Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology
Abstract
1. Introduction
1.1. Secondary Mitochondrial Cascades in Traumatic Brain Injury
1.2. Role of mPT in TBI Pathophysiology
1.3. Therapeutic Targeting of mPT by Cyclosporin A
1.4. Therapeutic Targeting of mPT by NIM811
1.5. The Effects of CypD Knockout (KO) in Neurodegenerative Diseases
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Controlled Cortical Impact
2.3. Mitochondrial Isolation and Respirometry Analysis
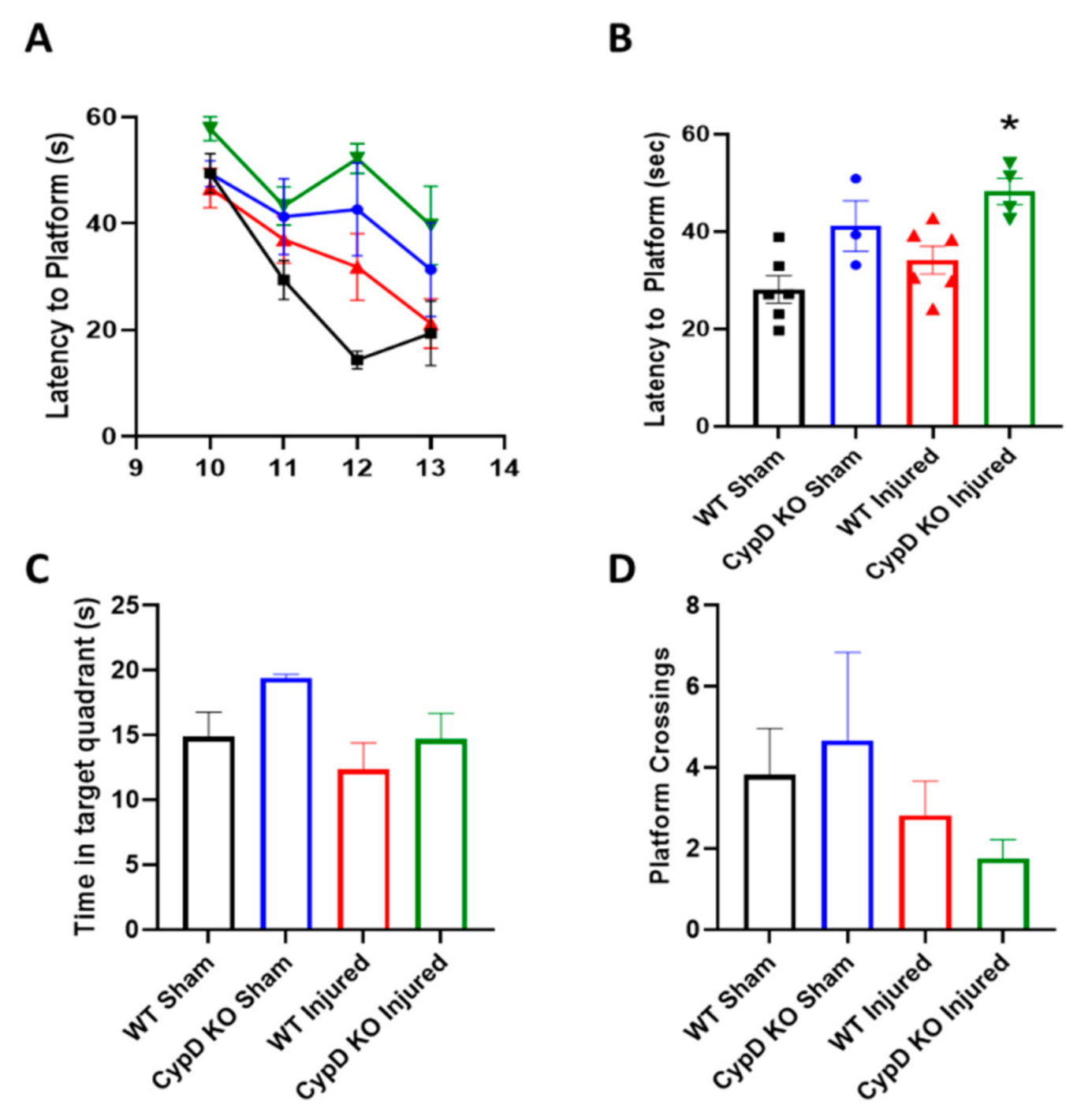
2.4. Morris Water Maze
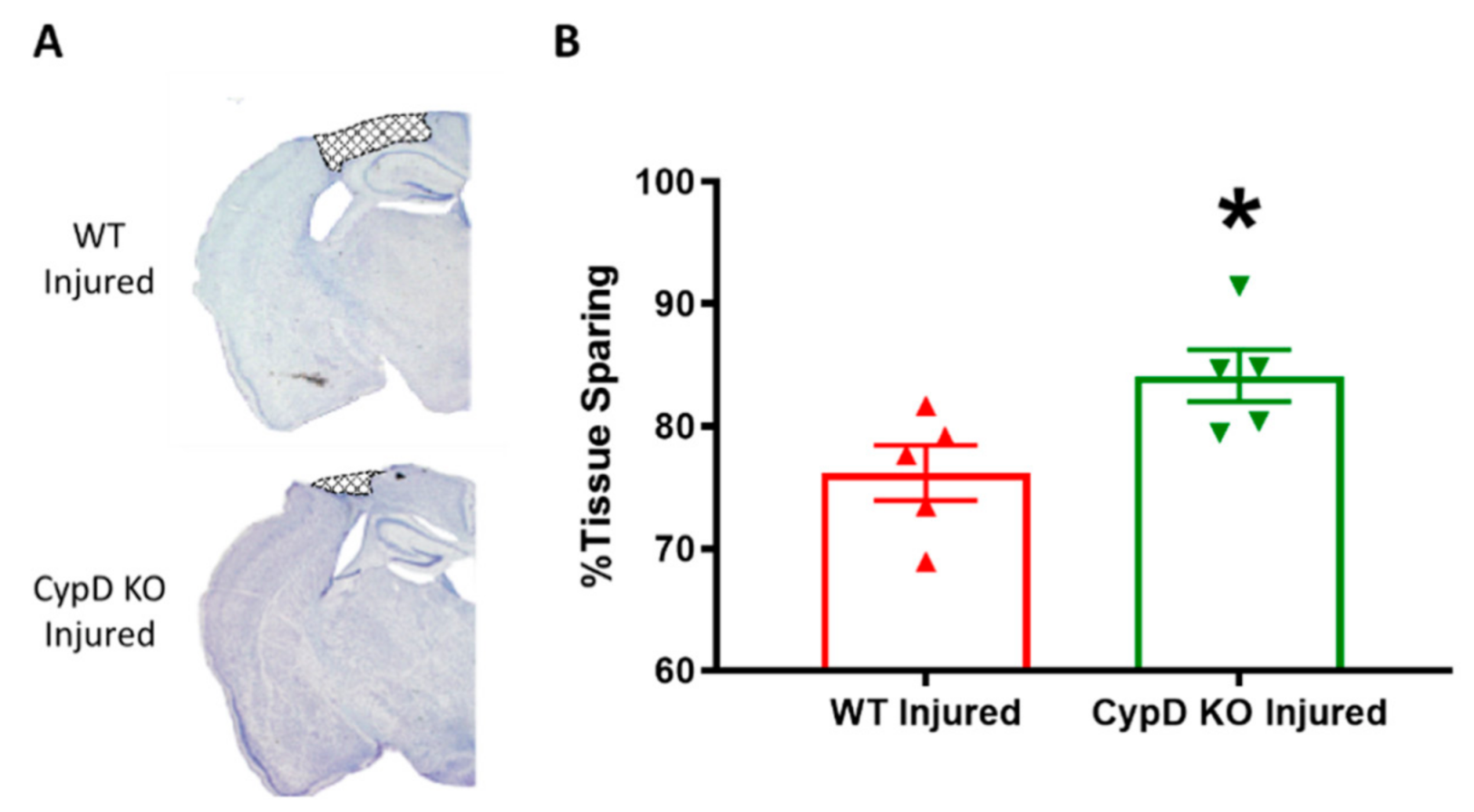
2.5. Tissue Processing and Tissue Sparing Measurement
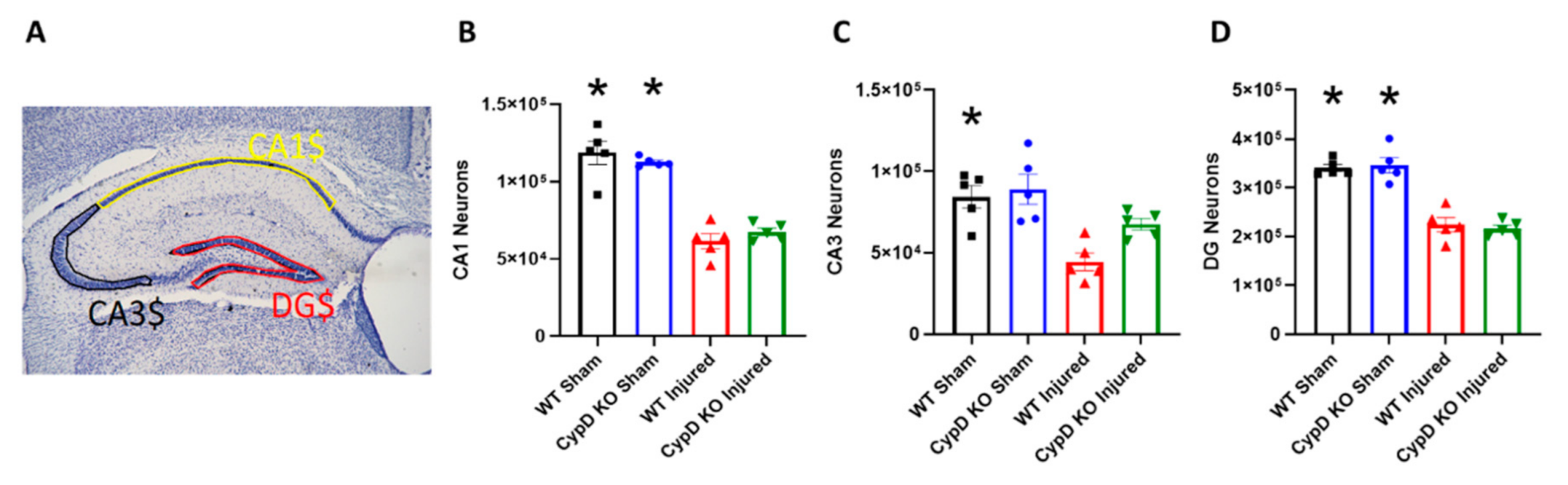
2.6. Stereology—Hippocampal Cell Counts
2.7. Statistics
3. Results
3.1. Cyclophilin D Knockout Improves Mitochondrial Bioenergetics
3.2. Cyclophilin D Knockout Alleviates TBI-Related Reduction of CA3 Hippocampal Neurons
3.3. Cyclophilin D knockout Increases Tissue Sparing Following TBI
3.4. Cyclophilin D Knockout Does Not Improve Cognition Following TBI
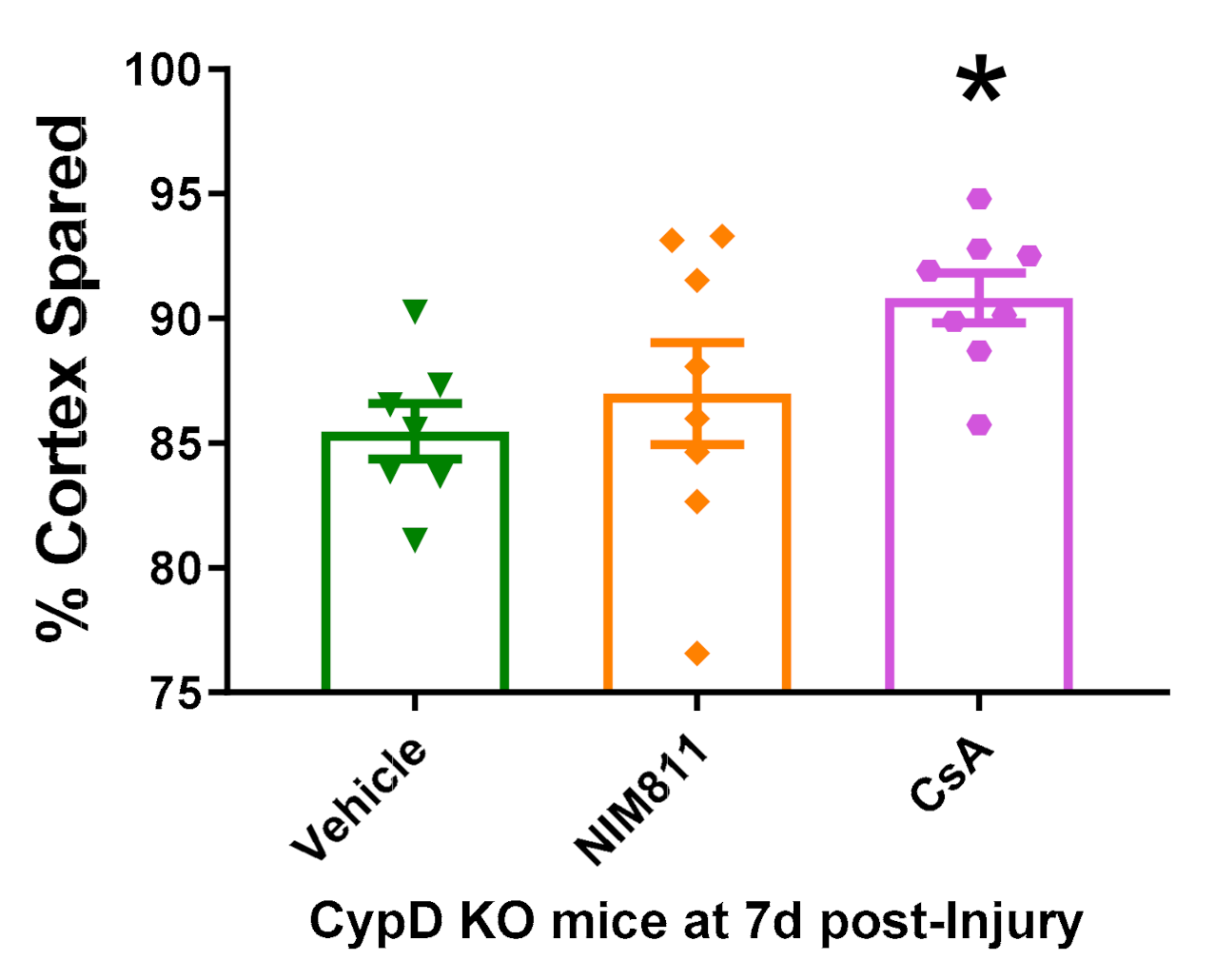
3.5. Cyclosporin A Provides Neuroprotection in CypD KO Mice after TBI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. TBI Data and Statistics. Available online: https://www.cdc.gov/traumaticbraininjury/data/ (accessed on 26 October 2020).
- Yonutas, H.; Vekaria, H.; Sullivan, P.G. Mitochondrial specific therapeutic targets following brain injury. Brain Res. 2016, 1640, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Kulbe, J.R.; Hill, R.L.; Singh, I.N.; Wang, J.A.; Hall, E.D. Synaptic Mitochondria Sustain More Damage than Non-Synaptic Mitochondria after Traumatic Brain Injury and Are Protected by Cyclosporine A. J. Neurotrauma 2017, 34, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.E.; Azbill, D.; Knapp, P.E. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat. Med. 1999, 5, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Keller, J.N.; Bussen, W.L.; Scheff, S.W. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002, 949, 88–96. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Vekaria, H.J.; Sullivan, P.G. Chapter 17—Mitochondrial drug delivery systems: Therapeutic application for clinical bioenergetics in neurodegenerative disease. In Clinical Bioenergetics; Ostojic, S., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 385–409. [Google Scholar] [CrossRef]
- Crompton, M.; Virji, S.; Ward, J.M. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. JBIC J. Biol. Inorg. Chem. 1998, 258, 729–735. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Davidson, A.M. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 1990, 268, 153–160. [Google Scholar] [CrossRef]
- Neginskaya, M.A.; Solesio, M.E.; Berezhnaya, E.V.; Amodeo, G.F.; Mnatsakanyan, N.; Jonas, E.A.; Pavlov, E.V. ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel but Lack the Permeability Transition Pore. Cell Rep. 2019, 26, 11–17. [Google Scholar] [CrossRef]
- Karch, J.M.; Bround, M.J.; Khalil, H.; Sargent, M.A.; Latchman, N.; Terada, N.; Peixoto, P.; Molkentin, J.D. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 2019, 5, eaaw4597. [Google Scholar] [CrossRef]
- Krauskopf, A.; Eriksson, O.; Craigen, W.J.; Forte, M.A.; Bernardi, P. Properties of the permeability transition in VDAC1−/−mitochondria. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 590–595. [Google Scholar] [CrossRef]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Wallace, D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nat. Cell Biol. 2004, 427, 461–465. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Llaguno, M.C.; Yang, Y.; Yan, Y.; Weber, J.; Sigworth, F.J.; Jonas, E.A. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; Bisetto, E.; Soriano, M.E.; Dabbeni-Sala, F.; Basso, E.; Petronilli, V.; Forte, M.A.; Bernardi, P.; Lippe, G. Cyclophilin D Modulates Mitochondrial F0F1-ATP Synthase by Interacting with the Lateral Stalk of the Complex. J. Biol. Chem. 2009, 284, 33982–33988. [Google Scholar] [CrossRef] [PubMed]
- Urbani, A.; Giorgio, V.; Carrer, A.; Franchin, C.; Arrigoni, G.; Jiko, C.; Abe, K.; Maeda, S.; Shinzawa-Itoh, K.; Bogers, J.F.M.; et al. Purified F-ATP synthase forms a Ca2+-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 9086–9091. [Google Scholar] [CrossRef]
- He, J.; Ford, H.C.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 3409–3414. [Google Scholar] [CrossRef]
- Zhou, W.; Marinelli, F.; Nief, C.; Faraldo-Gómez, J.D. Atomistic simulations indicate the c-subunit ring of the F1Fo ATP synthase is not the mitochondrial permeability transition pore. eLife 2017, 6, 10580. [Google Scholar] [CrossRef]
- Carroll, J.; He, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase. Proc. Natl. Acad. Sci. USA 2019, 116, 12816–12821. [Google Scholar] [CrossRef]
- Tanveer, A.; Virji, S.; Andreeva, L.; Totty, N.F.; Hsuan, J.J.; Ward, J.M.; Crompton, M. Involvement of Cyclophilin D in the Activation of A mitochondrial Pore by Ca2+ and Oxidant Stress. JBIC J. Biol. Inorg. Chem. 1996, 238, 166–172. [Google Scholar] [CrossRef]
- Connern, C.P.; Halestrap, A.P. Purification and N-terminal sequencing of peptidyl-prolyl cis-trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem. J. 1992, 284, 381–385. [Google Scholar] [CrossRef]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nat. Cell Biol. 2005, 434, 652–658. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Ii, G.W.D.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nat. Cell Biol. 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Fournier, N.; Ducet, G.; Crevat, A. Action of cyclosporine on mitochondrial calcium fluxes. J. Bioenerg. Biomembr. 1987, 19, 297–303. [Google Scholar] [CrossRef]
- Scheff, S.W.; Sullivan, P.G.; Sullivan, S.W.S.G. Cyclosporin A Significantly Ameliorates Cortical Damage Following Experimental Traumatic Brain Injury in Rodents. J. Neurotrauma 1999, 16, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C.; Sullivan, P.G.; Thompson, M.B.; Scheff, S.W.P.; Mattson, M.P. Cyclosporin Ameliorates Traumatic Brain-Injury-Induced Alterations of Hippocampal Synaptic Plasticity. Exp. Neurol. 2000, 162, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, B.; Rice, A.C.; Levasseur, J.; de Ford, M.; Hamm, R.J.; Bullock, M.R. Cyclosporin A Improves Brain Tissue Oxygen Consumption and Learning/Memory Performance after Lateral Fluid Percussion Injury in Rats. J. Neurotrauma 2002, 19, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Büki, A.; Okonkwo, D.O.; Povlishock, J.T. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma 1999, 16, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, D.O.; Büki, A.; Siman, R.; Povlishock, J.T. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. NeuroReport 1999, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, D.O.; Povlishock, J.T. An Intrathecal Bolus of Cyclosporin a before Injury Preserves Mitochondrial Integrity and Attenuates Axonal Disruption in Traumatic Brain Injury. Br. J. Pharmacol. 1999, 19, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Kilbaugh, T.J.; Bhandare, S.; Lorom, D.H.; Saraswati, M.; Robertson, C.L.; Margulies, S.S. Cyclosporin A Preserves Mitochondrial Function after Traumatic Brain Injury in the Immature Rat and Piglet. J. Neurotrauma 2011, 28, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.S.; Kilbaugh, T.; Sullivan, S.; Smith, C.; Propert, K.; Byro, M.; Saliga, K.; Costine, B.A.; Duhaime, A.-C. Establishing a Clinically Relevant Large Animal Model Platform for TBI Therapy Development: Using Cyclosporin A as a Case Study. Brain Pathol. 2015, 25, 289–303. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Thompson, M.; Scheff, S.W. Continuous Infusion of Cyclosporin a Postinjury Significantly Ameliorates Cortical Damage Following Traumatic Brain Injury. Exp. Neurol. 2000, 161, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Thompson, M.B.; Scheff, S.W. Cyclosporin A Attenuates Acute Mitochondrial Dysfunction Following Traumatic Brain Injury. Exp. Neurol. 1999, 160, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Sebastian, A.H.; Hall, E.D. Therapeutic Window Analysis of the Neuroprotective Effects of Cyclosporine A after Traumatic Brain Injury. J. Neurotrauma 2011, 28, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Riess, P.; Bareyre, F.M.; Saatman, K.; Cheney, J.; Lifshitz, J.; Raghupathi, R.; Grady, M.S.; Neugebauer, E.; McIntosh, T.K. Effects of chronic, post-injury Cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor. Neurol. Neurosci. 2001, 18, 1–8. [Google Scholar]
- Berden, J.; Hoitsma, A.; Merx, J.; Keyser, A. Severe central-nervous-system toxicity associated with cyclosporin. Lancet 1985, 325, 219–220. [Google Scholar] [CrossRef]
- de Groen, P.C.; Aksamit, A.J.; Rakela, J.; Forbes, G.S.; Krom, R.A.F. Central Nervous System Toxicity after Liver Transplantation. N. Engl. J. Med. 1987, 317, 861–866. [Google Scholar] [CrossRef]
- Hatton, J.; Rosbolt, B.; Empey, P.; Kryscio, R.; Young, B. Dosing and safety of cyclosporine in patients with severe brain injury. J. Neurosurg. 2008, 109, 699–707. [Google Scholar] [CrossRef]
- Mazzeo, A.T.; Brophy, G.M.; Gilman, C.B.; Alves-Óscar, L.; Robles, J.R.; Hayes, R.L.; Povlishock, J.T.; Bullock, M.R. Safety and Tolerability of Cyclosporin A in Severe Traumatic Brain Injury Patients: Results from a Prospective Randomized Trial. J. Neurotrauma 2009, 26, 2195–2206. [Google Scholar] [CrossRef]
- Rabchevsky, A.G.; Fugaccia, I.; Sullivan, P.G.; Scheff, S.W. Cyclosporin A Treatment Following Spinal Cord Injury to the Rat: Behavioral Effects and Stereological Assessment of Tissue Sparing. J. Neurotrauma 2001, 18, 513–522. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rabchevsky, A.G.; Keller, J.N.; Lovell, M.; Sodhi, A.; Hart, R.P.; Scheff, S.W. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 2004, 474, 524–534. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rabchevsky, A.; Waldmeier, P.; Springer, J. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J. Neurosci. Res. 2004, 79, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Waldmeier, P.C.; Zimmermann, K.; Qian, T.; Tintelnot-Blomley, M.; Lemasters, J.J. Cyclophilin D as a Drug Target. Curr. Med. Chem. 2003, 10, 1485–1506. [Google Scholar] [CrossRef] [PubMed]
- Waldmeier, P.C.; Feldtrauer, J.-J.; Qian, T.; Lemasters, J.J. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 2002, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Readnower, R.D.; Pandya, J.D.; McEwen, M.L.; Pauly, J.R.; Springer, J.E.; Sullivan, P.G. Post-Injury Administration of the Mitochondrial Permeability Transition Pore Inhibitor, NIM811, Is Neuroprotective and Improves Cognition after Traumatic Brain Injury in Rats. J. Neurotrauma 2011, 28, 1845–1853. [Google Scholar] [CrossRef]
- Mbye, L.H.A.N.; Singh, I.N.; Carrico, K.M.; Saatman, K.E.; Hall, E.D. Comparative Neuroprotective Effects of Cyclosporin a and NIM811, a Nonimmunosuppressive Cyclosporin a Analog, following Traumatic Brain Injury. Br. J. Pharmacol. 2008, 29, 87–97. [Google Scholar] [CrossRef]
- Mbye, L.; Singh, I.; Sullivan, P.; Springer, J.; Hall, E. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 2008, 209, 243–253. [Google Scholar] [CrossRef]
- Springer, J.E.; Visavadiya, N.P.; Sullivan, P.G.; Hall, E.D. Post-Injury Treatment with NIM811 Promotes Recovery of Function in Adult Female Rats after Spinal Cord Contusion: A Dose-Response Study. J. Neurotrauma 2018, 35, 492–499. [Google Scholar] [CrossRef]
- McEwen, M.L.; Sullivan, P.G.; Springer, J.E. Pretreatment with the Cyclosporin Derivative, NIM811, Improves the Function of Synaptic Mitochondria following Spinal Cord Contusion in Rats. J. Neurotrauma 2007, 24, 613–624. [Google Scholar] [CrossRef]
- Ravikumar, R.; McEwen, M.L.; Springer, J.E. Post-Treatment with the Cyclosporin Derivative, NIM811, Reduced Indices of Cell Death and Increased the Volume of Spared Tissue in the Acute Period following Spinal Cord Contusion. J. Neurotrauma 2007, 24, 1618–1630. [Google Scholar] [CrossRef]
- Nicolli, A.; Basso, E.; Petronilli, V.; Wenger, R.M.; Bernardi, P. Interactions of Cyclophilin with the Mitochondrial Inner Membrane and Regulation of the Permeability Transition Pore, a Cyclosporin A-sensitive Channel. J. Biol. Chem. 1996, 271, 2185–2192. [Google Scholar] [CrossRef]
- Basso, E.; Fante, L.; Fowlkes, J.; Petronilli, V.; Forte, M.A.; Bernardi, P. Properties of the Permeability Transition Pore in Mitochondria Devoid of Cyclophilin D. J. Biol. Chem. 2005, 280, 18558–18561. [Google Scholar] [CrossRef] [PubMed]
- Gainutdinov, T.; Molkentin, J.D.; Siemen, D.; Ziemer, M.; Debska-Vielhaber, G.; Vielhaber, S.; Gizatullina, Z.; Orynbayeva, Z.; Gellerich, F.N. Knockout of cyclophilin D in Ppif−/− mice increases stability of brain mitochondria against Ca2+ stress. Arch. Biochem. Biophys. 2015, 579, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, V.; Brustovetsky, T.; Brustovetsky, N. Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp. Neurol. 2009, 218, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, W.B.; Harwood, C.L.; Geisler, J.G.; Vekaria, H.J.; Sullivan, P.G. Mitochondrial uncoupling prodrug improves tissue sparing, cognitive outcome, and mitochondrial bioenergetics after traumatic brain injury in male mice. J. Neurosci. Res. 2018, 96, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Bryant, Y.D.; Cho, W.; Sullivan, P.G. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 2008, 25, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Yonutas, H.M.; Hubbard, W.B.; Pandya, J.D.; Vekaria, H.J.; Geldenhuys, W.J.; Sullivan, P.G. Bioenergetic restoration and neuroprotection after therapeutic targeting of mitoNEET: New mechanism of pioglitazone following traumatic brain injury. Exp. Neurol. 2020, 327, 113243. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Harwood, C.L.; Prajapati, P.; Springer, J.E.; Saatman, K.E.; Sullivan, P.G. Fractionated mitochondrial magnetic separation for isolation of synaptic mitochondria from brain tissue. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Joseph, B.; Spry, M.; Vekaria, H.J.; Saatman, K.E.; Sullivan, P.G.; Sulllivan, P.G. Acute Mitochondrial Impairment Underlies Prolonged Cellular Dysfunction after Repeated Mild Traumatic Brain Injuries. J. Neurotrauma 2019, 36, 1252–1263. [Google Scholar] [CrossRef]
- Vekaria, H.J.; Hubbard, W.B.; Scholpa, N.E.; Spry, M.L.; Gooch, J.L.; Prince, S.J.; Schnellmann, R.G.; Sullivan, P.G. Formoterol, a β2-adrenoreceptor agonist, induces mitochondrial biogenesis and promotes cognitive recovery after traumatic brain injury. Neurobiol. Dis. 2020, 140, 104866. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, F.; Zou, X.; Torbey, M.T. Comparison of unbiased estimation of neuronal number in the rat hippocampus with different staining methods. J. Neurosci. Methods 2015, 254, 73–79. [Google Scholar] [CrossRef]
- Baldwin, S.A.; Gibson, T.; Callihan, C.T.; Sullivan, P.G.; Palmer, E.; Scheff, S.W. Neuronal Cell Loss in the CA3 Subfield of the Hippocampus Following Cortical Contusion Utilizing the Optical Disector Method for Cell Counting. J. Neurotrauma 1997, 14, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Kirino, T.; Sano, K. Fine structural nature of delayed neuronal death following ischemia in the gerbil hippocampus. Acta Neuropathol. 1984, 62, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Davolio, C.; Greenamyre, J.T. Selective vulnerability of the CA1 region of hippocampus to the indirect excitotoxic effects of malonic acid. Neurosci. Lett. 1995, 192, 29–32. [Google Scholar] [CrossRef]
- Mao, H.; Elkin, B.S.; Genthikatti, V.V.; Morrison, B.; Yang, K.H. Why Is CA3 More Vulnerable Than CA1 in Experimental Models of Controlled Cortical Impact-Induced Brain Injury? J. Neurotrauma 2013, 30, 1521–1530. [Google Scholar] [CrossRef]
- Hånell, A.; Greer, J.E.; McGinn, M.J.; Povlishock, J.T. Traumatic brain injury-induced axonal phenotypes react differently to treatment. Acta Neuropathol. 2014, 129, 317–332. [Google Scholar] [CrossRef]
- Hazelton, J.L.; Petrasheuskaya, M.; Fiskum, G.; Kristián, T. Cyclophilin D is expressed predominantly in mitochondria of γ-aminobutyric acidergic interneurons. J. Neurosci. Res. 2008, 87, 1250–1259. [Google Scholar] [CrossRef]
- Florian, C.; Roullet, P. Hippocampal CA3-region is crucial for acquisition and memory consolidation in Morris water maze task in mice. Behav. Brain Res. 2004, 154, 365–374. [Google Scholar] [CrossRef]
- Mouri, A.; Noda, Y.; Shimizu, S.; Tsujimoto, Y.; Nabeshima, T. The role of Cyclophilin D in learning and memory. Hippocampus 2009, 20, 293–304. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Fang, F.; Chen, D.; Sosunov, A.A.; McKhann, G.M.; Yan, Y.; Wang, C.; Zhang, H.; Molkentin, J.D.; et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008, 14, 1097–1105. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Zhang, W.; Rydzewska, M.; Yan, S.S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging 2011, 32, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Carlsson, Y.; Basso, E.; Zhu, C.; Rousset, C.I.; Rasola, A.; Johansson, B.R.; Blomgren, K.; Mallard, C.; Bernardi, P.; et al. Developmental Shift of Cyclophilin D Contribution to Hypoxic-Ischemic Brain Injury. J. Neurosci. 2009, 29, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Gauba, E.; Guo, L.; Du, H. Cyclophilin D Promotes Brain Mitochondrial F1FO ATP Synthase Dysfunction in Aging Mice. J. Alzheimer’s Dis. 2016, 55, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.; Brooks, W.; Vukas, R.; Pierce, J.; Harris, J.L. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Readnower, R.D.; Hubbard, W.B.; Kalimon, O.J.; Geddes, J.W.; Sullivan, P.G. Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology. Cells 2021, 10, 199. https://doi.org/10.3390/cells10020199
Readnower RD, Hubbard WB, Kalimon OJ, Geddes JW, Sullivan PG. Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology. Cells. 2021; 10(2):199. https://doi.org/10.3390/cells10020199
Chicago/Turabian StyleReadnower, Ryan D., William Brad Hubbard, Olivia J. Kalimon, James W. Geddes, and Patrick G. Sullivan. 2021. "Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology" Cells 10, no. 2: 199. https://doi.org/10.3390/cells10020199
APA StyleReadnower, R. D., Hubbard, W. B., Kalimon, O. J., Geddes, J. W., & Sullivan, P. G. (2021). Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology. Cells, 10(2), 199. https://doi.org/10.3390/cells10020199





