PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples and Data Collection
2.2. Tissue Microarray (TMA) Construction
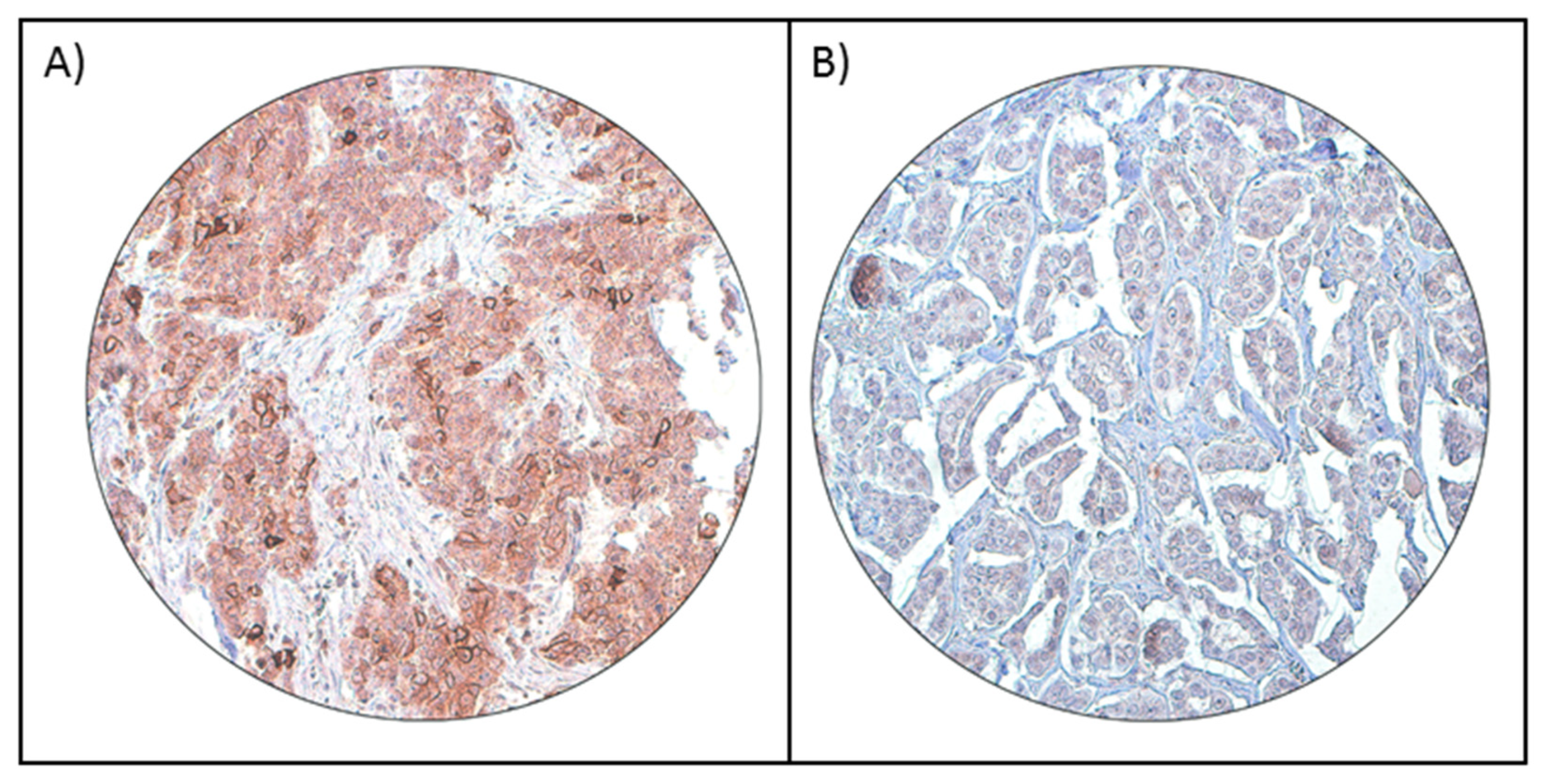
2.3. Immunohistochemistry (IHC) Staining and Evaluation
2.4. Statistical Analysis
3. Results
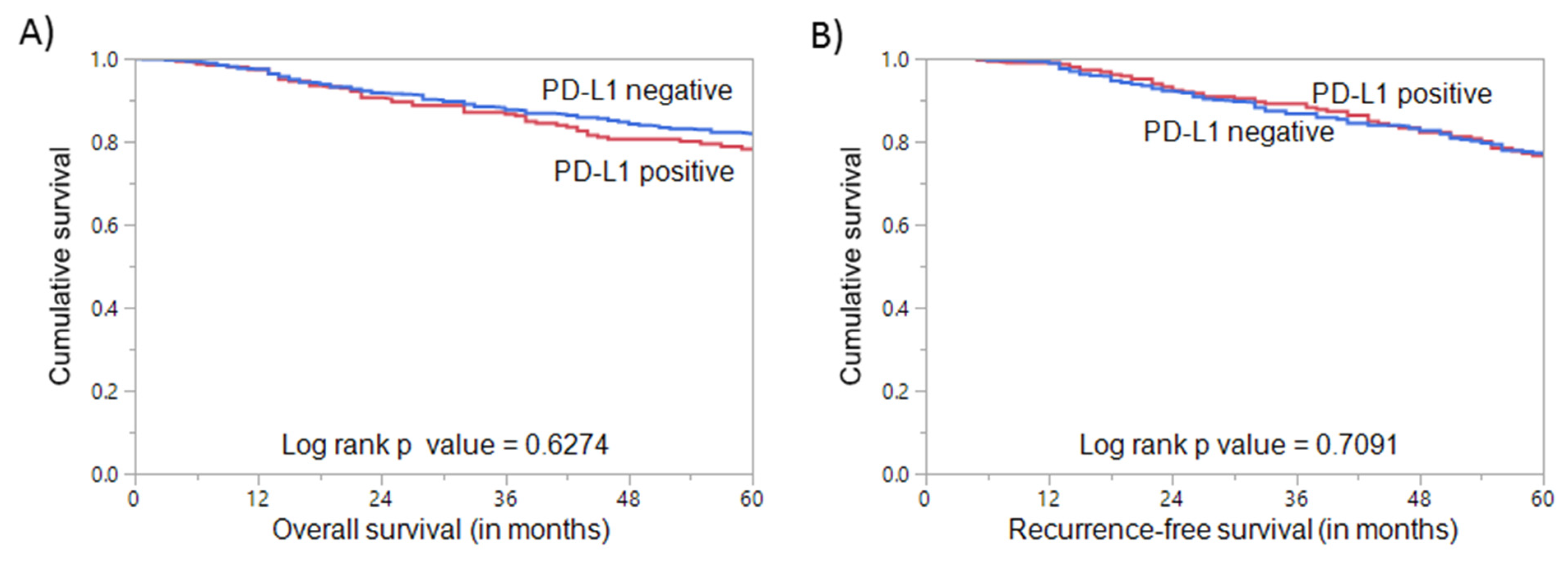
3.1. PD-L1 Expression in Breast Cancer and Its Clinico-Pathological Associations
3.2. PD-L1 Expression in Triple-Negative Breast Cancer
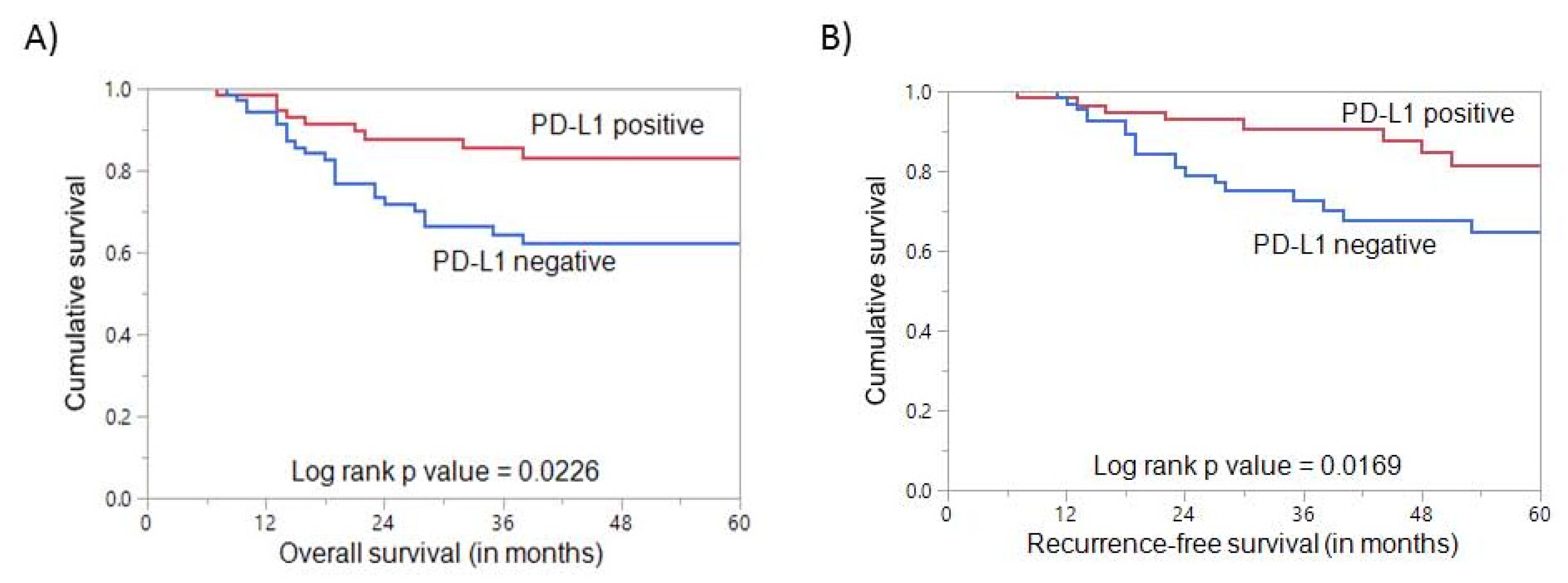
3.3. PD-L1 Expression and Clinical Outcome in Triple Negative Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alrawaji, A.; Alshahrani, Z.; Alzahrani, W.; Alomran, F.; Almadouj, A.; Alshehri, S.; Alzahrani, A.; Bazarbashi, S.; Alhashmi, H.; Almutlaq, H.; et al. Cancer Incidence Report Saudi Arabia 2015; Saudi Cancer Registry, Saudi Health Council: Riyadh, Saudi Arabia, 2018.
- Weigelt, B.; Peterse, J.L.; Veer, L.J.V. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thürlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results from the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin. Cancer Biol. 2020, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar]
- He, J.; Hu, Y.; Hu, M.; Li, B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2015, 5, srep13110. [Google Scholar] [CrossRef]
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019, 5, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yan, X.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Li, S.; Mao, L.; Lian, B.; Wang, X.; et al. Axitinib in Combination with Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients with Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J. Clin. Oncol. 2019, 37, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.O.; Ogunniyi, A.; Barbee, M.S.; Drilon, A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2016, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, D.; Li, L.; Chai, Y.; Huang, J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS ONE 2015, 10, e0131403. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Dai, W.; Cai, G.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 2016, 15, 1–15. [Google Scholar] [CrossRef]
- Aust, S.; Felix, S.; Auer, K.; Bachmayr-Heyda, A.; Kenner, L.; Dekan, S.; Meier, S.M.; Gerner, C.; Grimm, C.; Pils, D. Absence of PD-L1 on tumor cells is associated with reduced MHC I expression and PD-L1 expression increases in recurrent serous ovarian cancer. Sci. Rep. 2017, 7, srep42929. [Google Scholar] [CrossRef]
- Yu, W.; Hua, Y.; Qiu, H.; Hao, J.; Zou, K.; Li, Z.; Hu, S.; Guo, P.; Chen, M.; Sui, S.; et al. PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Qin, T.; Zeng, Y.-D.; Qin, G.; Xu, F.; Lu, J.-B.; Fang, W.-F.; Xue, C.; Zhan, J.-H.; Zhang, X.-K.; Zheng, Q.-F.; et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 2015, 6, 33972–33981. [Google Scholar] [CrossRef]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef]
- Huang, W.; Ran, R.; Shao, B.; Li, H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019, 178, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Vassallo, J.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Botti, G.; Collina, F.; Scognamiglio, G.; Rao, F.; Peluso, V.; De Cecio, R.; Piezzo, M.; Landi, G.; De Laurentiis, M.; Cantile, M.; et al. Programmed Death Ligand 1 (PD-L1) Tumor Expression Is Associated with a Better Prognosis and Diabetic Disease in Triple Negative Breast Cancer Patients. Int. J. Mol. Sci. 2017, 18, 459. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Milne, K.; DeRocher, H.; Webb, J.R.; Nelson, B.H.; Watson, P.H. PD-L1 and intratumoral immune response in breast cancer. Oncotarget 2017, 8, 51641–51651. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.T.; Lenkiewicz, E.; Malasi, S.; Basu, A.; Yearley, J.; Annamalai, L.; McCullough, A.E.; Kosiorek, H.E.; Narang, P.; Sayres, M.A.W.; et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018, 20, 1–15. [Google Scholar] [CrossRef]
- Bavi, P.; Jehan, Z.; Atizado, V.; Al-Dossari, H.; Al-Dayel, F.; Tulbah, A.; Amr, S.S.; Sheikh, S.S.; Ezzat, A.; El-Solh, H.; et al. Prevalence of Fragile Histidine Triad Expression in Tumors from Saudi Arabia: A Tissue Microarray Analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1708–1718. [Google Scholar] [CrossRef][Green Version]
- Mesnage, S.J.L.; Auguste, A.; Genestie, C.; Dunant, A.; Pain, E.; Drusch, F.; Gouy, S.; Morice, P.; Bentivegna, E.; Lhomme, C.; et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann. Oncol. 2017, 28, 651–657. [Google Scholar] [CrossRef]
- Siraj, A.K.; Beg, S.; Jehan, Z.; Prabhakaran, S.; Ahmed, M.; Hussain, A.R.; Al-Dayel, F.; Tulbah, A.; Ajarim, D.; Al-Kuraya, K.S. ALK alteration is a frequent event in aggressive breast cancers. Breast Cancer Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Beg, S.; Siraj, A.K.; Prabhakaran, S.; Jehan, Z.; Ajarim, D.; Al-Dayel, F.; Tulbah, A.; Al-Kuraya, K.S. Loss of PTEN expression is associated with aggressive behavior and poor prognosis in Middle Eastern triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 151, 541–553. [Google Scholar] [CrossRef]
- Siraj, A.K.; Prabhakaran, S.; Bavi, P.; Bu, R.; Beg, S.; Al Hazmi, M.; Al-Rasheed, M.; Al-Assiri, M.; Sairafi, R.; Al-Dayel, F.; et al. Prevalence of Lynch syndrome in a Middle Eastern population with colorectal cancer. Cancer 2015, 121, 1762–1771. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Carvajal-Hausdorf, D.; Altan, M.; Wasserman, B.; Carvajal-Hausdorf, C.; Wimberly, H.; Brown, J.; Lannin, D.R.; Pusztai, L.; Rimm, D.L. Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Res. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Opyrchal, M.; Yao, S.; Peng, X.; Yan, L.; Jabbour, H.; Khoury, T. The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene. Breast Cancer Res. Treat. 2018, 170, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, P.; Ren, M.; Song, Y.; Qian, X.; Yang, Y.; Li, S.; Zhang, X.; Liu, F. PD-L1 Expression Is Associated with Tumor FOXP3+ Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J. Cancer 2016, 7, 784–793. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.-X.; Liu, Y.; Yang, W.-T.; Shao, Z.-M. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int. J. Cancer 2017, 140, 1384–1395. [Google Scholar] [CrossRef]
- Mills, A.M.; Dill, E.A.; Moskaluk, C.A.; Dziegielewski, J.; Bullock, T.N.; Dillon, P. The Relationship Between Mismatch Repair Deficiency and PD-L1 Expression in Breast Carcinoma. Am. J. Surg. Pathol. 2018, 42, 183–191. [Google Scholar] [CrossRef]
- Lemery, S.; Keegan, P.; Pazdur, R. First FDA Approval Agnostic of Cancer Site—When a Biomarker Defines the Indication. N. Engl. J. Med. 2017, 377, 1409–1412. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Ren, X.; Wu, H.; Lu, J.; Zhang, Y.; Luo, Y.; Xu, Q.; Shen, S.; Liang, Z. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol. Ther. 2018, 19, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Cao, S.; Li, N.; Jiang, L.; Sun, T. PD-1 and PD-L1 correlated gene expression profiles and their association with clinical outcomes of breast cancer. Cancer Cell Int. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.-P. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Q.; Wang, D.; Liu, C.; Han, B.; Yang, J. Expression of PD-L1 Attenuates the Positive Impacts of High-level Tumor-infiltrating Lymphocytes on Prognosis of Triple-negative Breast Cancer. Cancer Biol. Ther. 2019, 20, 1105–1112. [Google Scholar] [CrossRef]
- Desmedt, C.; Haibe-Kains, B.; Wirapati, P.; Buyse, M.; Larsimont, D.; Bontempi, G.; Delorenzi, M.; Piccart, M.; Sotiriou, C. Biological Processes Associated with Breast Cancer Clinical Outcome Depend on the Molecular Subtypes. Clin. Cancer Res. 2008, 14, 5158–5165. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Foukakis, T.; Lövrot, J.; Matikas, A.; Zerdes, I.; Lorent, J.; Tobin, N.; Suzuki, C.; Brage, S.E.; Carlsson, L.; Einbeigi, Z.; et al. Immune gene expression and response to chemotherapy in advanced breast cancer. Br. J. Cancer 2018, 118, 480–488. [Google Scholar] [CrossRef]
| Clinico-Pathologic Variables | n (%) |
|---|---|
| Age (years) | |
| ≤50 | 686 (68.0) |
| >50 | 323 (32.0) |
| Median (in years) | 45.0 |
| Range (IQR) ^ | 39.0–54.0 |
| Histological Type | |
| Infiltrating ductal carcinoma | 913 (90.5) |
| Infiltrating lobular carcinoma | 44 (4.4) |
| Mucinous carcinoma | 16 (1.6) |
| Others | 36 (3.5) |
| Tumor stage | |
| I | 91 (9.0) |
| II | 401 (39.7) |
| III | 379 (37.6) |
| IV | 91 (9.0) |
| Unknown | 47 (4.7) |
| Histologic grade | |
| Well-differentiated | 77 (7.6) |
| Moderately differentiated | 514 (50.9) |
| Poorly differentiated | 405 (40.2) |
| Unknown | 13 (1.3) |
| Estrogen receptor | |
| Positive | 662 (65.6) |
| Negative | 346 (34.3) |
| Unknown | 1 (0.1) |
| Progesterone receptor | |
| Positive | 579 (57.4) |
| Negative | 426 (42.2) |
| Unknown | 4 (0.4) |
| Her-2 neu | |
| Positive | 379 (37.6) |
| Negative | 628 (62.2) |
| Unknown | 2 (0.2) |
| Triple negative breast cancer | |
| Yes | 149 (14.8) |
| No | 852 (84.4) |
| Unknown | 8 (0.8) |
| Survival duration (in months) | |
| Median | 48.0 |
| Range (IQR) ^ | 26.0–74.0 |
| Clinico-Pathologic Variables | Total | PD-L1 Positive | PD-L1 Negative | p Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total Number of Cases | 1003 | 329 | 32.8 | 674 | 67.2 | ||
| Age Groups | |||||||
| ≤50 | 680 | 67.8 | 237 | 34.9 | 443 | 65.1 | 0.0432 * |
| >50 | 323 | 32.2 | 92 | 28.5 | 231 | 71.5 | |
| Histology | |||||||
| Infiltrating ductal carcinoma | 909 | 94.0 | 296 | 32.6 | 613 | 67.4 | 0.6176 |
| Infiltrating lobular carcinoma | 42 | 4.3 | 11 | 26.2 | 31 | 73.8 | |
| Mucinous carcinoma | 16 | 1.7 | 6 | 37.5 | 10 | 62.5 | |
| Histological Grade | |||||||
| Well-differentiated | 76 | 7.7 | 17 | 22.4 | 59 | 77.6 | 0.0025 * |
| Moderately differentiated | 511 | 51.5 | 151 | 29.6 | 360 | 70.4 | |
| Poorly differentiated | 404 | 40.8 | 155 | 38.4 | 249 | 61.6 | |
| pT | |||||||
| T1 | 213 | 22.1 | 68 | 31.9 | 145 | 68.1 | 0.8039 |
| T2 | 484 | 50.2 | 163 | 33.7 | 321 | 66.3 | |
| T3 | 143 | 14.8 | 42 | 29.4 | 101 | 70.6 | |
| T4 | 124 | 12.9 | 40 | 32.3 | 84 | 67.7 | |
| pN | |||||||
| N0 | 307 | 33.2 | 102 | 33.2 | 205 | 66.8 | 0.2121 |
| N1 | 297 | 32.1 | 102 | 34.3 | 195 | 65.7 | |
| N2 | 192 | 20.8 | 50 | 26.0 | 142 | 74.0 | |
| N3 | 128 | 13.9 | 44 | 34.4 | 84 | 65.6 | |
| pM | |||||||
| M0 | 808 | 89.9 | 265 | 32.8 | 543 | 67.2 | 0.2968 |
| M1 | 91 | 10.1 | 25 | 27.5 | 66 | 72.5 | |
| Tumor Stage | |||||||
| I | 91 | 9.5 | 33 | 36.3 | 58 | 63.7 | 0.6161 |
| II | 398 | 41.5 | 128 | 32.2 | 270 | 67.8 | |
| III | 378 | 39.5 | 126 | 33.3 | 252 | 66.7 | |
| IV | 91 | 9.5 | 25 | 27.5 | 66 | 72.5 | |
| Estrogen Receptor | |||||||
| Positive | 656 | 65.5 | 184 | 28.1 | 472 | 71.9 | <0.0001 * |
| Negative | 346 | 34.5 | 145 | 41.9 | 201 | 58.1 | |
| Progesterone Receptor | |||||||
| Positive | 575 | 57.6 | 161 | 28.0 | 414 | 72.0 | 0.0001 * |
| Negative | 424 | 42.4 | 168 | 39.6 | 256 | 60.4 | |
| Her-2 neu | |||||||
| Positive | 379 | 37.9 | 131 | 34.6 | 248 | 65.4 | 0.3729 |
| Negative | 622 | 62.1 | 198 | 31.8 | 424 | 68.2 | |
| Triple-Negative Breast Cancer | |||||||
| Yes | 149 | 15.0 | 64 | 43.0 | 85 | 57.0 | 0.0062 * |
| No | 846 | 85.0 | 265 | 31.3 | 581 | 68.7 | |
| Ki-67 | |||||||
| High | 630 | 64.1 | 238 | 37.8 | 392 | 62.2 | <0.0001 * |
| Low | 352 | 35.9 | 88 | 25.0 | 264 | 75.0 | |
| MMR Protein Expression | |||||||
| Deficient MMR | 33 | 3.3 | 20 | 60.6 | 13 | 39.4 | 0.0009 * |
| Proficient MMR | 970 | 96.7 | 309 | 31.9 | 661 | 68.1 | |
| Clinico-Pathologic Variables | Total | PD-L1 Positive | PD-L1 Negative | p Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total Number of Cases | 149 | 67 | 45.0 | 82 | 55.0 | ||
| Age Groups | |||||||
| ≤50 | 113 | 75.8 | 52 | 46.0 | 61 | 54.0 | 0.6471 |
| >50 | 36 | 24.2 | 15 | 41.7 | 21 | 58.3 | |
| Histology | |||||||
| Infiltrating Ductal Carcinoma | 139 | 98.6 | 61 | 43.9 | 78 | 56.1 | 0.0711 |
| Infiltrating Lobular Carcinoma | 2 | 1.4 | 2 | 100.0 | 0 | 0.0 | |
| Histological Grade | |||||||
| Moderately differentiated | 41 | 27.7 | 15 | 36.6 | 26 | 63.4 | 0.2225 |
| Poorly differentiated | 107 | 72.3 | 51 | 47.7 | 56 | 52.3 | |
| pT | |||||||
| T1 | 23 | 16.2 | 11 | 47.8 | 12 | 52.2 | 0.6633 |
| T2 | 75 | 52.8 | 33 | 44.0 | 42 | 56.0 | |
| T3 | 22 | 15.5 | 12 | 54.5 | 10 | 45.5 | |
| T4 | 22 | 15.5 | 8 | 36.4 | 14 | 63.6 | |
| pN | |||||||
| N0 | 59 | 44.0 | 22 | 37.3 | 37 | 62.7 | 0.0459 * |
| N1 | 40 | 29.8 | 25 | 62.5 | 15 | 37.5 | |
| N2 | 21 | 15.7 | 7 | 33.3 | 14 | 66.7 | |
| N3 | 14 | 10.5 | 5 | 35.7 | 9 | 64.3 | |
| pM | |||||||
| M0 | 114 | 83.8 | 53 | 46.5 | 61 | 53.5 | 0.1987 |
| M1 | 22 | 16.2 | 7 | 31.8 | 15 | 68.2 | |
| Tumor Stage | |||||||
| I | 13 | 9.3 | 8 | 61.5 | 5 | 38.5 | 0.3864 |
| II | 57 | 40.7 | 26 | 45.6 | 31 | 54.4 | |
| III | 48 | 34.3 | 21 | 43.8 | 27 | 56.2 | |
| IV | 22 | 15.7 | 7 | 31.8 | 15 | 68.2 | |
| Ki-67 | |||||||
| High | 137 | 91.9 | 61 | 44.5 | 76 | 55.5 | 0.7153 |
| Low | 12 | 8.1 | 6 | 50.0 | 6 | 50.0 | |
| MMR Protein Expression | |||||||
| Deficient MMR | 4 | 2.7 | 3 | 75.0 | 1 | 25.0 | 0.2159 |
| Proficient MMR | 145 | 97.3 | 64 | 44.1 | 81 | 55.9 | |
| Overall Survival | 83.1 | 62.3 | 0.0226 * | ||||
| Recurrence-Free Survival | 81.5 | 64.6 | 0.0169 * | ||||
| Overall Survival | Recurrence-Free Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||
| Clinico-Pathological Variables | Number of Events per Covariate | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Number of Events per Covariate | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Age (years) >50 ( vs. ≤ 50) | 10 (vs. 27) | 0.73 (0.29–1.57) | 0.4519 | 0.27 (0.09–0.80) | 0.0175 * | 11 (vs. 28) | 0.74 (0.30–1.60) | 0.4821 | 0.35 (0.11–0.86) | 0.0355 * |
| Histology IDC (vs. others) | 26 (vs. 11) | 0.48 (0.03–2.23) | 0.4706 | 0.81 (0.10–6.67) | 0.8426 | 28 (vs. 11) | 0.26 (0.01–1.27) | 0.1922 | 0.18 (0.01–1.03) | 0.1199 |
| Grade 3 (vs. 1–2) | 25 (vs. 12) | 0.61 (0.31–1.27) | 0.1660 | 0.54 (0.25–1.18) | 0.1241 | 29 (vs. 10) | 1.02 (0.47–2.54) | 0.9716 | 0.68 (0.28–1.82) | 0.4119 |
| Lymph Node Metastasis N1-3 (vs. N0) | 25 (vs. 12) | 3.34 (1.52–8.38) | 0.0050 * | 4.98 (1.95–12.75) | 0.0008 * | 24 (vs. 15) | 1.82 (0.92–3.79) | 0.0943 | 2.70 (1.31–5.87) | 0.0089 * |
| Stage IV (vs. I–III) | 11 (vs. 26) | 3.91 (1.82–7.92) | 0.0002 * | 2.80 (1.21–6.45) | 0.0159 * | 10 (vs. 29) | 0.82 (0.20–2.33) | 0.7481 | 0.76 (0.18–2.23) | 0.6593 |
| PD-L1 Positive (vs. Negative) | 11 (vs. 26) | 0.45 (0.21–0.89) | 0.0272 * | 0.28 (0.11–0.64) | 0.0043 * | 14 (vs. 25) | 0.42 (0.20–0.86) | 0.0205 * | 0.31 (0.13–0.67) | 0.0043 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvathareddy, S.K.; Siraj, A.K.; Ahmed, S.O.; Ghazwani, L.O.; Aldughaither, S.M.; Al-Dayel, F.; Tulbah, A.; Ajarim, D.; Al-Kuraya, K.S. PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer. Cells 2021, 10, 229. https://doi.org/10.3390/cells10020229
Parvathareddy SK, Siraj AK, Ahmed SO, Ghazwani LO, Aldughaither SM, Al-Dayel F, Tulbah A, Ajarim D, Al-Kuraya KS. PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer. Cells. 2021; 10(2):229. https://doi.org/10.3390/cells10020229
Chicago/Turabian StyleParvathareddy, Sandeep Kumar, Abdul K. Siraj, Saeeda O. Ahmed, Laila Omar Ghazwani, Saud M. Aldughaither, Fouad Al-Dayel, Asma Tulbah, Dahish Ajarim, and Khawla S. Al-Kuraya. 2021. "PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer" Cells 10, no. 2: 229. https://doi.org/10.3390/cells10020229
APA StyleParvathareddy, S. K., Siraj, A. K., Ahmed, S. O., Ghazwani, L. O., Aldughaither, S. M., Al-Dayel, F., Tulbah, A., Ajarim, D., & Al-Kuraya, K. S. (2021). PD-L1 Protein Expression in Middle Eastern Breast Cancer Predicts Favorable Outcome in Triple-Negative Breast Cancer. Cells, 10(2), 229. https://doi.org/10.3390/cells10020229





