Abstract
Stroke remains the number one cause of morbidity in the United States. Within weeks to months after an ischemic event, there is a resolution of inflammation and evidence of neurogenesis; however, years following a stroke, there is evidence of chronic inflammation in the central nervous system, possibly by the persistence of an autoimmune response to brain antigens as a result of ischemia. The mechanisms underlying the involvement of macrophage and microglial activation after stroke are widely acknowledged as having a role in ischemic stroke pathology; thus, modulating inflammation and neurological recovery is a hopeful strategy for treating the long-term outcomes after ischemic injury. Current treatments fail to provide neuroprotective or neurorestorative benefits after stroke; therefore, to ameliorate brain injury-induced deficits, therapies must alter both the initial response to injury and the subsequent inflammatory process. This review will address differences in macrophage and microglia nomenclature and summarize recent work in elucidating the mechanisms of macrophage and microglial participation in antigen presentation, neuroprotection, angiogenesis, neurogenesis, synaptic remodeling, and immune modulating strategies for treating the long-term outcomes after ischemic injury.
Keywords:
stroke; macrophage; microglia; neurogenesis; neuroprotection; inflammation; transplantation; therapies 1. Introduction
In the United States, stroke is the number one cause of morbidity and the third highest cause of death. It is estimated that over 800,000 people have a stroke each year, equating more than one stroke-per-minute. Of these 800,000 strokes, 87% are ischemic in nature, resulting from a clot or mass blocking a blood vessel, and 13% are hemorrhagic caused by a weakened blood vessel that ruptures [1]. Immediately after a stroke, damage-associated molecular patterns (DAMPs) activate microglia. At the injury site, cytokines and chemokines produced by activated microglia, endothelial cells, and astrocytes allow for transendothelial migration of monocytes and macrophages through the compromised blood–brain barrier (BBB) [2]. The ensuing neuroinflammation causes motor and cognitive deficits and are linked to increased risk of developing neurodegenerative disorders and chronic encephalopathy.
Despite decades of research, tissue plasminogen activator (tPA) remains the only drug approved by the U.S. Food and Drug Administration for treating stroke. Effective only if administered 3–4.5 h after the onset of stroke, the vast majority of patients are not able to receive tPA [3]. Advances in endovascular approaches such as mechanical thrombectomy have shown to be effective at reducing post-stroke disability in patients when treated within the first 8 h of symptom onset, but only a small number of patients are eligible to receive this treatment [4]. There is a need for therapies that offer neuroprotective and neurorestorative benefits within the limitation of narrow treatment windows.
Current treatments fail to provide neuroprotective or neurorestorative benefits; therefore, to ameliorate brain injury-induced deficits, therapies must target both the initial response to injury and the subsequent inflammatory process [5]. Recent data from experimental and clinical stroke studies have further elucidated the complex pathophysiology of ischemic stroke. The mechanisms underlying the involvement of microglia and macrophages in both neuroprotection and neurogenesis after stroke are widely acknowledged as having a role in ischemic stroke pathology [6,7,8,9] Since microglia and macrophages are regarded as major players in the pathological progression of ischemic stroke, modulating inflammation and neurological recovery is a hopeful strategy for treating the long-term outcomes after ischemic injury.
2. Nomenclature
2.1. Microglial Classification
Microglia are the resident macrophages in the central nervous system (CNS) and are a part of the mononuclear phagocyte system. Similar to other tissue-specific macrophages of embryonic origin, microglia originate from the yolk sack and are established approximately at the same time as neurons during the early prenatal period, where they participate in the developmental maintenance of neurons throughout life [10]. However, unlike peripheral macrophages, microglia are the only adult population with an early erythroid myeloid progenitor origin, which originate in the yolk sac [11]. Due to the maturation of the BBB, the CNS does not receive additional precursors from postnatal hematopoiesis; therefore, microglia remain yolk sac-derived myeloid cells exclusively in the CNS [12,13,14].
2.2. Macrophage Classification
Macrophages that reside in the CNS-border, which includes the choroid plexus, perivascular spaces, and subdural meninges, express distinct transcriptional signatures from microglia and infiltrating macrophages [15,16]. Through fluorescence-activated cell sorting, other microglia-specific genes have been identified as Tmem119, P2ry12, and P2ry13 [17], while systemic macrophage-specific genes include CD163 and Fabp4. Under physiological conditions, macrophages, except microglia and monocytes, express CD163 [18]. Different inflammatory signals will either upregulate (anti-inflammatory) or downregulate (pro-inflammatory) CD163 expression [19], in which CD163+ cells proliferate in the injured brain 3–4 days following ischemia [16]. Various gating strategies can be used to further separate macrophages at CNS interfaces from microglia [15].
2.3. Discrepancies in Nomenclature
Although current advances in technology have defined microglia and macrophages as having transcriptionally distinct profiles, identification of microglia and macrophages in the brain through the use of various cellular markers remains unclear and contentious in the literature (Table 1). Many groups consider all macrophages in the brain as microglia and utilize ionized calcium binding adaptor molecule 1 (Iba1)+ staining as evidence, but Iba1 can label both resident microglia and macrophages. Similarly, many other macrophage populations including microglia and CNS-associated myeloid cells express CD11b, CD115, Iba1, and F4/80 [20,21]. Due to the historical discrepancies in nomenclature and inability to distinguish between microglia and macrophages, many groups refer to CNS mononuclear phagocytes and recruited peripheral blood monocytes collectively as “microglia/macrophages.” More recently, cellular profiling can also be determined through single cell RNA-Seq and flow cytometry, whereas CD11b+CD45high populations are identified as macrophages and CD11b+CD45low populations as microglia. However, it is important to note that microglia can upregulate CD45 after ischemic stroke and become indistinguishable from CD11b+CD45high cells in the border regions of the CNS or the periphery [21,22].

Table 1.
Microglia and macrophage markers. Many studies utilize a variety of microglia and macrophage markers. Some of these markers may overlap between the two cell types.
In this review, we will distinctly denote microglia and macrophage nomenclature based on the methods by which each research study used to make its conclusions, with additional comments on the authors’ chosen nomenclature and any potential discrepancies with the study’s interpretation resulting from that choice.
3. Dichotomous Role of Microglia and Macrophages
3.1. Microglial Activation during Stroke
After the onset of brain ischemia, microglia undergo morphological and functional changes in the penumbra, while macrophages also infiltrate the brain parenchyma and migrate toward the infarct area. Studies have shown that activated microglia can have both a beneficial and detrimental effect during all stages of ischemic stroke; however, the timing and kinetics at which specific anti- and proinflammatory events occur may influence the nature of the outcome (Figure 1). Activated microglia release cytotoxic factors that can exacerbate ischemia and enable poststroke inflammation, displaying elevated levels of reactive oxygen species and TNF [43]. Several reports maintain this detrimental role of microglia in stroke; however, these reports are not consistent with their markers, often looking broadly at Iba1+ or CD11b+ cells, and sometimes using glial fibrillary acidic protein (GFAP), a marker often used to label reactive astrocytes to identify microglia.
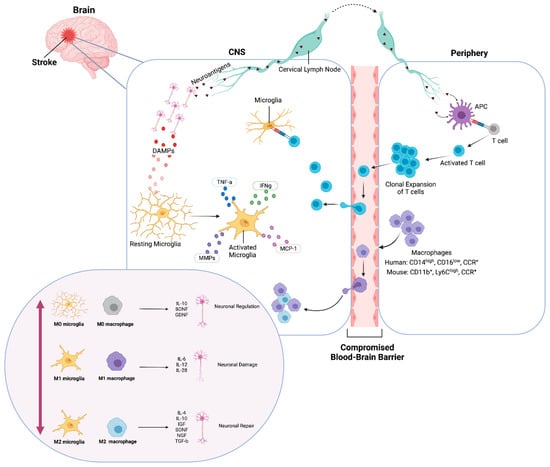
Figure 1.
Ischemic inflammatory response of microglia and macrophages. Immediately after an ischemic event, DAMPs activate resting (M0) microglia and neuroantigens are released. Microglia produce cytokines and chemokines. Transendothelial migration of monocytes and macrophages occurs through the compromised BBB. Neuroantigens are processed and presented by APCs and activate CD4+ T-cells, which undergo clonal expansion, promoting inflammation and neuronal damage. Classically activated (M1) microglia and macrophages release pro-inflammatory factors and contribute to neuronal damage. Conversely, alternatively activated (M2) microglia and macrophages release anti-inflammatory factors and contribute to neuronal repair and neurogenesis. Created with BioRender.com.
While the detrimental effects of microglia have been emphasized in many studies, Szalay et al. showed that a selective elimination of microglia using a CSF1R antagonist PLX3397 prior to a middle cerebral artery occlusion (MCAO) led to a 60% increase in infarct size. The increase in infarct volume was reversed with microglial repopulation [43]. Similarly, Herbert et al. reported a neuroprotective role of proliferating microglia in stroke after selectively ablating proliferating microglia using the Galectin-3 (Mac-2) marker to preferentially label resident microglia [44]. The result was an altered proinflammatory brain response and exacerbation of ischemic injury. Notably, these studies abrogated the microglial response to stroke early in the injury timeline, resulting in detrimental effects.
3.2. Macrophage Activation Profiles
There has been evidence of infiltration of peripheral monocytes to the brain in response to DAMPs produced after ischemic stroke, with the presence of neutrophils and macrophages in the site of CNS injury [45,46,47,48] (Figure 1). Activated macrophages can be categorized on a spectrum of functional activity, starting at resting (M0), classical activation (M1), which promotes inflammatory responses, to alternate activation (M2), which promotes tissue remodeling, wound healing, and immune regulation [49,50].
This switch in macrophage phenotype has been explored in ischemic models. For example, monocyte chemoattractant protein-1 (MCP-1) possesses cytokine-like properties and plays a significant role in macrophage and Ly-6Chi (CCR2+) proinflammatory monocyte migration to injury sites. The increase in CCL2 (MCP-1) expression in the ischemic hemisphere leads to an increased infarct volume [51,52], while the inhibition of CCR2 or MCP-1 resulted in a reduced infarct size [53,54]. In contrast, Ly-6Clo monocytes that do not express CCR2, but express the CX3CR1 receptor for CXC3CL1 fractalkine, develop into M2 macrophages after recruitment to normal tissues [55]. This understanding has led to many studies to focus on skewing microglia and macrophage toward the M2 phenotype rather than M1.
It is important to note that the M0/M1/M2 trichotomy is an oversimplification of activation states as the status of macrophages may include overlapping functional phenotype as well as different ones. For example, the M2 phenotype includes subpopulations such as M2a, M2b, M2c, and Mox, each with distinct physiological functions [56]. Not all current studies in CNS injuries have characterized these subpopulations, so while the M0/M1/M2 classification is broad, it is still a meaningful concept to facilitate our understanding of the functional status of macrophages.
4. Neuroprotection after Stroke
Depending on the ischemic milieu, macrophages can transform to different functional phenotypes in the hemisphere of infarction [57]. In response to signals from the microenvironment, microglia and recruited macrophages are alternatively activated and express an M2 phenotype during the early stages of ischemic stroke and then transform over time into an M1 phenotype in the infarct lesion [58]. Others have suggested the opposite effect, in which Ly6Chi (CCR2+) macrophages express the M1 phenotype in the ischemic hemisphere, but convert to an alternate phenotype at the injury site 48 h after stroke. These proinflammatory cells downregulate Ly6C and CCR2 expression, enhancing the resolution of inflammation by releasing vascular endothelial growth factor and TGF-β [59,60]. Zhang et al. reported the reprogramming of infiltrating macrophages that strongly favored efferocytic activity 3–7 days after brain ischemia and were potentially regulated by PPARy and STAT6 [61]. Similarly, Wang et al. also reported RNA transcriptome profiles that were related to the regulation of cell migration and mobilization and pro-neurovascular remodeling effects in macrophages as early as five days following ischemic stroke [21].
4.1. Angiogenesis
Microglia are known to influence angiogenesis, whereas the elimination of microglia or deficiency in macrophage colony-stimulating factory causes a reduction in retinal vasculature [62,63]. During ischemic stroke, Iba1+ microglia and macrophages cluster around the vasculature and release vascular endothelial growth factor to promote the reconstruction of blood vessels following stroke [64,65,66]. Pro-angiogenic microglia and macrophages may enhance neural proliferation and differentiation following stroke, possibly contributing to neurogenesis and CNS repair.
4.2. Synaptic Remodeling
It is often reported that ischemic stroke can lead to synaptic dysfunction; however, it is known that microglia and macrophages participate in synaptic remodeling and refining neural circuitry [67]. Microglia promote spine formation and synaptic maturation through CX3CR1 and complement proteins as well as synaptic pruning in the brain during development [68,69,70]. Modulation of synaptic function and the neural circuitry is also dependent on the activation state of microglia and macrophages. For example, one of the inflammatory mediators of neurotoxicity in stroke is NADPH oxidase, which is triggered by CR3 activation on macrophages and microglia, resulting in long-term synaptic depression [71,72]. Modulation of activation states of both microglia and macrophages may thus change the inflammatory environment following ischemic injury, encouraging neuroprotection and angiogenesis.
5. Neurogenesis after Stroke
Macrophage and microglial activation have been linked to changes in neurogenesis following ischemic stroke in mammals [9,73]. It has been suggested that newly generated neuroblasts from the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles can migrate and terminally differentiate into specific cell types in order to generate new granule cell neurons in the hippocampal dentate gyrus as anatomical substrates for learning and memory [74], or replenish the loss of neurons in other regions of the adult brain [75,76], respectively. Some studies suggest that the brain is capable of self-repair after insults of extensive neuronal death through a number of compensatory neurogenesis mechanisms after stroke [77,78,79]. Additionally, enhanced SVZ neurogenesis has been observed after stroke [80,81], and newborn neurons have been found in the ischemic penumbra [82,83].
Microglia and macrophages may participate in regulating neurogenesis by supporting axonal regrowth and regeneration to allow for functional recovery after stroke. Their production of local trophic gradients helps to stimulate axonal sprouting toward the infarct area [84]. Microglia also are necessary for regulating synaptic maturation, while in the setting of microglial injury, there can be synaptic dysfunction [85]. This active regulation of functional synapses in the CNS through axon guidance, synaptic patterning, and cell migration is evidence of their role in modulating neurogenesis [86,87,88].
The increase in neurogenesis after stroke may only be transient, and similar to what is seen in traumatic brain injury models, appears to be disrupted by a secondary inflammatory response [89]. While the production of trophic factors is essential for the migration of newborn neurons [90], activation of microglia and macrophages can also mediate inflammation that is detrimental to neurogenesis [7,91]. CD4+ T cells are essential to maintain homeostatic neurogenesis [92], and they also contribute to learning and memory [93]; however, activated cells responding to the injury may inhibit neurogenesis [94]. New treatment modalities can arise from shedding light on the mechanisms that modulate neurogenesis after stroke, as currently there are none.
6. Therapeutic Perspectives
Although neurons in the stroke lesion core cannot be rescued, improvement in stroke outcome can be achieved through reduction in secondary brain injury due to inflammation. Based on our developing knowledge of microglia and macrophage involvement in the pathophysiological processes of stroke, many novel therapeutic approaches have emerged to promote remodeling of the injured brain in stroke models by altering the activation phenotypes of these cells.
6.1. Polarization via Small Molecules
Modulating microglial and macrophage activation and polarization through use of various pharmacological and small molecules has been a popular area of study (Figure 2). Minocycline, a tetracycline antibiotic commonly used as an inhibitor of microglial activation, administered five times a week in amyotrophic lateral sclerosis (ALS) mice from 8–24 weeks of age, diminished the expression of M1 microglia and macrophages but not M2 microglia and macrophages [95]. Additionally, treatment with minocycline for one week, beginning at four days after reperfusion injury in rats, preserved adult new neurons, reduced reactive astrocytes, and improved dentate gyrus neurogenesis and neurological function [96]. Typically, ischemic stroke is characterized by the downregulation of the Wnt/beta-catenin signaling pathway; however, activation of Wnt/beta-catenin signaling through TWS119 attenuated neuroinflammation after stroke, by driving microglial anti-inflammatory activation, promotes angiogenesis [97,98].
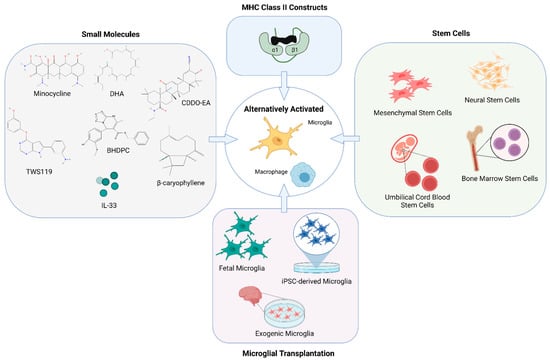
Figure 2.
Modulating microglia and macrophage for therapy. Many novel therapeutic approaches have emerged to promote remodeling of the injured brain in stroke models by altering the activation phenotype of microglia and macrophages. Methods for alternative activation of microglia and macrophages include but are not limited to the use of various small molecules, partial MHC class II constructs, stem cell therapies as well as transplantation of microglia as a treatment for both the acute and chronic effects of stroke. Created with BioRender.com.
The nature of the ischemic environment is a key determinant in driving microglia and macrophage function and their activation phenotype. For example, microglia and macrophages are highly susceptible to energy deficits, leading to activation and recruitment; thus, metabolic status of the lesion environment is a major factor in determining the nature of microglial response [99,100]. A number of studies have examined the effects of small molecules on the shift in microglial polarization toward an M2 phenotype after an ischemic event. One study found that a pyrimidine derivative, BHDPC, could confer neuroprotective actions and suppress both microglial and macrophage activation by inactivating NF-κB signaling, but promoted M2 polarization through BHDPC-enhanced phosphorylation of protein kinase A and cAMP-response element-bind protein in rats [101].
Microglia and macrophage activation could also be modulated toward the M2 phenotype through post-stroke treatment with docosahexaenoic acid (DHA) administered immediately after reperfusion and daily for three days thereafter. Treatment with DHA also significantly inhibits infiltration of neutrophils, T, and B lymphocytes [102]. A polarization shift of microglia and macrophages toward the M2 phenotype with a reduction in neurological deficit and infarct volume was also seen when a redox transcription factor NFE2 related factor 2 activator, CDDO-EA, was administered 30 min after the end of the ischemic period in mice [103]. Modulation of microglial M2 polarization via the toll-like receptor 4 pathway, a regulator of macrophage activation and polarization after injury, through the use of β-caryophyllene, a natural bicyclic sesquiterpene, also reduced infarct volume and neurologic deficits in mice after a transient MCAO [104]. Additionally, human stroke patients have increased plasma levels of sST2, an inhibitory IL-33 receptor; while in mice, treatment with IL-33, a cytokine known to induce a shift toward M2 polarization, increased peripheral levels of IL-4 in the spleen and peri-infarct area [105].
Angiogenesis can also be stimulated by modulating microglial polarization toward M2 through AMPK signaling after stroke. The treatment of human umbilical vein endothelial cells (HUVECs) in vitro with conditioned media collected from BV-2 microglial cells in culture incubated in berberine, an isoquinoline alkaloid extract from traditional Chinese medicine, facilitated angiogenesis of HUVEC cells [106]. Moreover, oral administration of berberine in mice subjected to transient MCAO resulted in angiogenesis as revealed by PET/CT imaging.
Additionally, the use of partial major histocompatibility complex (MHC) class II constructs as a novel treatment for ischemic stroke presents hopeful outcomes (Figure 2). These constructs have demonstrated a reduction in infarct volume and long-term neurological deficit in both young and aging mice ischemic models [107]. The mechanism of action of partial MHC class II constructs involves inhibiting the infiltration of immune cells into the CNS and reversing stroke-associated splenic atrophy, thereby also providing a therapeutic effect in the periphery [108]. Partial MHC class II constructs have been shown to enhance alternatively activated M2 microglia and macrophages [109].
It is also worth mentioning that there is accruing evidence suggesting oxidative stress activated mechanisms playing a role in the impairment of function after stroke. It is known that M1 microglia produce free radicals and oxidants, leading to long-term deficits in stroke patients [110,111,112,113,114]. When oxidative enzyme myeloperoxidase (MPO) activity was inhibited after stroke, there was a noticeable reduction in the number of M1 microglial cells, but there was no impact on M2 microglia. Additionally, there was an increase in proliferation and differentiation of neural stem cells and protection of exogenous neural cells [111,115]. Modulating microglial phenotypes by mediating oxidative stress and neuroinflammation can affect adult neurogenesis in the post-stroke brain.
6.2. Stem Cell Therapies
Stem cell therapies for stroke have the potential to provide neurorestorative benefits. The type of stem cell and route of administration determines the efficacy of stem cell-based therapies in mediating a therapeutic effect (Figure 2). The therapeutic of effect of neural stem cells have been attributed to the promotion of neurogenesis and regeneration.
Neural stem cell (NSC) transplants have been shown to improve behavioral outcomes and angiogenesis in rat models of ischemic stroke, leading to studies interrogating human NSCs (hNSC) [116,117]. Grafted hNSCs can differentiate into neurons, astrocytes, and oligodendrocytes in the ischemic brain, even making direct contact with stroke-damaged vasculature and participating in remyelination, respectively [118]. More recently, administration of clinical-grade hNSC line CTX0E03 by intracerebral implantation has been shown to improve upper limb function in stroke patients [119]. Success in previous clinical trials will allow researchers to utilize this cell line for other neurological diseases and disorders.
Bone marrow stem cells (BMSC) have been shown to migrate to the site of ischemia and differentiate into neural cells [120]. More recently, bone marrow-derived mesenchymal stem cells (MSC) SB632 were associated with improvement in clinical outcome in stroke patients with chronic motor deficits [121]. These cells were intracerebrally transplanted and well tolerated after 12 months. The advantages of MSC transplantation for functional recovery, angiogenesis, and endogenous neurogenesis have also been shown in animal stroke models [122,123,124,125]. However, one major limitation of the current stem cell therapies is the sparse migration of MCSs to the ischemic brain regions after transplantation.
Umbilical cord blood stem cells (UCBSC) offer many advantages over other types of stem cells. The immune properties of UCBSCs allow for increased tolerance for human leukocyte antigen mismatches and decreased incidence of graft-versus-host disease as well as ease of procurement and availability [126,127]. Non-hematopoietic umbilical cord blood stem cell (nh-UCBSC) has also been shown to ameliorate ischemic brain injury by reducing the number of macrophages and microglia and normalizing the number of B cells and T cells in the brain following stroke when administered 48 h after the ischemic injury [128,129]. The major alterations in gene expression profiles after the administration of nh-UCBSCs has the potential for altering microglia and macrophage activation to improve macrophage-induced neural damage.
7. Chronic Stroke
A significant subset of stroke patients experience progressive cognitive decline. Levine et al. conducted a prospective study of cognitive function in 23,572 patients aged 46 years or older who were followed for an average of six years post stroke [130]. These investigators reported a decline in global cognition, new learning, and verbal learning during the acute phase following stroke. During the subsequent years of monitoring, they found that individuals with stroke exhibited faster rates of decline, global cognition, and executive function when compared to individuals without stroke. Pendlebury and Rothwell published a meta-analysis of 7511 subjects in 73 reports of stroke and dementia [131]. Of these stroke subjects, 10% developed dementia after their initial stroke, while approximately 33% developed dementia after a recurrent stroke. Contributions to cognitive decline may stem from the interaction of immune cells, the presence of neuroantigens, and sustained microglial activation, resulting in chronic inflammation.
Antigen Presentation and Cognitive Decline
After an ischemic event, antigen-presenting cells (APCs) accumulate in the brain parenchyma and express MHC class I and II cell surface molecules [132,133,134,135]. APCs engulf and process the peptides of damaged and dying cells and then present these peptides to CD8+ T cells, in association with MHC class I, and to CD4 T+ cells, in association with MHC class II (Figure 1). The activation of the T cell population to target self-antigens in the CNS can potentially contribute to the progressive, cognitive decline seen in many patients after stroke. Macrophage populations of dendritic cells that express both Iba1 and CD11lb markers migrate in the choroid plexus in mice and behave as APCs [136,137]. A population of Iba1+CD11b+CD45int are a subset of microglial cells that can also upregulate antigen presentation and activation markers [138,139,140]. While CD4+ T-cell mediated neuroprotection is initiated by APCs, MHC class II+ microglia APCs are required for a secondary restimulation and can drive antigen-specific neuroprotection [141], demonstrating a close relationship between these two cell types. For example, in the brain of Alzheimer’s disease (AD) patients, the transition into AD dementia correlates with increased MHC class II+ microglia-mediated immunity and a decrease in T cell number [141,142,143].
The volume of infarction and severity of stroke is correlated with the concentration of neural antigens found in the serum of patients after stroke [144,145]. Autoantibodies against brain antigens have been found in the CSF of patients with stroke, possibly contributing to the development of post-stroke cognitive impairment [146,147,148]. A series of studies demonstrated the reversal of stroke-associated splenic atrophy 96 h after MCAO by administering partial MHC class II constructs starting at 4 h after the onset of ischemia [107,109,149,150,151]. These MHC class II constructs inhibited neuroantigen-specific T-cells and blocked the binding of macrophage migration inhibitory factor to its CD74 receptor, promoting M2 macrophage and microglia phenotypes in the CNS and also improving long-term cognitive outcomes 28 days after stroke [109,151]. Thus, the balance between macrophage and microglia phenotypes and presence of neuroantigens following a stroke is critical to the outcome of ischemic brain injury.
8. Microglial Transplantation as a Treatment for Chronic Stroke
Regulation of microglial function as a means to attenuate neurological dysfunction seen in chronic stroke has become a promising strategy in pre-clinical research [152]. Cell transplantation therapies involving microglia have been shown to exert a therapeutic effect for Alzheimer’s disease [153]. While other transplanted cells types such as mesenchymal stem cells are more common [154], the therapeutic potential of microglia transplantation in treating chronic stroke is an emerging field (Figure 2).
Positron emission tomography imaging studies in patients with chronic stroke and cognitive decline revealed persistent microglia activation [155]. Preclinical animal models of chronic stroke support the contention that chronic inflammation and sustained microglia activation may be an underlying factor in the progressive deterioration observed in the clinical setting. Basu et al. demonstrated that IL-1 and IL-1R signaling is essential for progressive neurodegeneration that arises subsequent to ischemic brain injury [156]. In addition, Yang et al. reported that the ST2 receptor, a member of the IL-1R receptor family, is critical for microglial signaling related to ischemic brain injury [157]. Together, these results suggest that targeting microglial signaling pathways or replacing chronically active inflammatory microglia may offer a therapeutic strategy for ameliorating progressive neurodegeneration and cognitive decline resulting from ischemic stroke.
8.1. Transplantation of Fetal Microglia
Transplantation of fetal microglia has been demonstrated to improve ischemia-induced functional changes and apoptotic events after stroke. Ischemia was induced in rats by MCAO and human microglial cells (HMO6) from fetal telencephalon tissue were transplanted into a treated group [158]. Animals that received HMO6 transplantation showed significantly reduced infarct volume and apoptotic cells in the infarct core and penumbra when compared to the control group that did not receive the transplant. Gene expression analysis showed that HMO6 cells migrated to the ischemic area and produced neurotrophic factors such as GDNF and BDNF and anti-inflammatory cytokines IL-4 and IL-5, which reduced the endogenous glial response. The accumulation of transplanted microglia in the lesion core suggests that microglia can potentially be used in gene therapy as a vehicle for the transfer of therapeutic genes.
Fetal microglial transplantation may improve stroke outcomes by modulating inflammation and facilitating angiogenesis. Expression of IL-1β is high in HMO6 and has been shown to increase VEGF mRNA expression in HMO6 cell lines when co-cultured with a mesenchymal stem cell line B10 [159]. White matter lesions (WMLs) as a result of chronic cerebral ischemia are thought to contribute to vascular dementia. HMO6 cells were injected intravenously and WML development was assessed in a chronic cerebral hypoperfusion rat model induced by bilateral common carotid artery occlusion (BCAO) [160]. The authors found that the transplantation of HMO6 inhibited BCAO-induced WMLs and displayed an early and prolonged improvement in WMLs. Both glial activation and astrocyte and microglial accumulation was inhibited in the site of BCAO after HMO6 transplantation, and this effect was more robust compared to animals that received B10 transplantation. Additionally, the expression of microglial proteases MMP-2, MMP-9, and cathepsin B, all of which influence WML pathology, was also inhibited after transplantation. The capacity of transplanted fetal microglial cells to reduce the severity of WMLs through glial activation should be considered as a potential therapy.
8.2. Transplantation of iPSC Derived Microglia
Microglia derived from iPSCs are an important consideration for translational neuroimmunology research and hold great potential for therapy. iPSC-microglia are able to recapitulate the inflammatory-modulating properties of brain-resident microglia by resembling the in vivo phenotypical profiles of microglia within stroke lesions and responding to IL-13 stimulation [161]. While primary microglia grown in culture tend to have major differences in morphology and gene expression when compared to resident-microglia in the brain, single-cell RNA-sequencing of transplanted iPSC-derived microglia showed similarities to healthy primary microglia in both gene expression profiles and phenotypic morphology and are able to functionally integrate in the chimeric mouse brain [162,163]. Beyond iPSC-derived microglia serving as a suitable representation of human microglial cells, the cells have also demonstrated the ability to mitigate neuronal loss after stroke in rats aged 24 months. Animals that received iPSCs also performed better at the cylinder test at four and seven weeks after the ischemic event when compared to animals injected with only the vehicle, suggesting the ability of iPSCs to ameliorate functional deficits in a stroke-injured aged brain [164]. Additionally, it has been shown that IPSC-derived microglia can be transplanted into the brain through a transnasal route, offering a noninvasive method to deliver potential therapies [165].
8.3. Prospects for Transplanting Exogenic Microglia
Exogenous microglia also have the potential to protect against neuronal damage after stroke by exhibiting an affinity for ischemic brain lesions. Injection of exogenous microglia was show to promote CA1 cell survival by migrating to the CA1 cell layer and increasing the expression of BDNF and GDNF in the ischemic hippocampus [166]. Cell therapies involving hypoxic preconditioning is becoming a popular strategy for treating ischemic stroke. Microglia subjected to oxygen-glucose deprivation before transplantation can induce anti-inflammatory microglia and result in the overexpression of remodeling factors such as MMP-9, VEGF, and TGF-β in the injured brain parenchyma [167]. Such therapeutic potential has led to hypoxic preconditioning of stem cells to facilitate the switching of microglia toward an anti-inflammatory polarization for use in alleviating ischemic injury [168].
9. Conclusions and Perspectives
Ischemic injury is characterized by the time dependent activation of microglia and infiltration of macrophages into the penumbral tissue. Lack of effective treatment options is partially attributed to the dynamic pathophysiology of ischemic stroke; however, future research aimed at elucidating mechanisms underlying the involvement of these cell types in a time-dependent manner is a promising strategy for treating the long-term outcomes after ischemic injury.
The studies reviewed here suggest the importance of microglia and macrophages in both neuroprotection and neurogenesis after stroke and the potential use of these cell types for therapy, especially for treating long-term cognitive deficits. Further discrimination of this relationship may include careful distinction between different populations of macrophage and microglia due to their temporal differences after the onset of stroke. Currently in the literature, there is a lack of consistent agreement in nomenclature used to distinguish differences between microglia and macrophage populations. This aspect will be important to accurately describe the role of these cells over time, shortly before and following the induction of stroke.
While alternative activation and modulation of the macrophage and microglial response may stimulate neurogenesis, angiogenesis, and synaptic remodeling in the injured brain, it is worth considering other immune cells, factors, and molecules that are influenced by inflammatory microglia and macrophages. Investigation of functional recovery and improvement of long-term cognitive outcomes may be dependent on the relationship between macrophage and microglia with MHC class II molecules, T cells, and the presence of neuroantigens.
Understanding the timing and kinetics at which specific anti- and proinflammatory events occur will affect stroke outcome, and delineating the role of macrophage and microglia and their signaling pathways over time could provide a host of candidates for therapeutic interventions after an ischemic event. Transplantation studies demonstrate the potential of targeting signaling pathways and using microglia in gene therapy as a vehicle for the transfer of therapeutic genes as a promising therapy for treating chronic stroke.
The role of microglia and macrophages in stroke-induced neurogenesis is multifaceted and complex: production of trophic factors is essential for the migration of newborn neurons; however, activation of microglia and macrophages can mediate inflammation that is detrimental to neurogenesis. Their interaction with other immune cells such as lymphocytes contributes to the complexity of the microenvironment generated by DAMPs and neuroantigens, and consequently alters the macrophage and microglial response to injury (Figure 1). Since there is a dichotomy in the consequences of microglia- and macrophage-induced neurogenesis and involvement in inflammation through its different activation phenotypes, future research aimed at elucidating the temporal characteristics of activated microglia and macrophages to determine their beneficial role in stimulating neuroprotection and neurogenesis after stroke would be critical for developing novel therapeutic strategies. Strategies targeted toward modulating microglia and macrophage activation of inflammatory pathways have the potential to be advantageous in controlling injury and improving clinical outcomes following stroke.
Author Contributions
Conceptualization, M.C.C. and W.C.L.; literature search and data curation, S.R.V.; writing—original draft preparation, S.R.V.; writing—review and editing, A.W.G., W.C.L., M.C.C.; visualization A.V.S.; supervision, M.C.C.; project administration, M.C.C.; funding acquisition, W.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by NIH training grant T32 DA007097, NIH grant R42 NS 112070, and the Suzanne Schwarz Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef]
- Venkat, P.; Shen, Y.; Chopp, M.; Chen, J. Cell-based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology 2018, 134, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Linfante, I.; Cipolla, M.J. Improving Reperfusion Therapies in the Era of Mechanical Thrombectomy. Transl. Stroke Res. 2016, 7, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Jean, W.C.; Spellman, S.R.; Nussbaum, E.S.; Low, W.C. Reperfusion Injury after Focal Cerebral Ischemia: The Role Inflammation and the therapeutic Horizon. Neurosurgery 1998, 43, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.-Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2016, 157, 247–272. [Google Scholar] [CrossRef]
- Zhao, S.-C.; Ma, L.-S.; Chu, Z.-H.; Xu, H.; Wu, W.-Q.; Liu, F. Regulation of microglial activation in stroke. Acta Pharmacol. Sin. 2017, 38, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-Y.; Liu, L.; Yang, Q.-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef]
- Greter, M.; Lelios, I.; Croxford, A.L. Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation. Front. Immunol. 2015, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef]
- Hagemeyer, N.; Hanft, K.-M.; Akriditou, M.-A.; Unger, N.; Park, E.S.; Stanley, E.R.; Staszewski, O.; Dimou, L.; Prinz, M. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017, 134, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Ginhoux, F. Ontogeny of Tissue-Resident Macrophages. Front. Immunol. 2015, 6, 486. [Google Scholar] [CrossRef]
- Grassivaro, F.; Menon, R.; Acquaviva, M.; Ottoboni, L.; Ruffini, F.; Bergamaschi, A.; Muzio, L.; Farina, C.; Martino, G. Convergence between Microglia and Peripheral Macrophages Phenotype during Development and Neuroinflammation. J. Neurosci. 2019, 40, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Wieghofer, P.; Jordão, M.J.C.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Rajan, W.D.; Wojtas, B.; Gielniewski, B.; Miro-Mur, F.A.; Pedragosa, J.; Zawadzka, M.; Pilanc, P.; Planas, A.M.; Kaminska, B. Defining molecular identity and fates of CNS-border associated macrophages after ischemic stroke in rodents and humans. Neurobiol. Dis. 2020, 137, 104722. [Google Scholar] [CrossRef]
- Ritzel, R.; Patel, A.R.; Grenier, J.M.; Crapser, J.; Verma, R.; Jellison, E.R.; McCullough, L.D. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflamm. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Abtin, A.; Jain, R.; Mitchell, A.J.; Roediger, B.; Brzoska, A.J.; Tikoo, S.; Cheng, Q.; Ng, L.G.; Cavanagh, L.L.; Von Andrian, U.H.; et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat. Immunol. 2013, 15, 45–53. [Google Scholar] [CrossRef]
- Van Gorp, H.; Delputte, P.L.; Nauwynck, H.J. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol. Immunol. 2010, 47, 1650–1660. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, N.; Yenari, M.A. Mechanisms and Potential Therapeutic Applications of Microglial Activation after Brain Injury. CNS Neurosci. Ther. 2014, 21, 309–319. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Ye, Q.; Hassan, S.H.; Zhao, J.; Li, S.; Hu, X.; Leak, R.; Rocha, M.; Wechsler, L.R.; et al. RNA sequencing reveals novel macrophage transcriptome favoring neurovascular plasticity after ischemic stroke. Br. J. Pharmacol. 2019, 40, 720–738. [Google Scholar] [CrossRef]
- Hickman, S.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.-C.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Chu, S.-H.; Hernandez, M.X.; Fang, M.J.; Modarresi, L.; Selvan, P.; MacGregor, G.R.; Tenner, A.J. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J. Neuroinflamm. 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat. Neurosci. 2013, 17, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Kuil, L.E.; Martí, A.L.; Mascaro, A.C.; van den Bosch, J.C.; van den Berg, P.; van der Linde, H.C.; Schoonderwoerd, K.; Ruijter, G.J.G.; van Ham, T.J. Hexb enzyme deficiency leads to lysosomal abnormalities in radial glia and microglia in zebrafish brain development. Glia 2019, 67, 1705–1718. [Google Scholar] [CrossRef]
- Abo-Ouf, H.; Hooper, A.W.; White, E.J.; van Rensburg, H.J.; Trigatti, B.L.; Igdoura, S.A. Deletion of tumor necrosis factor-α ameliorates neurodegeneration in Sandhoff disease mice. Hum. Mol. Genet. 2013, 22, 3960–3975. [Google Scholar] [CrossRef]
- Satoh, J.-I.; Kino, Y.; Asahina, N.; Takitani, M.; Miyoshi, J.; Ishida, T.; Saito, Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology 2015, 36, 39–49. [Google Scholar] [CrossRef]
- Tanaka, J. Favorable and unfavorable roles of microglia and macrophages in the pathologic central nervous system. Neuroimmunol. Neuroinflamm. 2020, 2020, 73–91. [Google Scholar] [CrossRef][Green Version]
- Amici, S.A.; Dong, J.; Guerau-De-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef] [PubMed]
- Cosenza-Nashat, M.A.; Kim, M.-O.; Zhao, M.-L.; Suh, H.-S.; Lee, S.C. CD45 Isoform Expression in Microglia and Inflammatory Cells in HIV-1 Encephalitis. Brain Pathol. 2006, 16, 256–265. [Google Scholar] [CrossRef]
- Hellwig, S.; Brioschi, S.; Dieni, S.; Frings, L.; Masuch, A.; Blank, T.; Biber, K. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 2016, 55, 126–137. [Google Scholar] [CrossRef]
- Dos Anjos Cassado, A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. In Results and Problems in Cell Differentiation; Springer: Amsterdam, The Netherlands, 2017; Volume 62, pp. 161–179. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef]
- O’Neill, A.S.G.; Berg, T.K.V.D.; Mullen, G.E.D. Sialoadhesin—A macrophage-restricted marker of immunoregulation and inflammation. Immunology 2013, 138, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Claflin, J.; Wang, X.; Lengi, A.; Kikuchi, T. Microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int. J. Parasitol. 2005, 35, 83–90. [Google Scholar] [CrossRef]
- Bertani, F.R.; Mozetic, P.; Fioramonti, M.; Iuliani, M.; Ribelli, G.; Pantano, F.; Santini, D.; Tonini, G.; Trombetta, M.; Businaro, L.; et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci. Rep. 2017, 7, 8965. [Google Scholar] [CrossRef]
- Zhang, Y.; Sime, W.; Juhas, M.; Sjölander, A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur. J. Cancer 2013, 49, 3320–3334. [Google Scholar] [CrossRef]
- Terra, X.; Quintero, Y.; Auguet, T.; Porras, J.A.; Hernández, M.; Sabench, F.; Aguilar, C.; Luna, A.M.; Del Castillo, D.; Richart, C. FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. Eur. J. Endocrinol. 2011, 164, 539–547. [Google Scholar] [CrossRef]
- Steen, K.A.; Xu, H.; Bernlohr, D.A. FABP4/aP2 Regulates Macrophage Redox Signaling and Inflammasome Activation via Control of UCP2. Mol. Cell. Biol. 2017, 37, e00282-16. [Google Scholar] [CrossRef]
- Szalay, G.; Martinecz, B.; Lénárt, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B.L.; Katona, G.; et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016, 7, 11499. [Google Scholar] [CrossRef] [PubMed]
- Lalancette-Hébert, M.; Gowing, G.; Simard, A.; Weng, Y.C.; Kriz, J. Selective Ablation of Proliferating Microglial Cells Exacerbates Ischemic Injury in the Brain. J. Neurosci. 2007, 27, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Nappi, G.; Bernardi, G.; Bagetta, G.; Corasaniti, M.T. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2008, 276, 13–26. [Google Scholar] [CrossRef]
- Stevens, S.L.; Bao, J.; Hollis, J.; Lessov, N.S.; Clark, W.M.; Stenzel-Poore, M.P. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002, 932, 110–119. [Google Scholar] [CrossRef]
- Schilling, M.; Strecker, J.-K.; Ringelstein, E.B.; Schäbitz, W.-R.; Kiefer, R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009, 1289, 79–84. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Tian, P.-X.; Han, F.; Zheng, J.; Xia, X.-X.; Xue, W.-J.; Ding, X.-M.; Ding, C.-G. Comparison of the characteristics of macrophages derived from murine spleen, peritoneal cavity, and bone marrow. J. Zhejiang Univ. Sci. B 2017, 18, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hallenbeck, J.M.; Ruetzler, C.; Bol, D.; Thomas, K.; Berman, N.E.J.; Vogel, S.N. Overexpression of Monocyte Chemoattractant Protein 1 in the Brain Exacerbates Ischemic Brain Injury and is Associated with Recruitment of Inflammatory Cells. J. Cereb. Blood Flow Metab. 2003, 23, 748–755. [Google Scholar] [CrossRef]
- Schuette-Nuetgen, K.; Strecker, J.-K.; Minnerup, J.; Ringelstein, E.B.; Schilling, M. MCP-1/CCR-2-double-deficiency severely impairs the migration of hematogenous inflammatory cells following transient cerebral ischemia in mice. Exp. Neurol. 2012, 233, 849–858. [Google Scholar] [CrossRef]
- Hughes, P.M.; Allegrini, P.R.; Rudin, M.; Perry, V.H.; Mir, A.K.; Wiessner, C. Monocyte Chemoattractant Protein-1 Deficiency is Protective in a Murine Stroke Model. J. Cereb. Blood Flow Metab. 2002, 22, 308–317. [Google Scholar] [CrossRef]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.; Andjelkovic, A.V. Absence of the Chemokine Receptor CCR2 Protects Against Cerebral Ischemia/Reperfusion Injury in Mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; De Simoni, M.-G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.J.; Daley, J.M.; Van Houtte, O.; Brancato, S.K.; Jr, W.L.H.; Albina, J.E. The Monocyte to Macrophage Transition in the Murine Sterile Wound. PLoS ONE 2014, 9, e86660. [Google Scholar] [CrossRef]
- Wattananit, S.; Tornero, D.; Graubardt, N.; Memanishvili, T.; Monni, E.; Tatarishvili, J.; Miskinyte, G.; Ge, R.; Ahlenius, H.; Lindvall, O.; et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 2016, 36, 4182–4195. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Wang, R.; Jiang, M.; Ye, Q.; Smith, A.D.; Chen, J.; Shi, Y. Macrophages reprogram after ischemic stroke and promote efferocytosis and inflammation resolution in the mouse brain. CNS Neurosci. Ther. 2019, 25, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Takubo, K.; Shimizu, T.; Ohno, H.; Kishi, K.; Shibuya, M.; Saya, H.; Suda, T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Cell Biol. 2009, 185, i6. [Google Scholar] [CrossRef]
- Zhao, X.; Eyo, U.B.; Murugan, M.; Wu, L.-J.; Murguan, M. Microglial interactions with the neurovascular system in physiology and pathology. Dev. Neurobiol. 2018, 78, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Mao, X.; Jin, K.; Greenberg, D.A. Vascular endothelial growth factor-B expression in postischemic rat brain. Vasc. Cell 2013, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; van Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Jolivel, V.; Bicker, F.; Binamé, F.; Ploen, R.; Keller, S.; Gollan, R.; Jurek, B.; Birkenstock, J.; Poisa-Beiro, L.; Bruttger, J.; et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2014, 129, 279–295. [Google Scholar] [CrossRef]
- Nie, J.; Yang, X. Modulation of Synaptic Plasticity by Exercise Training as a Basis for Ischemic Stroke Rehabilitation. Cell. Mol. Neurobiol. 2016, 37, 5–16. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.W.H.; Koizumi, K.S.S.; Moorhouse, A.; Yoshimura, A.W.I.Y.; Nabekura, A.M. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016, 7, 12540. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Duan, X.; Tian, X.; Shen, H.; Sun, Q.; Chen, G. NADPH Oxidase: A Potential Target for Treatment of Stroke. Oxid. Med. Cell. Longev. 2016, 2016, 5026984. [Google Scholar] [CrossRef]
- Zhang, J.; Malik, A.; Choi, H.B.; Ko, R.W.; Dissing-Olesen, L.; MacVicar, B.A. Microglial CR3 Activation Triggers Long-Term Synaptic Depression in the Hippocampus via NADPH Oxidase. Neuron 2014, 82, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.-Q.; Ma, X.-T.; Hu, Z.-W.; Yang, S.; Chen, M.; Bosco, D.; Wu, L.-J.; Tian, D.S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.R.; Schoenfeld, T.J.; Karlsson, R.-M.; Bannerman, D.M.; Cameron, H.A. Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues. PLoS Biol. 2017, 15, e2001154. [Google Scholar] [CrossRef] [PubMed]
- Kerschensteiner, M.; Meinl, E.; Hohlfeld, R. Neuro-Immune Crosstalk in CNS Diseases. Results Probl. Cell Differ. 2010, 51, 197–216. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Lin, R.; Iacovitti, L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015, 1628, 327–342. [Google Scholar] [CrossRef]
- Otero, L.; Zurita, M.; Bonilla, C.; Rico, M.A.; Aguayo, C.; Rodriguez, A.; Vaquero, J. Endogenous neurogenesis after intracerebral hemorrhage. Histol. Histopathol. 2012, 27, 303–315. [Google Scholar] [CrossRef]
- Tobin, M.K.; Bonds, J.A.; Minshall, R.D.; Pelligrino, D.A.; Testai, F.D.; Lazarov, O. Neurogenesis and Inflammation after Ischemic Stroke: What is Known and Where We Go from Here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Kreuzberg, M.; Kanov, E.; Timofeev, O.; Schwaninger, M.; Monyer, H.; Khodosevich, K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp. Neurol. 2010, 226, 90–99. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Neurogenesis following Stroke Affecting the Adult Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a019034. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, J.; Lin, X.; Wang, L.; Shao, B.; Jin, K.; Wang, Y.; Yang, G.-Y. Neural Stem Cell Protects Aged Rat Brain from Ischemia–Reperfusion Injury through Neurogenesis and Angiogenesis. J. Cereb. Blood Flow Metab. 2014, 34, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Nada, S.E.; Tulsulkar, J.; Shah, Z.A. Heme Oxygenase 1-Mediated Neurogenesis Is Enhanced by Ginkgo biloba (EGb 761®) After Permanent Ischemic Stroke in Mice. Mol. Neurobiol. 2013, 49, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, P.E.; Porritt, M.J.; Martinello, P.; Parish, C.L.; Liberatore, G.T.; Donnan, G.A.; Howells, D.W. Macrophages and Microglia Produce Local Trophic Gradients That Stimulate Axonal Sprouting Toward but Not beyond the Wound Edge. Mol. Cell. Neurosci. 2002, 21, 436–453. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ferretti, M.T. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef]
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia Actively Regulate the Number of Functional Synapses. PLoS ONE 2013, 8, e56293. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L. Microglia and Beyond: Innate Immune Cells as Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Yin, J.; Valin, K.L.; Dixon, M.L.; Leavenworth, J.W. The Role of Microglia and Macrophages in CNS Homeostasis, Autoimmunity, and Cancer. J. Immunol. Res. 2017, 2017, 5150678. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Grimmig, B.; Diamond, D.; Sanberg, P.R.; Bickford, P.; Kaneko, Y.; Borlongan, C.V. Long-Term Upregulation of Inflammation and Suppression of Cell Proliferation in the Brain of Adult Rats Exposed to Traumatic Brain Injury Using the Controlled Cortical Impact Model. PLoS ONE 2013, 8, e53376. [Google Scholar] [CrossRef]
- Rotschafer, J.H.; Hu, S.; Little, M.; Erickson, M.; Low, W.C.; Cheeran, M.C. Modulation of neural stem/progenitor cell proliferation during experimental Herpes Simplex encephalitis is mediated by differential FGF-2 expression in the adult brain. Neurobiol. Dis. 2013, 58, 144–155. [Google Scholar] [CrossRef]
- Fan, W.; Dai, Y.; Xu, H.; Zhu, X.; Cai, P.; Wang, L.; Sun, C.; Hu, C.; Zheng, P.; Zhao, B. Caspase-3 Modulates Regenerative Response After Stroke. Stem Cells 2013, 32, 473–486. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.-G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Baruch, K.; Ron-Harel, N.; Gal, H.; Deczkowska, A.; Shifrut, E.; Ndifon, W.; Mirlas-Neisberg, N.; Cardon, M.; Vaknin, I.; Cahalon, L.; et al. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc. Natl. Acad. Sci. USA 2013, 110, 2264–2269. [Google Scholar] [CrossRef]
- Hu, S.; Rotschafer, J.H.; Lokensgard, J.R.; Cheeran, M.C.-J. Activated CD8+ T Lymphocytes Inhibit Neural Stem/Progenitor Cell Proliferation: Role of Interferon-Gamma. PLoS ONE 2014, 9, e105219. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, Y.; Won, S.J.; Neumann, M.; Hu, D.; Zhou, L.; Weinstein, P.R.; Liu, J. Chronic Treatment with Minocycline Preserves Adult New Neurons and Reduces Functional Impairment After Focal Cerebral Ischemia. Stroke 2007, 38, 146–152. [Google Scholar] [CrossRef]
- Song, D.; Zhang, X.; Chen, J.; Liu, X.; Xue, J.; Zhang, L.; Lan, X. Wnt canonical pathway activator TWS119 drives microglial anti-inflammatory activation and facilitates neurological recovery following experimental stroke. J. Neuroinflamm. 2019, 16, 1–17. [Google Scholar] [CrossRef]
- Laksitorini, M.; Yathindranath, V.; Xiong, W.; Hombach-Klonisch, S.; Miller, D.W. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci. Rep. 2019, 9, 19718. [Google Scholar] [CrossRef]
- Fumagalli, S.; Perego, C.; Pischiutta, F.; Zanier, E.; de Simoni, M.G. The Ischemic Environment Drives Microglia and Macrophage Function. Front. Neurol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Bayón, J.; López-López, A.; Rodríguez, M.; Mahy, N. Glucose pathways adaptation supports acquisition of activated microglia phenotype. J. Neurosci. Res. 2014, 92, 723–731. [Google Scholar] [CrossRef]
- Li, C.; Bian, Y.; Feng, Y.; Tang, F.; Wang, L.; Hoi, M.P.M.; Ma, D.; Zhao, C.; Lee, S.M.Y. Neuroprotective Effects of BHDPC, a Novel Neuroprotectant, on Experimental Stroke by Modulating Microglia Polarization. ACS Chem. Neurosci. 2019, 10, 2434–2449. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.; Hu, M.; Sun, X.; Qiu, W.; Zheng, S.; Hu, X.; Lu, Z. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res. 2018, 9, 669–680. [Google Scholar] [CrossRef]
- Lei, X.; Li, H.; Li, M.; Dong, Q.; Zhao, H.; Zhang, Z.; Sun, B.; Mao, L. The novel Nrf2 activator CDDO-EA attenuates cerebral ischemic injury by promoting microglia/macrophage polarization toward M2 phenotype in mice. CNS Neurosci. Ther. 2020, 27, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, H.; Xiang, F.; Xu, L.; Dong, Z. β-Caryophyllene protects against ischemic stroke by promoting polarization of microglia toward M2 phenotype via the TLR4 pathway. Life Sci. 2019, 237, 116915. [Google Scholar] [CrossRef]
- Korhonen, P.; Kanninen, K.M.; Lehtonen, S.; Lemarchant, S.; Puttonen, K.; Oksanen, M.; Dhungana, H.; Loppi, S.; Pollari, E.; Wojciechowski, S.; et al. Immunomodulation by interleukin-33 is protective in stroke through modulation of inflammation. Brain Behav. Immun. 2015, 49, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cao, D.; Guo, C.; Liu, M.; Tao, Y.; Zhou, J.; Wang, F.; Zhao, Y.; Wei, J.; Zhang, Y.; et al. Berberine Facilitates Angiogenesis Against Ischemic Stroke Through Modulating Microglial Polarization via AMPK Signaling. Cell. Mol. Neurobiol. 2019, 39, 751–768. [Google Scholar] [CrossRef]
- Benedek, G.; Vandenbark, A.A.; Alkayed, N.J.; Offner, H. Partial MHC class II constructs as novel immunomodulatory therapy for stroke. Neurochem. Int. 2016, 107, 138–147. [Google Scholar] [CrossRef]
- Brown, J.; Kingsbury, C.; Lee, J.; Vandenbark, A.A.; Meza-Romero, R.; Offner, H.; Borlongan, C.V. Spleen participation in partial MHC class II construct neuroprotection in stroke. CNS Neurosci. Ther. 2020, 26, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Castelli, V.; Bonsack, B.; Coats, A.B.; Navarro-Torres, L.; Garcia-Sanchez, J.; Kingsbury, C.; Nguyen, H.; Vandenbark, A.A.; Meza-Romero, R.; et al. A Novel Partial MHC Class II Construct, DRmQ, Inhibits Central and Peripheral Inflammatory Responses to Promote Neuroprotection in Experimental Stroke. Transl. Stroke Res. 2020, 11, 831–836. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Berry, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [CrossRef]
- Yu, G.; Liang, Y.; Zheng, S.; Zhang, H. Inhibition of Myeloperoxidase by N-Acetyl Lysyltyrosylcysteine Amide Reduces Oxidative Stress-Mediated Inflammation, Neuronal Damage, and Neural Stem Cell Injury in a Murine Model of Stroke. J. Pharmacol. Exp. Ther. 2018, 364, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; McBride, D.W.; Zhang, J.H. Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-κB pathway after MCAO in rats. Neurobiol. Dis. 2017, 110, 59–67. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Y.; He, Q.; Li, L.; Xie, H.; Zhao, Y.; Zhao, J. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018, 336, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, W.-N.; Matei, N.; Li, X.; Pang, J.-W.; Mo, J.; Chen, S.-P.; Tang, J.-P.; Yan, M.; Zhang, J.H. Ezetimibe Attenuates Oxidative Stress and Neuroinflammation via the AMPK/Nrf2/TXNIP Pathway after MCAO in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 4717258. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Sakaguchi, M.; Kuroiwa, T.; Yamasaki, M.; Kanemura, Y.; Shizuko, I.; Shimazaki, T.; Onodera, M.; Okano, H.; Mizusawa, H. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J. Neurosci. Res. 2004, 78, 215–223. [Google Scholar] [CrossRef]
- Fukunaga, A.; Uchida, K.; Hara, K.; Kuroshima, Y.; Kawase, T. Differentiation and angiogenesis of central nervous system stem cells implanted with mesenchyme into ischemic rat brain. Cell Transplant. 1999, 8, 435–441. [Google Scholar] [CrossRef]
- Daadi, M.M.; Li, Z.; Arac, A.; Grueter, B.; Sofilos, M.; Malenka, R.C.; Wu, J.C.; Steinberg, G.K. Molecular and Magnetic Resonance Imaging of Human Embryonic Stem Cell–Derived Neural Stem Cell Grafts in Ischemic Rat Brain. Mol. Ther. 2009, 17, 1282–1291. [Google Scholar] [CrossRef]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Lu, M.; Zhang, X.; Chopp, M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 2001, 189, 49–57. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Bates, D.; King, B.; Case, C. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef]
- Luan, X.; Qiu, H.; Hong, X.; Wu, C.; Zhao, K.; Chen, H.; Zhu, Z.; Li, X.; Shen, H.; He, J. High serum nerve growth factor concentrations are associated with good functional outcome at 3 months following acute ischemic stroke. Clin. Chim. Acta 2018, 488, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.-J.; Li, Z.; Qiu, J.-Y.; Zheng, X.-X.; Bian, T.-T.; Gao, F.-L.; Yu, Y.-Y.; Yang, D.-Z.; Tang, D.-Q. Screening for Potential Bioactive Components in Ginkgo biloba Extract by the Rat Renal Tubular Epithelial Cell Extraction and LC-MS/MS. Comb. Chem. High Throughput Screen. 2015, 18, 514–523. [Google Scholar] [CrossRef]
- Tang, W.; Lv, X.; Huang, J.; Wang, B.; Lin, L.; Shen, Y.; Yao, Y. Neuroprotective Effect of Stroke Pretreated Mesenchymal Stem Cells Against Cerebral Ischemia/Reperfusion Injury in Rats. World Neurosurg. 2021. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Metheny, L.; Caimi, P.; De Lima, M. Cord Blood Transplantation: Can We Make it Better? Front. Oncol. 2013, 3, 238. [Google Scholar] [CrossRef]
- Gupta, A.O.; Wagner, J.E. Umbilical Cord Blood Transplants: Current Status and Evolving Therapies. Front. Pediatr. 2020, 8, 570282. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.L.; Yuan, C.; Crane, A.T.; Voth, J.; Juliano, M.; Stone, L.L.H.; Nan, Z.; Zhang, Y.; Kuzmin-Nichols, N.; Sanberg, P.R.; et al. Immunomodulation with Human Umbilical Cord Blood Stem Cells Ameliorates Ischemic Brain Injury—A Brain Transcriptome Profiling Analysis. Cell Transplant. 2019, 28, 864–873. [Google Scholar] [CrossRef]
- Stone, L.L.H.; Xiao, F.; Rotschafer, J.; Nan, Z.; Juliano, M.; Sanberg, C.D.; Sanberg, P.R.; Kuzmin-Nichols, N.; Grande, A.; Cheeran, M.; et al. Amelioration of Ischemic Brain Injury in Rats with Human Umbilical Cord Blood Stem Cells: Mechanisms of Action. Cell Transplant. 2016, 25, 1473–1488. [Google Scholar] [CrossRef]
- Levine, D.A.; Galecki, A.T.; Langa, K.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Wadley, V.G. Trajectory of Cognitive Decline After Incident Stroke. JAMA 2015, 314, 41–51. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Pösel, C.; Uri, A.; Schulz, I.; Boltze, J.; Weise, G.; Wagner, D.-C. Flow cytometric characterization of brain dendritic cell subsets after murine stroke. Exp. Transl. Stroke Med. 2014, 6, 11. [Google Scholar] [CrossRef][Green Version]
- Felger, J.C.; Abe, T.; Kaunzner, U.W.; Gottfried-Blackmore, A.; Gal-Toth, J.; McEwen, B.S.; Iadecola, C.; Bulloch, K. Brain dendritic cells in ischemic stroke: Time course, activation state, and origin. Brain Behav. Immun. 2010, 24, 724–737. [Google Scholar] [CrossRef]
- Schwab, J.; Nguyen, T.; Meyermann, R.; Schluesener, H. Human focal cerebral infarctions induce differential lesional interleukin-16 (IL-16) expression confined to infiltrating granulocytes, CD8+ T-lymphocytes and activated microglia/macrophages. J. Neuroimmunol. 2001, 114, 232–241. [Google Scholar] [CrossRef]
- Miro-Mur, F.A.; Urra, X.; Gallizioli, M.; Chamorro, A.; Planas, A.M. Antigen Presentation after Stroke. Neurotherapeutics 2016, 13, 719–728. [Google Scholar] [CrossRef]
- Gottfried-Blackmore, A.; Kaunzner, U.W.; Idoyaga, J.; Felger, J.C.; McEwen, B.S.; Bulloch, K. Acute in vivo exposure to interferon- enables resident brain dendritic cells to become effective antigen presenting cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20918–20923. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.A.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Naranjo Golborne, C.; Chinnery, H.R.; Ruitenberg, M.J.; McMenamin, P.G. A case of mistaken identity: CD11c-eYFP+ cells in the normal mouse brain parenchyma and neural retina display the phenotype of microglia, not dendritic cells. Glia 2016, 64, 1331–1349. [Google Scholar] [CrossRef] [PubMed]
- Gregerson, D.S.; Sam, T.N.; McPherson, S.W. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J. Immunol. 2004, 172, 6587–6597. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Løbner, M.; Cédile, O.; Owens, T. Comparison of microglia and infiltrating CD11c+ cells as antigen presenting cells for T cell proliferation and cytokine response. J. Neuroinflamm. 2014, 11, 57. [Google Scholar] [CrossRef]
- Schetters, S.T.T.; Gomez-Nicola, D.; Garcia-Vallejo, J.J.; Van Kooyk, Y. Neuroinflammation: Microglia and T Cells Get Ready to Tango. Front. Immunol. 2018, 8, 1905. [Google Scholar] [CrossRef]
- Parachikova, A.; Agadjanyan, M.; Cribbs, D.; Blurton-Jones, M.; Perreau, V.; Rogers, J.; Beach, T.; Cotman, C. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol. Aging 2007, 28, 1821–1833. [Google Scholar] [CrossRef]
- Rogers, J.; Luber-Narod, J.; Styren, S.D.; Civin, W.H. Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 1988, 9, 339–349. [Google Scholar] [CrossRef]
- Planas, A.M.; Gómez-Choco, M.; Urra, X.; Gorina, R.; Caballero, M.; Chamorro, Á. Brain-Derived Antigens in Lymphoid Tissue of Patients with Acute Stroke. J. Immunol. 2012, 188, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Lindsell, C.; Broderick, J.; Fagan, S.C.; Tilley, B.C.; Levine, S.R. Association of serial biochemical markers with acute ischemic stroke: The National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 2006, 37, 2508–2513. [Google Scholar] [CrossRef]
- Doyle, K.; Quach, L.N.; Solé, M.; Axtell, R.C.; Nguyen, T.-V.V.; Soler-Llavina, G.J.; Jurado, S.; Han, J.; Steinman, L.; Longo, F.M.; et al. B-Lymphocyte-Mediated Delayed Cognitive Impairment following Stroke. J. Neurosci. 2015, 35, 2133–2145. [Google Scholar] [CrossRef]
- Bornstein, N.; Aronovich, B.; Korczyn, A.; Shavit, S.; Michaelson, D.; Chapman, J. Antibodies to brain antigens following stroke. Neurology 2001, 56, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Prüss, H.; Iggena, D.; Baldinger, T.; Prinz, V.; Meisel, A.; Endres, M.; Dirnagl, U.; Schwab, J. Evidence of intrathecal immunoglobulin synthesis in stroke: A cohort study. Arch. Neurol. 2012, 69, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Dziennis, S.; Palmateer, J.; Ren, X.; Vandenbark, A.A.; Offner, H.; Herson, P.S.; Hurn, P.D. Recombinant T Cell Receptor Ligands Improve Outcome After Experimental Cerebral Ischemia. Transl. Stroke Res. 2011, 2, 404–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, W.; Casper, A.; Libal, N.L.; Murphy, S.J.; Bodhankar, S.; Offner, H.; Alkayed, N.J. Preclinical evaluation of recombinant T cell receptor ligand RTL1000 as a therapeutic agent in ischemic stroke. Transl. Stroke Res. 2014, 6, 60–68. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Q.; Xu, J.; Benedek, G.; Zhang, H.; Yang, Y.; Liu, H.; Meza-Romero, R.; Vandenbark, A.A.; Offner, H.; et al. DRα1-MOG-35-55 Reduces Permanent Ischemic Brain Injury. Transl. Stroke Res. 2016, 8, 284–293. [Google Scholar] [CrossRef]
- Kou, D.; Li, T.; Liu, H.; Liu, C.; Yin, Y.; Wu, X.; Yu, T. Transplantation of rat-derived microglial cells promotes functional recovery in a rat model of spinal cord injury. Braz. J. Med. Biol. Res. 2018, 51, e7076. [Google Scholar] [CrossRef]
- Takata, K.; Kitamura, Y.; Yanagisawa, D.; Morikawa, S.; Morita, M.; Inubushi, T.; Tsuchiya, D.; Chishiro, S.; Saeki, M.; Taniguchi, T.; et al. Microglial transplantation increases amyloid-β clearance in Alzheimer model rats. FEBS Lett. 2007, 581, 475–478. [Google Scholar] [CrossRef]
- Wei, L.; Wei, Z.Z.; Jiang, M.Q.; Mohamad, O.; Yu, S.P. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog. Neurobiol. 2017, 157, 49–78. [Google Scholar] [CrossRef]
- Thiel, A.; Cechetto, D.F.; Heiss, W.-D.; Hachinski, V.; Whitehead, S.N. Amyloid Burden, Neuroinflammation, and Links to Cognitive Decline after Ischemic Stroke. Stroke 2014, 45, 2825–2829. [Google Scholar] [CrossRef]
- Basu, A.; Lazovic, J.; Krady, J.K.; Mauger, D.T.; Rothstein, R.P.; Smith, M.B.; Levison, S. Interleukin-1 and the Interleukin-1 Type 1 Receptor are Essential for the Progressive Neurodegeneration that Ensues Subsequent to a Mild Hypoxic/Ischemic Injury. Br. J. Pharmacol. 2005, 25, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Zhang, H.; Ye, Q.; Wang, J.; Yang, B.; Mao, L.; Zhu, W.; Leak, R.; Xiao, B.; et al. ST2/IL-33-Dependent Microglial Response Limits Acute Ischemic Brain Injury. J. Neurosci. 2017, 37, 4692–4704. [Google Scholar] [CrossRef]
- Narantuya, D.; Nagai, A.; Sheikh, A.M.; Masuda, J.; Kobayashi, S.; Yamaguchi, S.; Kim, S.U. Human Microglia Transplanted in Rat Focal Ischemia Brain Induce Neuroprotection and Behavioral Improvement. PLoS ONE 2010, 5, e11746. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Yano, S.; Mitaki, S.; Haque, M.A.; Yamaguchi, S.; Nagai, A. A Mesenchymal stem cell line (B10) increases angiogenesis in a rat MCAO model. Exp. Neurol. 2018, 311, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Narantuya, D.; Nagai, A.; Sheikh, A.M.; Wakabayashi, K.; Shiota, Y.; Watanabe, T.; Masuda, J.; Kobayashi, S.; Kim, S.U.; Yamaguchi, S. Microglia transplantation attenuates white matter injury in rat chronic ischemia model via matrix metalloproteinase-2 inhibition. Brain Res. 2010, 1316, 145–152. [Google Scholar] [CrossRef]
- Quarta, A.; Le Blon, D.; D’Aes, T.; Pieters, Z.; Taj, S.H.; Miro-Mur, F.A.; Luyckx, E.; Van Breedam, E.; Daans, J.; Goossens, H.; et al. Murine iPSC-derived microglia and macrophage cell culture models recapitulate distinct phenotypical and functional properties of classical and alternative neuro-immune polarisation. Brain Behav. Immun. 2019, 82, 406–421. [Google Scholar] [CrossRef]
- Svoboda, D.S.; Barrasa, M.I.; Shu, J.; Rietjens, R.; Zhang, S.; Mitalipova, M.; Berube, P.; Fu, D.; Shultz, L.D.; Bell, G.W.; et al. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc. Natl. Acad. Sci. USA 2019, 116, 25293–25303. [Google Scholar] [CrossRef]
- Xu, R.; Li, X.; Boreland, A.; Posyton, A.; Kwan, K.; Hart, R.P.; Jiang, P. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tatarishvili, J.; Oki, K.; Monni, E.; Koch, P.; Memanishvili, T.; Buga, A.-M.; Verma, V.; Popa-Wagner, A.; Brüstle, O.; Lindvall, O.; et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor. Neurol. Neurosci. 2014, 32, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, B.; Saito, H.; Shinozaki, Y.; Shigetomi, E.; Miwa, H.; Yoneda, S.; Tanimura, M.; Omachi, S.; Asaki, T.; Takahashi, K.; et al. Transnasal transplantation of human induced pluripotent stem cell-derived microglia to the brain of immunocompetent mice. Glia 2021, 69, 2332–2348. [Google Scholar] [CrossRef] [PubMed]
- Imai, F.; Suzuki, H.; Oda, J.; Ninomiya, T.; Ono, K.; Sano, H.; Sawada, M. Neuroprotective Effect of Exogenous Microglia in Global Brain Ischemia. Br. J. Pharmacol. 2006, 27, 488–500. [Google Scholar] [CrossRef]
- Kanazawa, M.; Miura, M.; Toriyabe, M.; Koyama, M.; Hatakeyama, M.; Ishikawa, M.; Nakajima, T.; Onodera, O.; Takahashi, T.; Nishizawa, M.; et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 2017, 7, 42582. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Z.; Qu, G.; Wang, H.; Lin, L.; Li, X.; Xie, X.; Lei, Y.; He, X.; Chen, Y.; et al. Hypoxic Preconditioning Enhances the Efficacy of Mesenchymal Stem Cells-Derived Conditioned Medium in Switching Microglia toward Anti-inflammatory Polarization in Ischemia/Reperfusion. Cell. Mol. Neurobiol. 2020, 41, 505–524. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).