CERI, CEFX, and CPI: Largely Improved Positive Controls for Testing Antigen-Specific T Cell Function in PBMC Compared to CEF
Abstract
1. Introduction
2. Materials and Methods
2.1. PBMC
2.2. CD4 and CD8 Depletion of PBMC
2.3. Positive Control Antigens
2.3.1. CEF
2.3.2. CEFX
2.3.3. CPI
2.3.4. CERI
2.3.5. Anti-CD3
2.4. Human Interferon-γ ImmunoSpot® Assay
2.5. Statistical Analysis of ImmunoSpot SFU Counts
3. Results
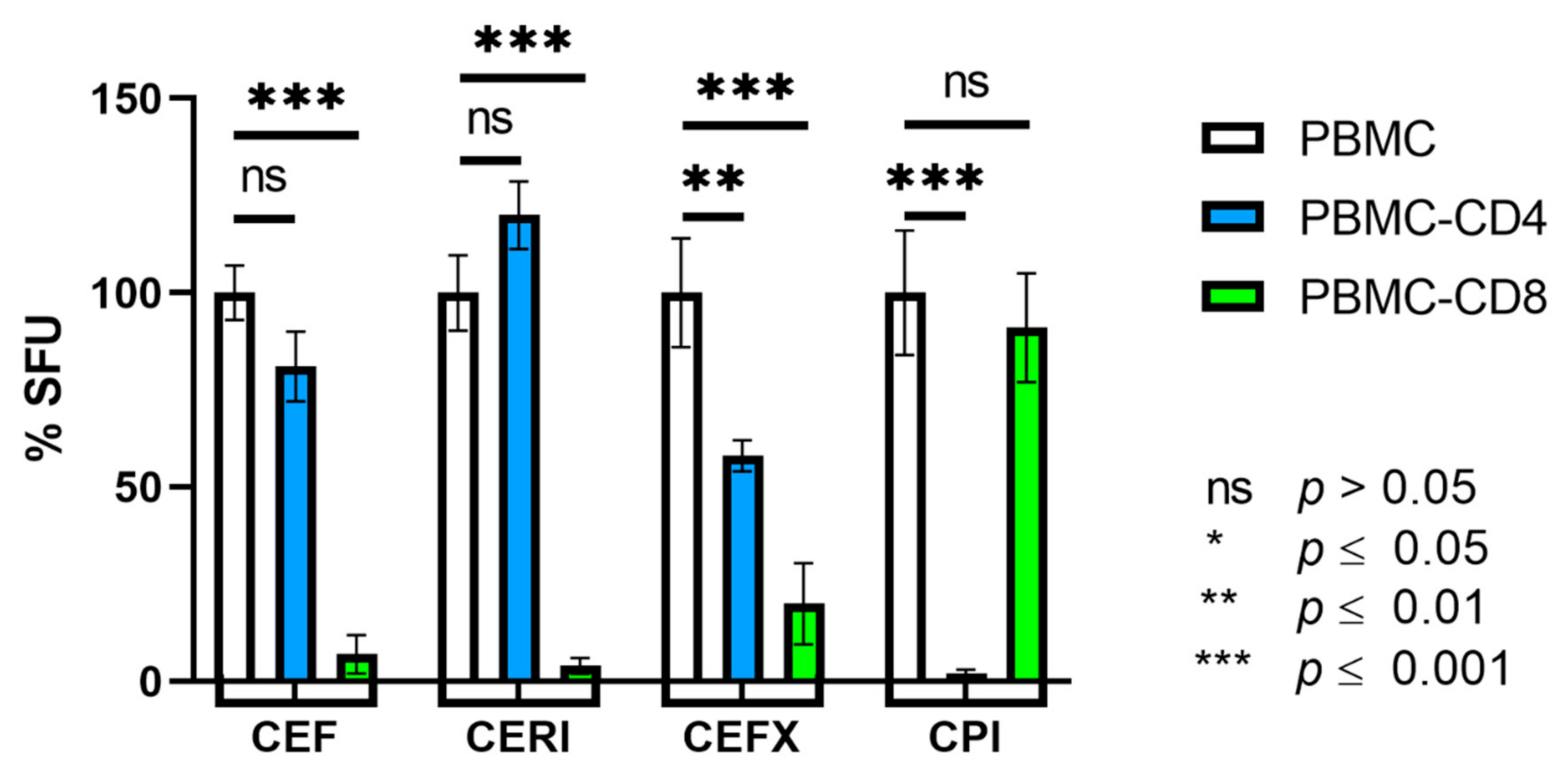
3.1. CEF and CERI Recall CD8+ T Cells, CPI Recalls CD4+ T Cells, and CEFX Recalls Both
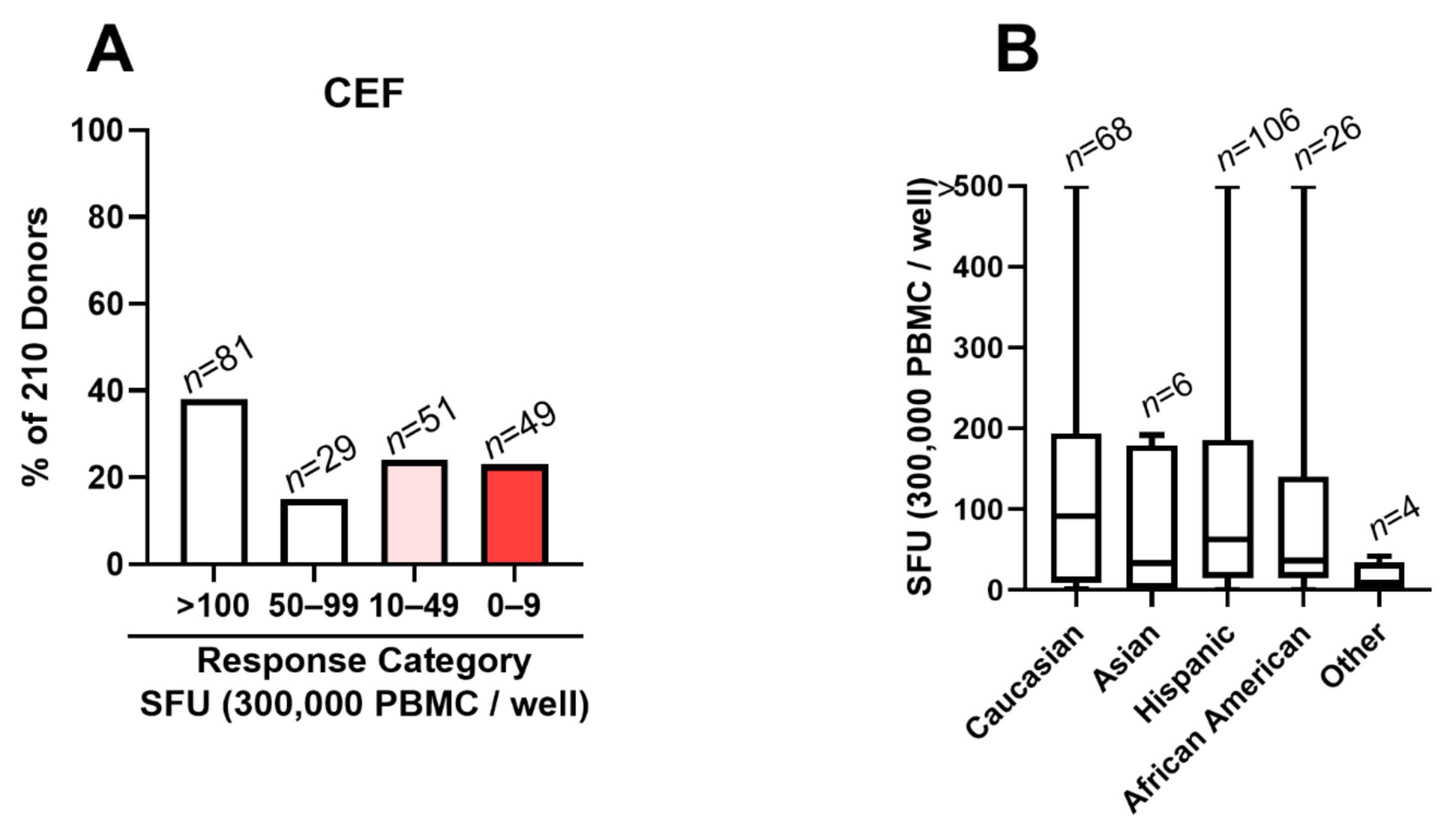
3.2. CEF Fails as a Positive Control in 48% of Test Subjects
3.3. CEF Non-Responder PBMCs Respond to Anti-CD3 Stimulation
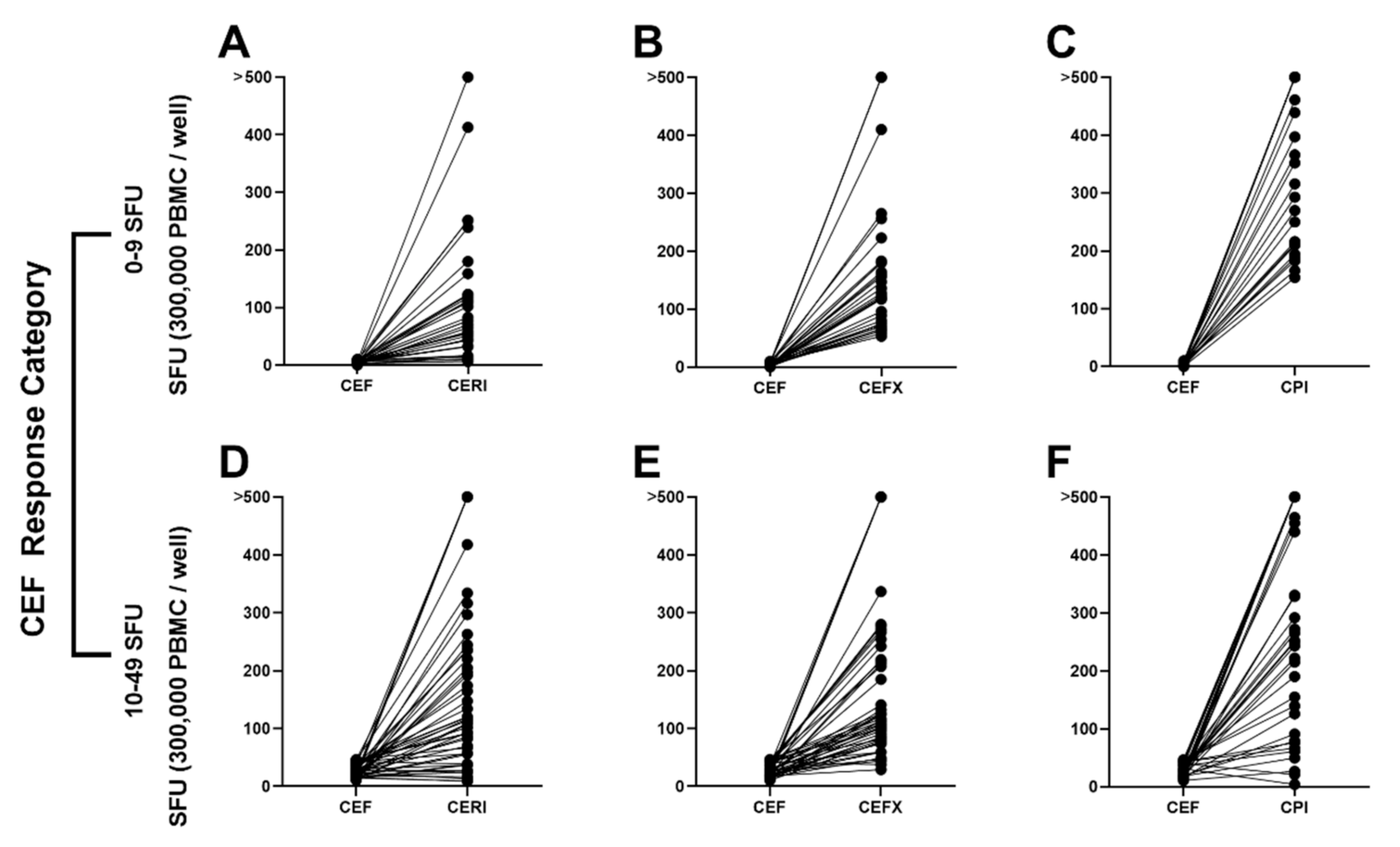
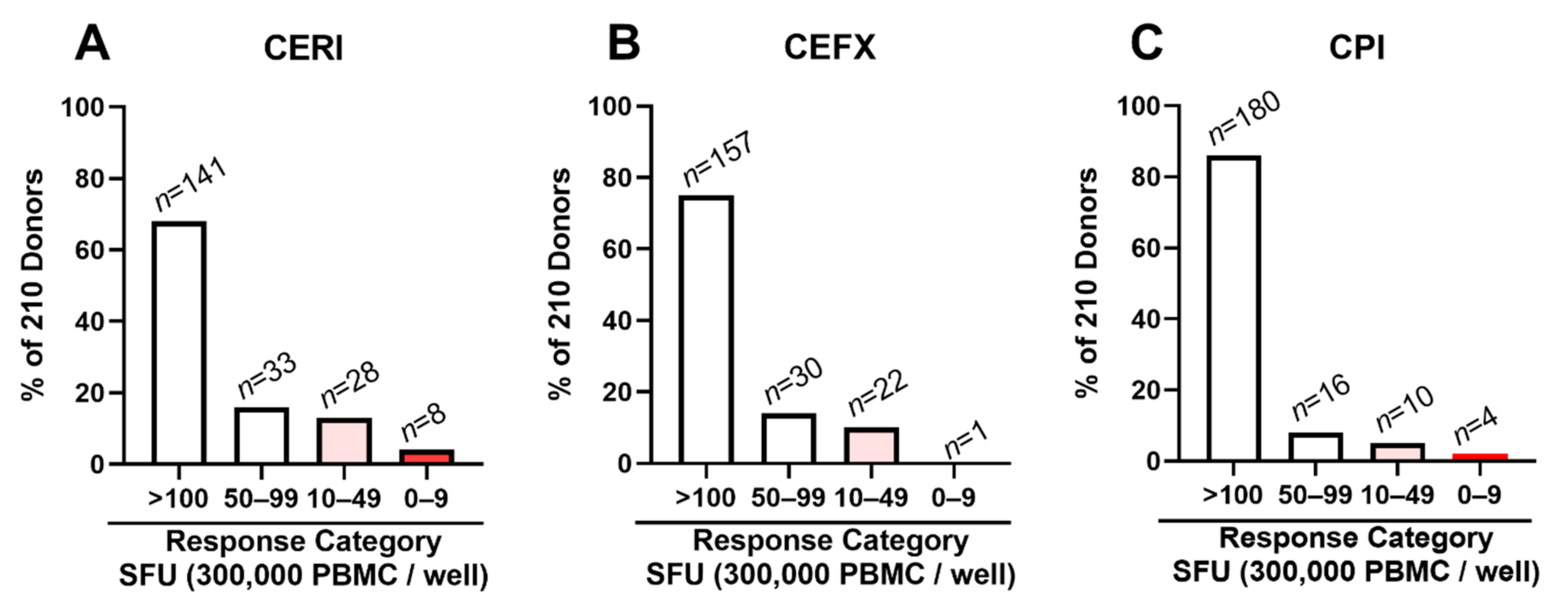
3.4. CEF Non-/Low-Responder PBMCs Respond to CERI, CEFX and CPI Stimulation
3.5. Low/Non-Responders to CERI, CEFX and CPI Are Rare
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tough, D.F.; Sprent, J. Life span of naive and memory T cells. Stem Cells 1995, 13, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Kelley, M. Validation of Cell-Based Assays in the GLP Setting: A Practical Guide; Wiley: Chichester, UK; John Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kreher, C.R.; Dittrich, M.T.; Guerkov, R.; Boehm, B.O.; Tary-Lehmann, M. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J. Immunol. Methods 2003, 278, 79–93. [Google Scholar] [CrossRef]
- Duechting, A.; Przybyla, A.; Kuerten, S.; Lehmann, P.V. Delayed Activation Kinetics of Th2- and Th17 Cells Compared to Th1 Cells. Cells 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, H.; Laux, J.; Moldovan, I.; Caspell, R.; Lehmann, P.V.; Subbramanian, R.A. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells 2012, 1, 313–324. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Pamer, E.; Cresswell, P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 1998, 16, 323–358. [Google Scholar] [CrossRef]
- Reche, P.A.; Reinherz, E.L. Sequence variability analysis of human class I and class II MHC molecules: Functional and structural correlates of amino acid polymorphisms. J. Mol. Biol. 2003, 331, 623–641. [Google Scholar] [CrossRef]
- Lehmann, P.V.; Lehmann, A.A. Aleatory epitope recognition prevails in human T cell responses? Crit Rev Immunol. 2020, 40, 225–235. [Google Scholar] [CrossRef]
- Currier, J.R.; Kuta, E.G.; Turk, E.; Earhart, L.B.; Loomis-Price, L.; Janetzki, S.; Ferrari, G.; Birx, D.L.; Cox, J.H. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 2002, 260, 157–172. [Google Scholar] [CrossRef]
- NIH AIDS Reagent Program. CEF Control Peptide Pool NIH, Ed. 2018. Available online: aidreagent.org (accessed on 20 October 2020).
- Lehmann, A.A.; Zhang, T.; Reche, P.A.; Lehmann, P.V. Discordance between the predicted vs. the actually recognized CD8+ T cell epitopes of HCMV pp65 antigen and aleatory epitope dominance. Front. Immunol. 2021, in press. [Google Scholar] [CrossRef]
- Schiller, A.; Zhang, T.; Li, R.; Duechting, A.; Sundararaman, S.; Przybyla, A.; Kuerten, S.; Lehmann, P.V. A positive control for detection of functional CD4 T cells in PBMC: The CPI pool. Cells 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Molero-Abraham, M.; Lafuente, E.M.; Flower, D.R.; Reche, P.A. Selection of conserved epitopes from hepatitis C virus for pan-populational stimulation of T-cell responses. Clin. Dev. Immunol. 2013, 2013, 601943. [Google Scholar] [CrossRef] [PubMed]
- Karulin, A.Y.; Karacsony, K.; Zhang, W.; Targoni, O.S.; Moldovan, I.; Dittrich, M.; Sundararaman, S.; Lehmann, P.V. ELISPOTs produced by CD8 and CD4 cells follow log normal size distribution permitting objective counting. Cells 2015, 4, 56–70. [Google Scholar] [CrossRef]
- Karulin, A.Y.; Caspell, R.; Dittrich, M.; Lehmann, P.V. Normal distribution of CD8+ T-cell-derived ELISPOT counts within replicates justifies the reliance on parametric statistics for identifying positive responses. Cells 2015, 4, 96–111. [Google Scholar] [CrossRef]
- Bjorkman, P.J.; Saper, M.A.; Samraoui, B.; Bennett, W.S.; Strominger, J.L.; Wiley, D.C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987, 329, 506–512. [Google Scholar] [CrossRef]
- Rock, K.L.; Reits, E.; Neefjes, J. Present yourself! By MHC class I and MHC class II molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Tary-Lehmann, M.; Lehmann, P.V. The secretory IFN-gamma response of single CD4 memory cells after activation on different antigen presenting cell types. Clin. Immunol. 2007, 124, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, I.; Targoni, O.; Zhang, W.; Sundararaman, S.; Lehmann, P.V. How frequently are predicted peptides actually recognized by CD8 cells? Cancer Immunol. Immunother. 2016, 65, 847–855. [Google Scholar] [CrossRef]
- Huppa, J.B.; Axmann, M.; Mörtelmaier, M.A.; Lillemeier, B.F.; Newell, E.W.; Brameshuber, M.; Klein, L.O.; Schütz, G.J.; Davis, M.M. TCR–peptide–MHC interactions in situ show accelerated kinetics and increased affinity. Nature 2010, 463, 963–967. [Google Scholar] [CrossRef]
- Norman, D.J. Mechanisms of action and overview of OKT3. Ther. Drug Monit. 1995, 17, 615–620. [Google Scholar] [CrossRef]
- Lehmann, P.V.; Suwansaard, M.; Zhang, T.; Roen, D.R.; Kirchenbaum, G.A.; Karulin, A.Y.; Lehmann, A.; Reche, P.A. Comprehensive evaluation of the expressed CD8+ T cell epitope space sing high-throughput epitope mapping. Front. Immunol. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
| Subject | Media | CEF | CERI | CEFX | CPI | Anti-CD3 |
|---|---|---|---|---|---|---|
| ID 30 | 1 | 8 | 117 | 96 | 212 | >500 |
| ID 51 | 2 | 7 | 138 | 41 | 268 | >500 |
| ID 68 | 2 | 2 | 413 | 265 | 270 | >500 |
| ID 69 | 0 | 0 | 2 | 4 | 6 | 436 |
| ID 71 | 2 | 4 | 51 | 160 | 352 | >500 |
| ID 82 | 1 | 1 | 15 | 27 | 4 | 129 |
| ID 87 | 2 | 1 | 48 | 17 | 158 | >500 |
| ID 98 | 2 | 3 | 252 | 118 | 250 | >500 |
| ID 101 | 1 | 6 | 72 | 29 | 29 | 233 |
| ID 103 | 1 | 1 | 112 | 180 | 194 | >500 |
| ID 118 | 0 | 2 | 32 | 18 | 212 | 243 |
| ID 123 | 2 | 4 | 180 | 58 | 366 | 348 |
| ID 128 | 1 | 7 | 121 | 53 | 183 | 459 |
| ID 131 | 1 | 9 | 47 | 23 | 37 | 260 |
| ID 133 | 3 | 2 | 159 | 89 | 316 | >500 |
| ID 138 | 1 | 5 | 32 | 123 | 461 | >500 |
| ID 144 | 0 | 7 | 55 | 127 | 166 | >500 |
| ID 147 | 1 | 5 | 77 | 156 | >500 | >500 |
| ID 150 | 0 | 8 | 83 | 410 | 216 | >500 |
| ID 160 | 1 | 2 | 12 | 43 | 27 | 495 |
| ID 168 | 3 | 6 | 33 | 136 | 439 | >500 |
| ID 169 | 2 | 8 | 102 | 70 | 293 | 381 |
| ID 191 | 1 | 0 | 70 | 75 | 154 | 403 |
| ID 196 | 1 | 5 | 32 | 20 | 154 | >500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, A.A.; Reche, P.A.; Zhang, T.; Suwansaard, M.; Lehmann, P.V. CERI, CEFX, and CPI: Largely Improved Positive Controls for Testing Antigen-Specific T Cell Function in PBMC Compared to CEF. Cells 2021, 10, 248. https://doi.org/10.3390/cells10020248
Lehmann AA, Reche PA, Zhang T, Suwansaard M, Lehmann PV. CERI, CEFX, and CPI: Largely Improved Positive Controls for Testing Antigen-Specific T Cell Function in PBMC Compared to CEF. Cells. 2021; 10(2):248. https://doi.org/10.3390/cells10020248
Chicago/Turabian StyleLehmann, Alexander A., Pedro A. Reche, Ting Zhang, Maneewan Suwansaard, and Paul V. Lehmann. 2021. "CERI, CEFX, and CPI: Largely Improved Positive Controls for Testing Antigen-Specific T Cell Function in PBMC Compared to CEF" Cells 10, no. 2: 248. https://doi.org/10.3390/cells10020248
APA StyleLehmann, A. A., Reche, P. A., Zhang, T., Suwansaard, M., & Lehmann, P. V. (2021). CERI, CEFX, and CPI: Largely Improved Positive Controls for Testing Antigen-Specific T Cell Function in PBMC Compared to CEF. Cells, 10(2), 248. https://doi.org/10.3390/cells10020248








