Longitudinal Multi-Parametric Liquid Biopsy Approach Identifies Unique Features of Circulating Tumor Cell, Extracellular Vesicle, and Cell-Free DNA Characterization for Disease Monitoring in Metastatic Breast Cancer Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Sample Collection and Liquid Biopsy Analyte Extraction
2.3. Quantitative PCR
2.4. Sequencing
2.5. Statistical Analysis
3. Results
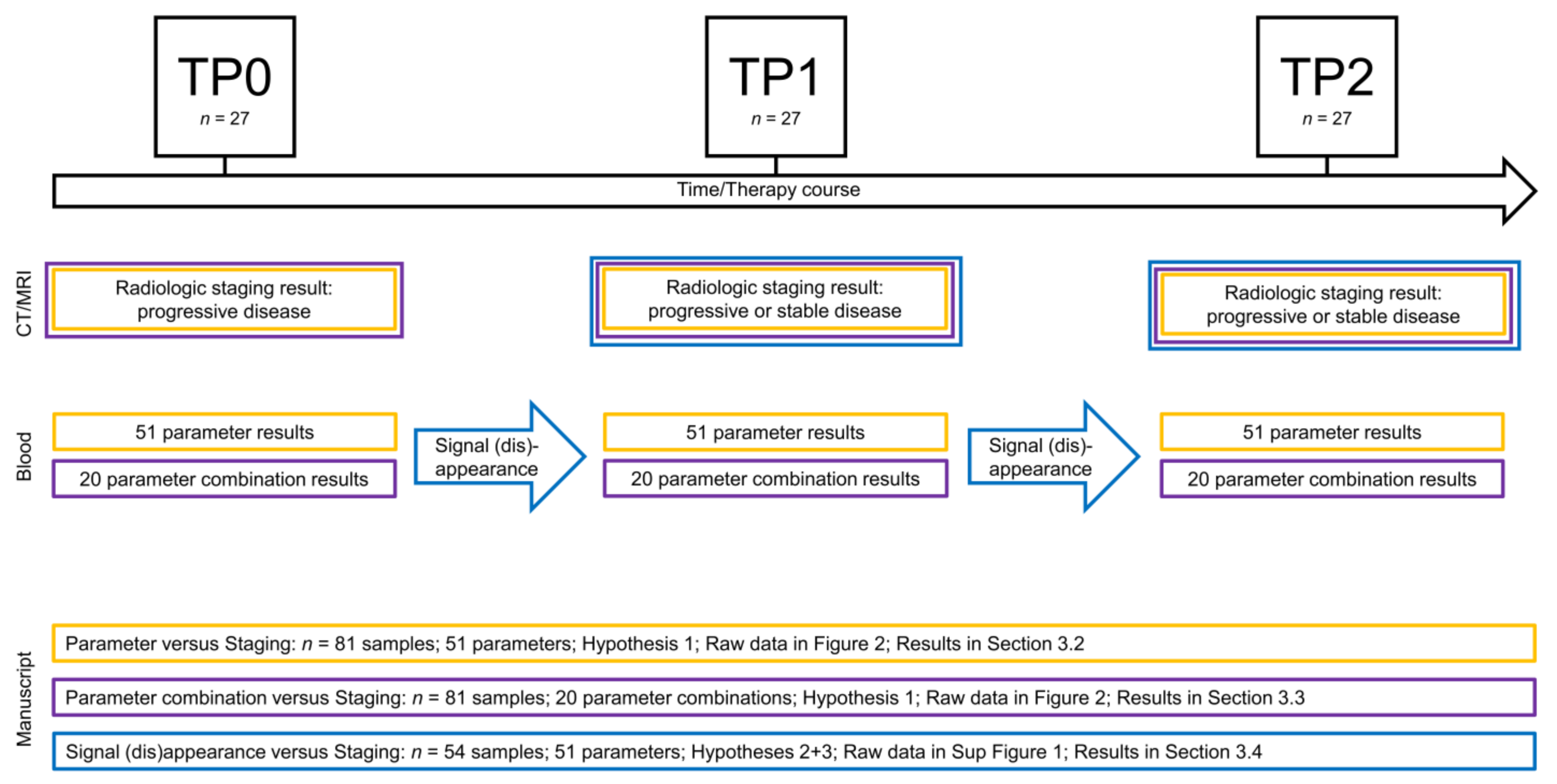
3.1. Longitudinal Multi-Parametric Liquid Biopsy Approach
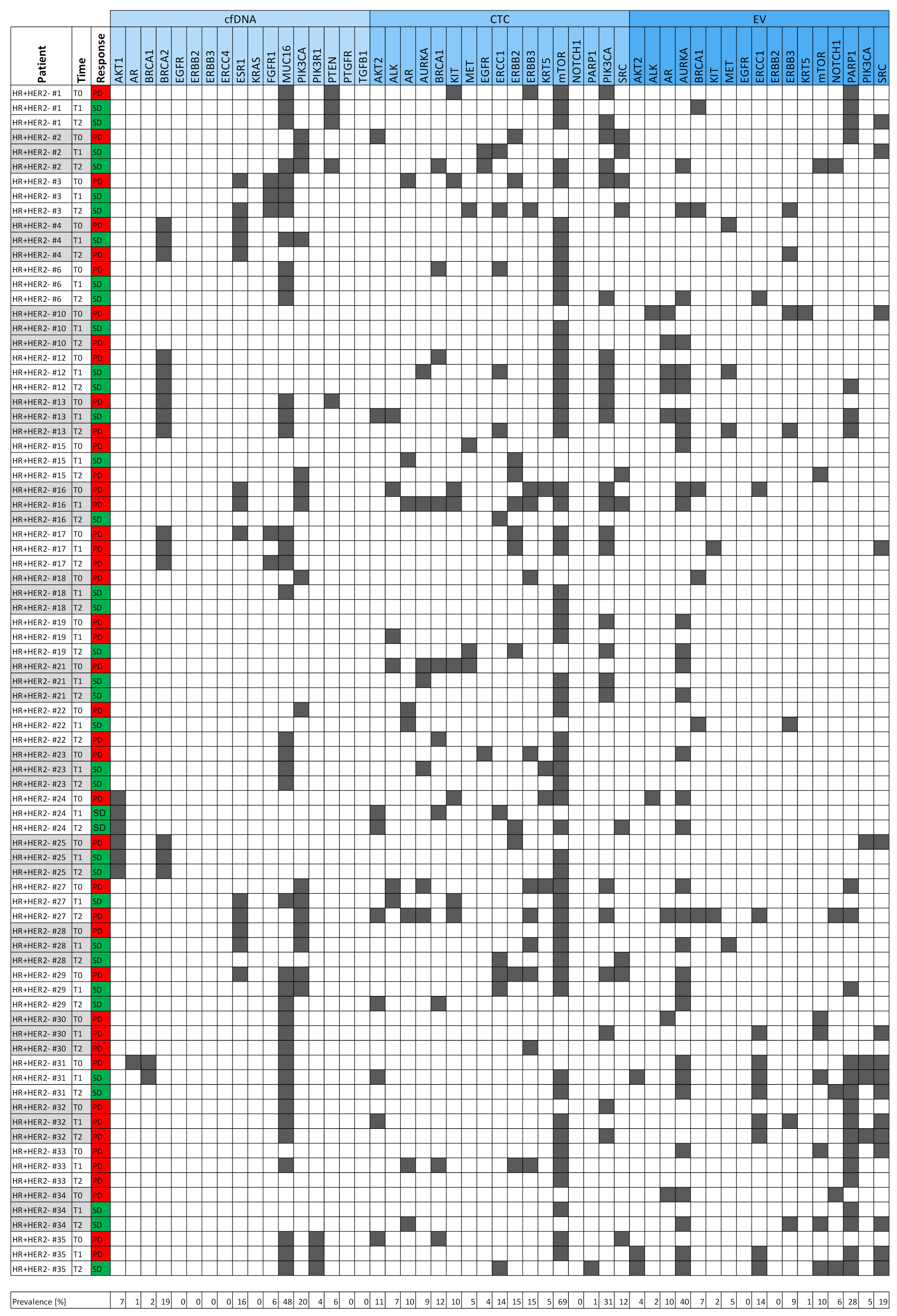
3.2. Signals as Monitoring Markers
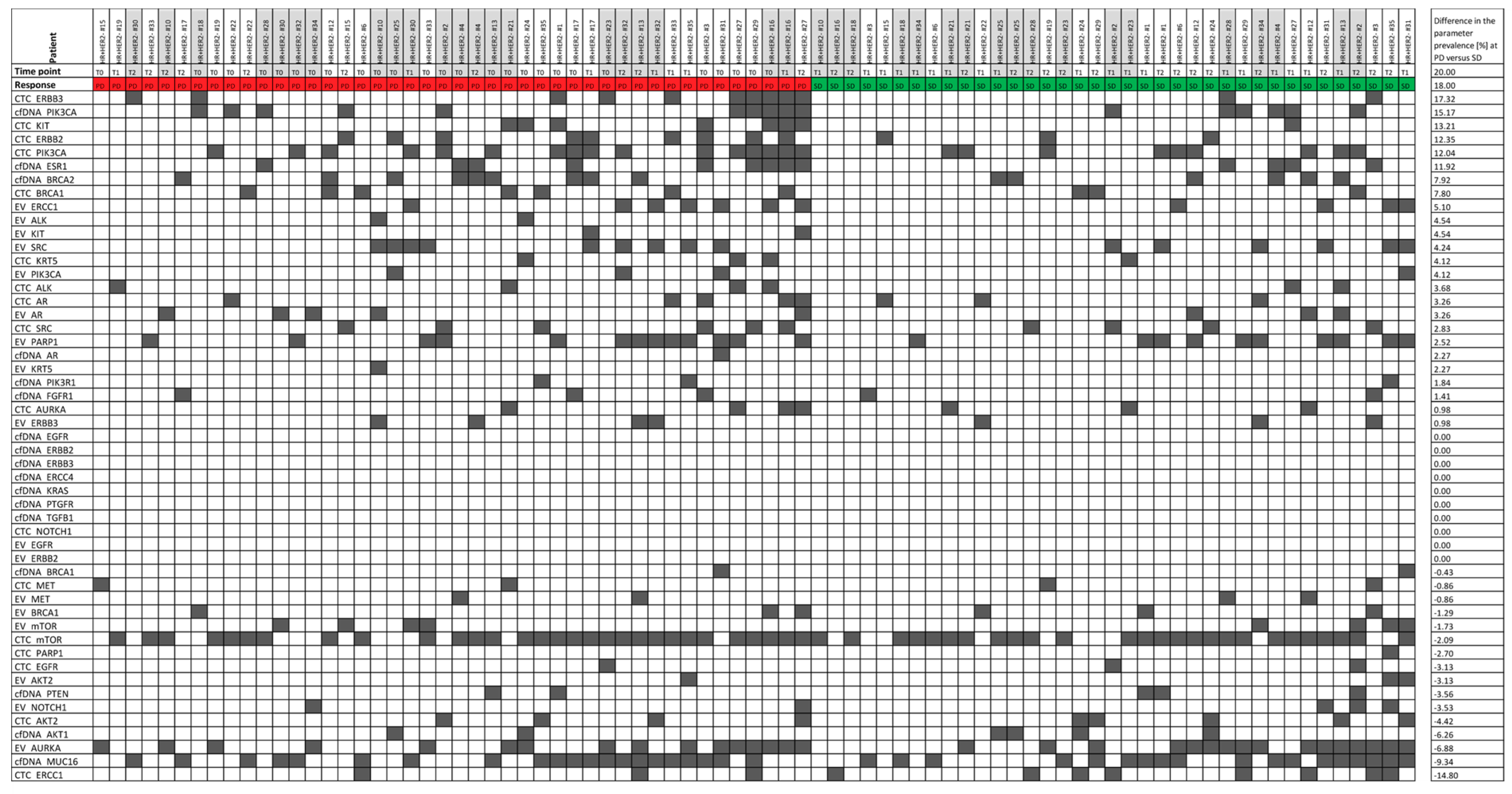
3.3. Parameter Combinations as Monitoring Markers
| EV_AURKA | OR | cfDNA_ERCC4 |
| EV_PARP | OR | cfDNA_ERCC4 |
| CTC_ERBB3 | OR | cfDNA_ERBB2 |
| CTC_ERBB3 | OR | cfDNA_ERBB3 |
| CTC_ERBB3 | OR | EV_ERBB2 |
| CTC_ERBB2 | OR | cfDNA_ERBB2 |
| CTC_ERBB2 | OR | cfDNA_ERBB3 |
| CTC_ERBB2 | OR | EV_ERBB2 |
| CTC_mTOR | OR | CTC_PIK3CA |
| CTC_PIK3CA | OR | cfDNA_PIK3CA |
| CTC_ERBB3 | OR | CTC_ERBB2 |
| cfDNA_ESR1 | OR | cfDNA_PIK3CA |
| cfDNA_AKT1 | OR | CTC_AKT2 | OR | EV_AKT2 |
| cfDNA_AR | OR | CTC_AR | OR | EV_AR |
| cfDNA_BRCA1 | OR | CTC_BRCA1 | OR | EV_BRCA1 |
| cfDNA_EGFR | OR | CTC_EGFR | OR | EV_EGFR |
| cfDNA_ERCC4 | OR | CTC_ERCC1 | OR | EV_ERCC1 |
| cfDNA_ERBB2 | OR | CTC_ERBB2 | OR | EV_ERBB2 |
| cfDNA_ERBB3 | OR | CTC_ERBB3 | OR | EV_ERBB3 |
| cfDNA_PIK3CA | OR | CTC_PIK3CA | OR | EV_PIK3CA |
3.4. Signal Appearance as Monitoring Marker
3.5. The Best Monitoring Markers
3.6. Allele Frequency Development of ESR1 and PIK3CA Variants
3.7. Six Index Patients
3.8. The Course of cfDNA PIK3CA and ESR1 Variants in Three Index Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| CDK 4/6 | Cyclin dependent kinase 4/6 |
| CEA | Carcinoembryonic antigen |
| CA 15-3 | Carcinoma antigen 15-3 |
| cfDNA | Cell-free DNA |
| CT | Computed tomography |
| CTCs | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| ΔVAF | Difference in variant allele frequencies |
| ECOG | Eastern Cooperative Oncology Group |
| ER | Estrogen receptor |
| EVs | Extracellular vesicles |
| HR | Hormone-receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| IVA | Ingenuity variant analysis |
| NGS | Next-generation sequencing |
| MRI | Magnetic resonance imaging |
| MBC | Metastatic breast cancer |
| MRD | Minimal residual disease |
| PD | Progressive disease progress |
| PFS | Progression-free survival |
| PR | Progesterone receptor |
| SD | Stable disease |
| VAF | Variant allele frequency |
| List of genes | |
| AKT1 | AKT serine/threonine kinase 1 |
| AKT2 | AKT serine/threonine kinase 2 |
| AR | Androgen receptor |
| AURKA | Aurora kinase A |
| BRCA1 | BRCA1 DNA repair associated |
| BRCA2 | BRCA2 DNA repair associated |
| EGFR | Epidermal growth factor receptor |
| EpCAM | Epithelial cell adhesion molecule |
| ERCC1 | ERCC excision repair 1, endonuclease non-catalytic subunit |
| ERCC4 | ERCC excision repair 4, endonuclease non-catalytic subunit |
| ERBB2 | Erb-b2 receptor tyrosine kinase 2 coding for the HER2 protein |
| ERBB3 | Erb-b2 receptor tyrosine kinase 3 |
| ESR1 | Estrogen receptor 1 |
| FGFR1 | Fibroblast growth factor receptor 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| KIT | KIT proto-oncogene receptor tyrosine kinase |
| KRT5 | Keratin 5 |
| KRAS | KRAS proto-oncogene, GTPase |
| MET | MET proto-oncogene, receptor tyrosine kinase |
| mTOR | Mechanistic target of rapamycin |
| MUC16 | Mucin 16, cell-surface associated |
| NOTCH1 | Notch 1 |
| PARP1 | Poly(ADP-ribose) polymerase 1 |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PIK3R1 | Phosphoinositide-3-kinase regulatory subunit 1 |
| PTEN | Phosphatase and tensin homolog |
| PTGFR | Prostaglandin F receptor |
| PTPRC | Protein tyrosine phosphatase, receptor type, C (also known as CD45) |
| SOX17 | SRY-box transcription factor 17 |
| SRC | SRC proto-oncogene, non-receptor tyrosine kinase |
| TGFB1 | Transforming growth factor beta 1 |
References
- Rossi, G.; Mu, Z.; Rademaker, A.W.; Austin, L.K.; Strickland, K.S.; Costa, R.L.B.; Nagy, R.J.; Zagonel, V.; Taxter, T.J.; Behdad, A.; et al. Cell-Free DNA and Circulating Tumor Cells: Comprehensive Liquid Biopsy Analysis in Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 560–568. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Venesio, T.; Siravegna, G.; Bardelli, A.; Sapino, A. Liquid Biopsies for Monitoring Temporal Genomic Heterogeneity in Breast and Colon Cancers. Pathobiology 2017. [Google Scholar] [CrossRef] [PubMed]
- Lianidou, E.; Pantel, K. Liquid Biopsies. Genes Chromosomes Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Budd, G.T.; Cristofanilli, M.; Ellis, M.J.; Stopeck, A.; Borden, E.; Miller, M.C.; Matera, J.; Repollet, M.; Doyle, G.V.; Terstappen, L.W.M.M.; et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006, 12, 6403–6409. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 2020, 7, 131. [Google Scholar] [CrossRef]
- Dawson, S.-J.; Tsui, D.W.Y.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.-F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef]
- Deutsch, T.M.; Stefanovic, S.; Feisst, M.; Fischer, C.; Riedel, F.; Fremd, C.; Domschke, C.; Pantel, K.; Hartkopf, A.D.; Sutterlin, M.; et al. Cut-Off Analysis of CTC Change under Systemic Therapy for Defining Early Therapy Response in Metastatic Breast Cancer. Cancers 2020, 12, 1055. [Google Scholar] [CrossRef]
- Saloustros, E.; Perraki, M.; Apostolaki, S.; Kallergi, G.; Xyrafas, A.; Kalbakis, K.; Agelaki, S.; Kalykaki, A.; Georgoulias, V.; Mavroudis, D. Cytokeratin-19 mRNA-positive circulating tumor cells during follow-up of patients with operable breast cancer: Prognostic relevance for late relapse. Breast Cancer Res 2011, 13, R60. [Google Scholar] [CrossRef]
- Stewart, C.M.; Kothari, P.D.; Mouliere, F.; Mair, R.; Somnay, S.; Benayed, R.; Zehir, A.; Weigelt, B.; Dawson, S.-J.; Arcila, M.E.; et al. The value of cell-free DNA for molecular pathology. J. Pathol. 2018, 244, 616–627. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Hrebien, S.; Morden, J.P.; Beaney, M.; Fribbens, C.; Huang, X.; Liu, Y.; Bartlett, C.H.; Koehler, M.; Cristofanilli, M.; et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat. Commun. 2018, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.; Dawson, S.-J.; Tsui, D.W.Y.; Gale, D.; Forshew, T.; Piskorz, A.M.; Parkinson, C.; Chin, S.-F.; Kingsbury, Z.; Wong, A.S.C.; et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013, 497, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Fiegl, H.; Millinger, S.; Mueller-Holzner, E.; Marth, C.; Ensinger, C.; Berger, A.; Klocker, H.; Goebel, G.; Widschwendter, M. Circulating tumor-specific DNA: A marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 2005, 65, 1141–1145. [Google Scholar] [CrossRef]
- Mastoraki, S.; Strati, A.; Tzanikou, E.; Chimonidou, M.; Politaki, E.; Voutsina, A.; Psyrri, A.; Georgoulias, V.; Lianidou, E. ESR1 Methylation: A Liquid Biopsy-Based Epigenetic Assay for the Follow-up of Patients with Metastatic Breast Cancer Receiving Endocrine Treatment. Clin. Cancer Res. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Clatot, F.; Perdrix, A.; Beaussire, L.; Lequesne, J.; Lévy, C.; Emile, G.; Bubenheim, M.; Lacaille, S.; Calbrix, C.; Augusto, L.; et al. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res 2020, 22, 56. [Google Scholar] [CrossRef]
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Tomiguchi, M.; Sueta, A.; Murakami, K.; Omoto, Y.; Iwase, H. Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget 2017, 8, 52142–52155. [Google Scholar] [CrossRef]
- Modi, S.; Andre, F.; Krop, I.E.; Saura, C.; Yamashita, T.; Kim, S.-B.; Tamura, K.; Chen, S.; Suto, F.; Kuwahara, Y.; et al. Trastuzumab deruxtecan for HER2-positive metastatic breast cancer: DESTINY-Breast01 subgroup analysis. J. Clin. Oncol. 2020, 38, 1036. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, T.; Yang, Z.; Zheng, Y.; Yu, R.; Wu, X.; Yan, J.; Shao, Y.W.; Shao, X.; Cao, W.; et al. Monitoring treatment efficacy and resistance in breast cancer patients via circulating tumor DNA genomic profiling. Mol. Genet. Genomic Med. 2020, 8, e1079. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Keup, C.; Storbeck, M.; Hauch, S.; Hahn, P.; Sprenger-Haussels, M.; Hoffmann, O.; Kimmig, R.; Kasimir-Bauer, S. Multimodal Targeted Deep Sequencing of Circulating Tumor Cells and Matched Cell-Free DNA Provides a More Comprehensive Tool to Identify Therapeutic Targets in Metastatic Breast Cancer Patients. Cancers 2020, 12, 1084. [Google Scholar] [CrossRef] [PubMed]
- Keup, C.; Mach, P.; Aktas, B.; Tewes, M.; Kolberg, H.-C.; Hauch, S.; Sprenger-Haussels, M.; Kimmig, R.; Kasimir-Bauer, S. RNA Profiles of Circulating Tumor Cells and Extracellular Vesicles for Therapy Stratification of Metastatic Breast Cancer Patients. Clin. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Keup, C.; Storbeck, M.; Hauch, S.; Hahn, P.; Sprenger-Haussels, M.; Tewes, M.; Mach, P.; Hoffmann, O.; Kimmig, R.; Kasimir-Bauer, S. Cell-Free DNA Variant Sequencing Using CTC-Depleted Blood for Comprehensive Liquid Biopsy Testing in Metastatic Breast Cancer. Cancers 2019, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Enderle, D.; Spiel, A.; Coticchia, C.M.; Berghoff, E.; Mueller, R.; Schlumpberger, M.; Sprenger-Haussels, M.; Shaffer, J.M.; Lader, E.; Skog, J.; et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS ONE 2015, 10, e0136133. [Google Scholar] [CrossRef] [PubMed]
- Keup, C.; Hahn, P.; Hauch, S.; Sprenger-Haussels, M.; Tewes, M.; Mach, P.; Bittner, A.-K.; Kimmig, R.; Kasimir-Bauer, S.; Benyaa, K. Targeted PCR-Based Deep Sequencing of cfDNA with Unique Molecular Indices by a Customized QIAseq Targeted DNA Panel; Protocols.io: Berkeley, CA, USA, 2018. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A.; et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Shirley, M. Epi proColon® for Colorectal Cancer Screening: A Profile of Its Use in the USA. Mol. Diagn. Ther. 2020, 24, 497–503. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Pereira-Veiga, T.; Martínez-Fernández, M.; Abuin, C.; Piñeiro, R.; Cebey, V.; Cueva, J.; Palacios, P.; Blanco, C.; Muinelo-Romay, L.; Abalo, A.; et al. CTCs Expression Profiling for Advanced Breast Cancer Monitoring. Cancers 2019, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shi, X.; Yuan, Y.; Peng, M.; Jin, H.; Qin, D. ERCC1 rs11615 polymorphism increases susceptibility to breast cancer: A meta-analysis of 4547 individuals. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- EL Baiomy, M.A.; El Kashef, W.F. ERCC1 Expression in Metastatic Triple Negative Breast Cancer Patients Treated with Platinum-Based Chemotherapy. Asian Pac. J. Cancer Prev. 2017, 18, 507–513. [Google Scholar] [CrossRef]
- Franken, A.; Behrens, B.; Reinhardt, F.; Yang, L.; Rivandi, M.; Cieslik, J.-P.; Dietzel, F.; Stoecklein, N.H.; Niederacher, D.; Fehm, T.; et al. A proof-of-concept study to analyze the clinical utility of a circulating tumor cell-based mutation analysis. In Proceedings of the 40 Jahrestagung der Deutschen Gesellschaft für Senologie e.V, München, Germany, 25–27 June 2020. [Google Scholar]
- Yanagita, M.; Redig, A.J.; Paweletz, C.P.; Dahlberg, S.E.; O’Connell, A.; Feeney, N.; Taibi, M.; Boucher, D.; Oxnard, G.R.; Johnson, B.E.; et al. A Prospective Evaluation of Circulating Tumor Cells and Cell-Free DNA in EGFR-Mutant Non-Small Cell Lung Cancer Patients Treated with Erlotinib on a Phase II Trial. Clin. Cancer Res. 2016, 22, 6010–6020. [Google Scholar] [CrossRef]
- Cheng, J.; Cao, Y.; MacLeay, A.; Lennerz, J.K.; Baig, A.; Frazier, R.P.; Lee, J.; Hu, K.; Pacula, M.; Meneses, E.; et al. Clinical Validation of a Cell-Free DNA Gene Panel. J. Mol. Diagn. 2019, 21, 632–645. [Google Scholar] [CrossRef]
- Keup, C.; Benyaa, K.; Hauch, S.; Sprenger-Haussels, M.; Tewes, M.; Mach, P.; Bittner, A.-K.; Kimmig, R.; Hahn, P.; Kasimir-Bauer, S. Targeted deep sequencing revealed variants in cell-free DNA of hormone receptor-positive metastatic breast cancer patients. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef]
- Chu, D.; Paoletti, C.; Gersch, C.; VanDenBerg, D.A.; Zabransky, D.J.; Cochran, R.L.; Wong, H.Y.; Toro, P.V.; Cidado, J.; Croessmann, S.; et al. ESR1 Mutations in Circulating Plasma Tumor DNA from Metastatic Breast Cancer Patients. Clin. Cancer Res. 2016, 22, 993–999. [Google Scholar] [CrossRef]
- Fribbens, C.; O’Leary, B.; Kilburn, L.; Hrebien, S.; Garcia-Murillas, I.; Beaney, M.; Cristofanilli, M.; Andre, F.; Loi, S.; Loibl, S.; et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J. Clin. Oncol. 2016, 34, 2961–2968. [Google Scholar] [CrossRef]
- Guttery, D.S.; Page, K.; Hills, A.; Woodley, L.; Marchese, S.D.; Rghebi, B.; Hastings, R.K.; Luo, J.; Pringle, J.H.; Stebbing, J.; et al. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin. Chem. 2015, 61, 974–982. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.-M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef]
- Schiavon, G.; Hrebien, S.; Garcia-Murillas, I.; Cutts, R.J.; Pearson, A.; Tarazona, N.; Fenwick, K.; Kozarewa, I.; Lopez-Knowles, E.; Ribas, R.; et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 2015, 7, 313ra182. [Google Scholar] [CrossRef] [PubMed]
- Kodahl, A.R.; Ehmsen, S.; Pallisgaard, N.; Jylling, A.M.B.; Jensen, J.D.; Laenkholm, A.-V.; Knoop, A.S.; Ditzel, H.J. Correlation between circulating cell-free PIK3CA tumor DNA levels and treatment response in patients with PIK3CA-mutated metastatic breast cancer. Mol. Oncol. 2018, 12, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Christenson, R.H.; Beck, J.; Walson, P.D. Plasma EGFR mutation testing in non-small cell lung cancer: A value proposition. Clin. Chim. Acta 2019, 495, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
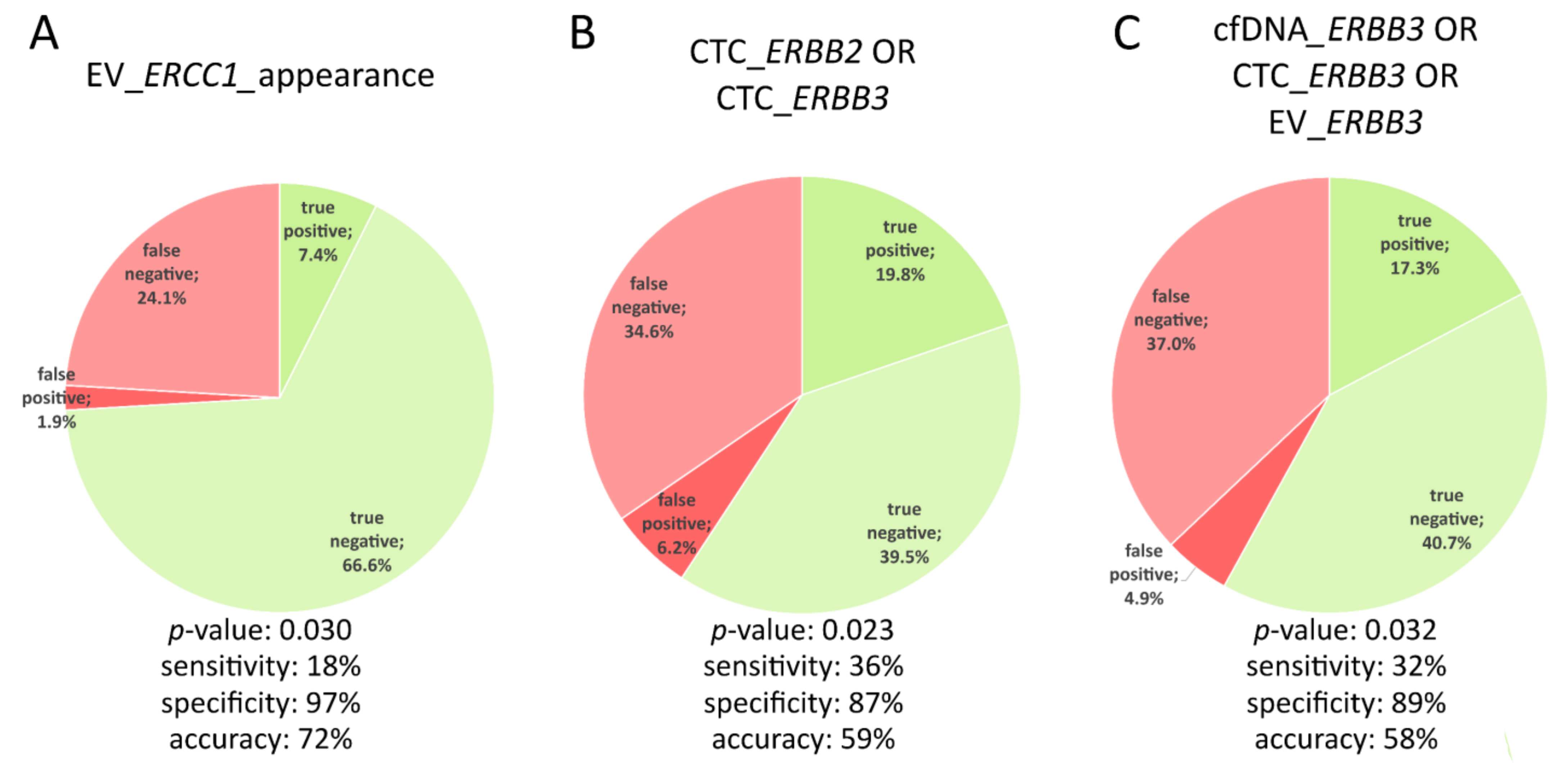
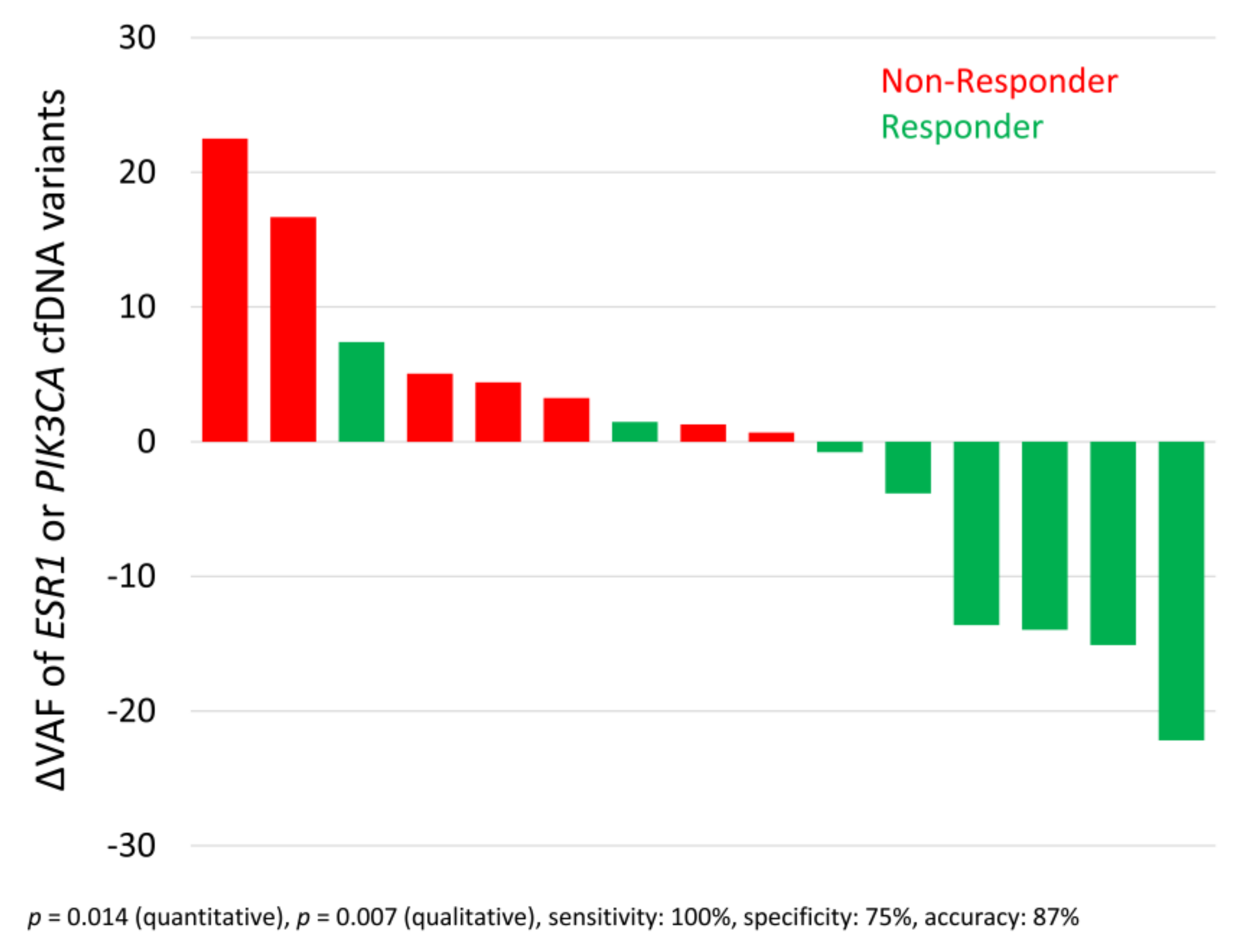
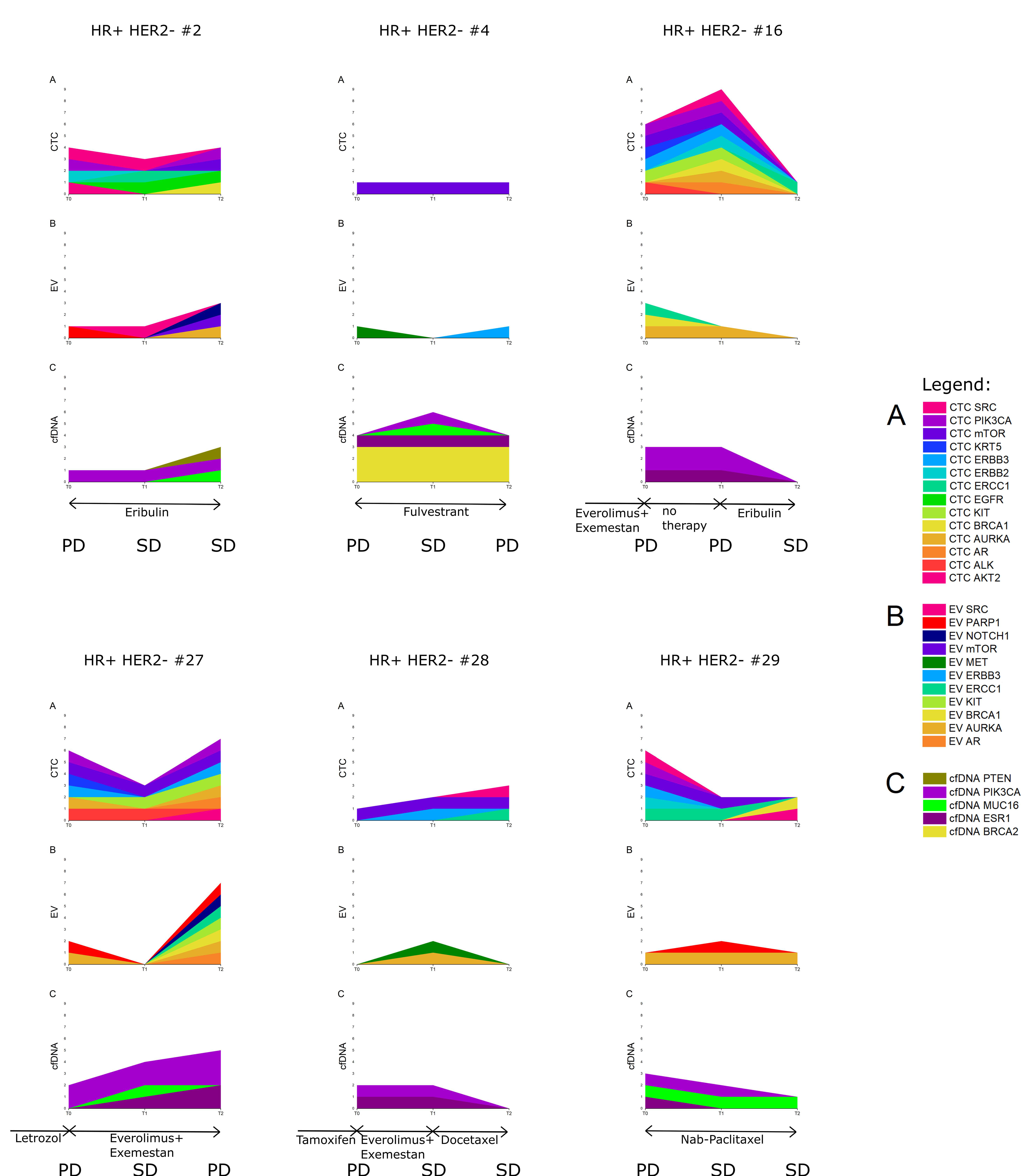
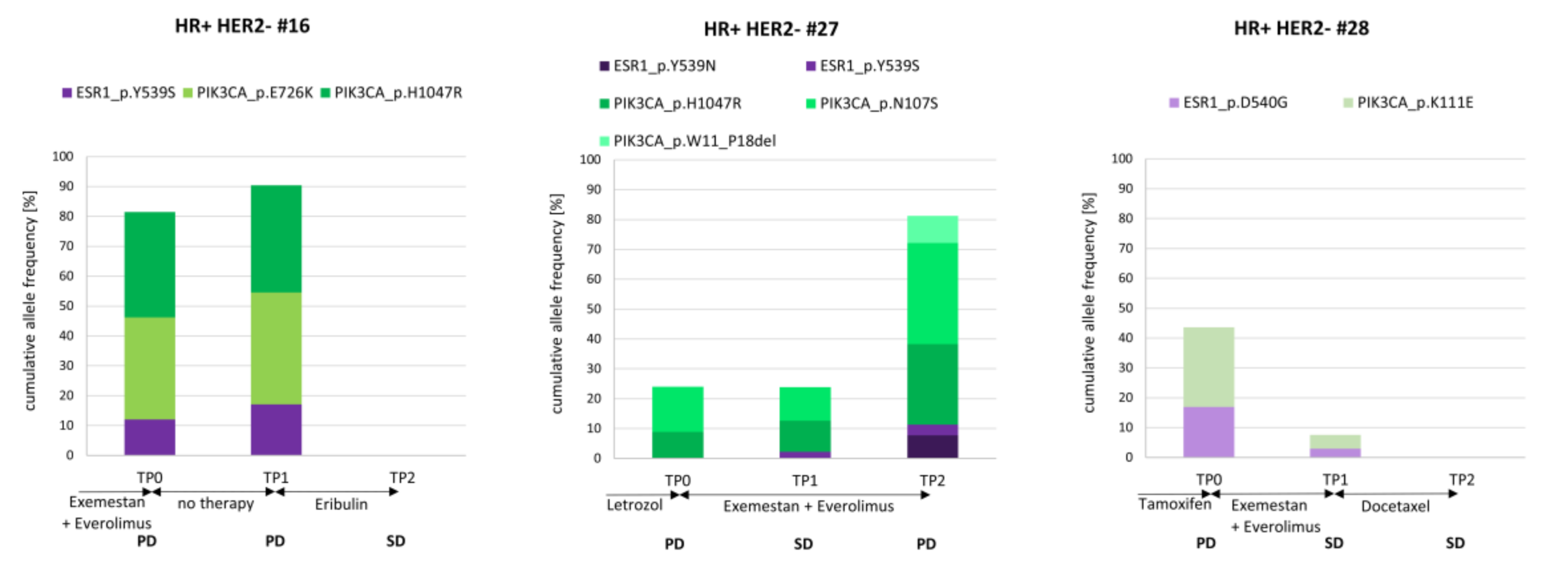
| Parameter | Sensitivity | Specificity | Accuracy | Sensitivity + Specificity + Accuracy |
|---|---|---|---|---|
| EV_ERCC1_appearance | 0.1765 | 0.9730 | 0.7222 | 1.8717 |
| EV_KIT_appearance | 0.1176 | 1.0000 | 0.7222 | 1.8399 |
| CTC_AR_appearance | 0.1765 | 0.9459 | 0.7037 | 1.8261 |
| CTC_ERBB3_appearance | 0.1765 | 0.9459 | 0.7037 | 1.8261 |
| CTC_ERBB3ORCTC_ERBB2 | 0.3636 | 0.8649 | 0.5926 | 1.8211 |
| cfDNA_PIK3CA_appearance | 0.1176 | 0.9730 | 0.7037 | 1.7943 |
| cfDNA_ERRB3ORCTC_ERBB3OREV_ERBB3 | 0.3182 | 0.8919 | 0.5802 | 1.7903 |
| CTC_BRCA1_appearance | 0.1765 | 0.9189 | 0.6852 | 1.7806 |
| EV_ERBB3_appearance | 0.1765 | 0.9189 | 0.6852 | 1.7806 |
| EV_SRC_appearance | 0.1765 | 0.9189 | 0.6852 | 1.7806 |
| cfDNA_FGFR1_appearance | 0.0588 | 1.0000 | 0.7037 | 1.7625 |
| EV_PIK3CA_appearance | 0.0588 | 1.0000 | 0.7037 | 1.7625 |
| CTC_AURKA_appearance | 0.1176 | 0.9459 | 0.6852 | 1.7488 |
| EV_AR_appearance | 0.1176 | 0.9459 | 0.6852 | 1.7488 |
| CTC_ERBB3 | 0.2273 | 0.9459 | 0.5556 | 1.7288 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keup, C.; Suryaprakash, V.; Storbeck, M.; Hoffmann, O.; Kimmig, R.; Kasimir-Bauer, S. Longitudinal Multi-Parametric Liquid Biopsy Approach Identifies Unique Features of Circulating Tumor Cell, Extracellular Vesicle, and Cell-Free DNA Characterization for Disease Monitoring in Metastatic Breast Cancer Patients. Cells 2021, 10, 212. https://doi.org/10.3390/cells10020212
Keup C, Suryaprakash V, Storbeck M, Hoffmann O, Kimmig R, Kasimir-Bauer S. Longitudinal Multi-Parametric Liquid Biopsy Approach Identifies Unique Features of Circulating Tumor Cell, Extracellular Vesicle, and Cell-Free DNA Characterization for Disease Monitoring in Metastatic Breast Cancer Patients. Cells. 2021; 10(2):212. https://doi.org/10.3390/cells10020212
Chicago/Turabian StyleKeup, Corinna, Vinay Suryaprakash, Markus Storbeck, Oliver Hoffmann, Rainer Kimmig, and Sabine Kasimir-Bauer. 2021. "Longitudinal Multi-Parametric Liquid Biopsy Approach Identifies Unique Features of Circulating Tumor Cell, Extracellular Vesicle, and Cell-Free DNA Characterization for Disease Monitoring in Metastatic Breast Cancer Patients" Cells 10, no. 2: 212. https://doi.org/10.3390/cells10020212
APA StyleKeup, C., Suryaprakash, V., Storbeck, M., Hoffmann, O., Kimmig, R., & Kasimir-Bauer, S. (2021). Longitudinal Multi-Parametric Liquid Biopsy Approach Identifies Unique Features of Circulating Tumor Cell, Extracellular Vesicle, and Cell-Free DNA Characterization for Disease Monitoring in Metastatic Breast Cancer Patients. Cells, 10(2), 212. https://doi.org/10.3390/cells10020212






