–444. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohoutova, D.; Worku, D.; Aziz, H.; Teare, J.; Weir, J.; Larkin, J. Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells 2021, 10, 327. https://doi.org/10.3390/cells10020327
Kohoutova D, Worku D, Aziz H, Teare J, Weir J, Larkin J. Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells. 2021; 10(2):327. https://doi.org/10.3390/cells10020327
Chicago/Turabian StyleKohoutova, Darina, Dominic Worku, Hala Aziz, Julian Teare, Justin Weir, and James Larkin. 2021. "Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options" Cells 10, no. 2: 327. https://doi.org/10.3390/cells10020327
APA StyleKohoutova, D., Worku, D., Aziz, H., Teare, J., Weir, J., & Larkin, J. (2021). Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells, 10(2), 327. https://doi.org/10.3390/cells10020327




