Microfluidic Characterization of Red Blood Cells Microcirculation under Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
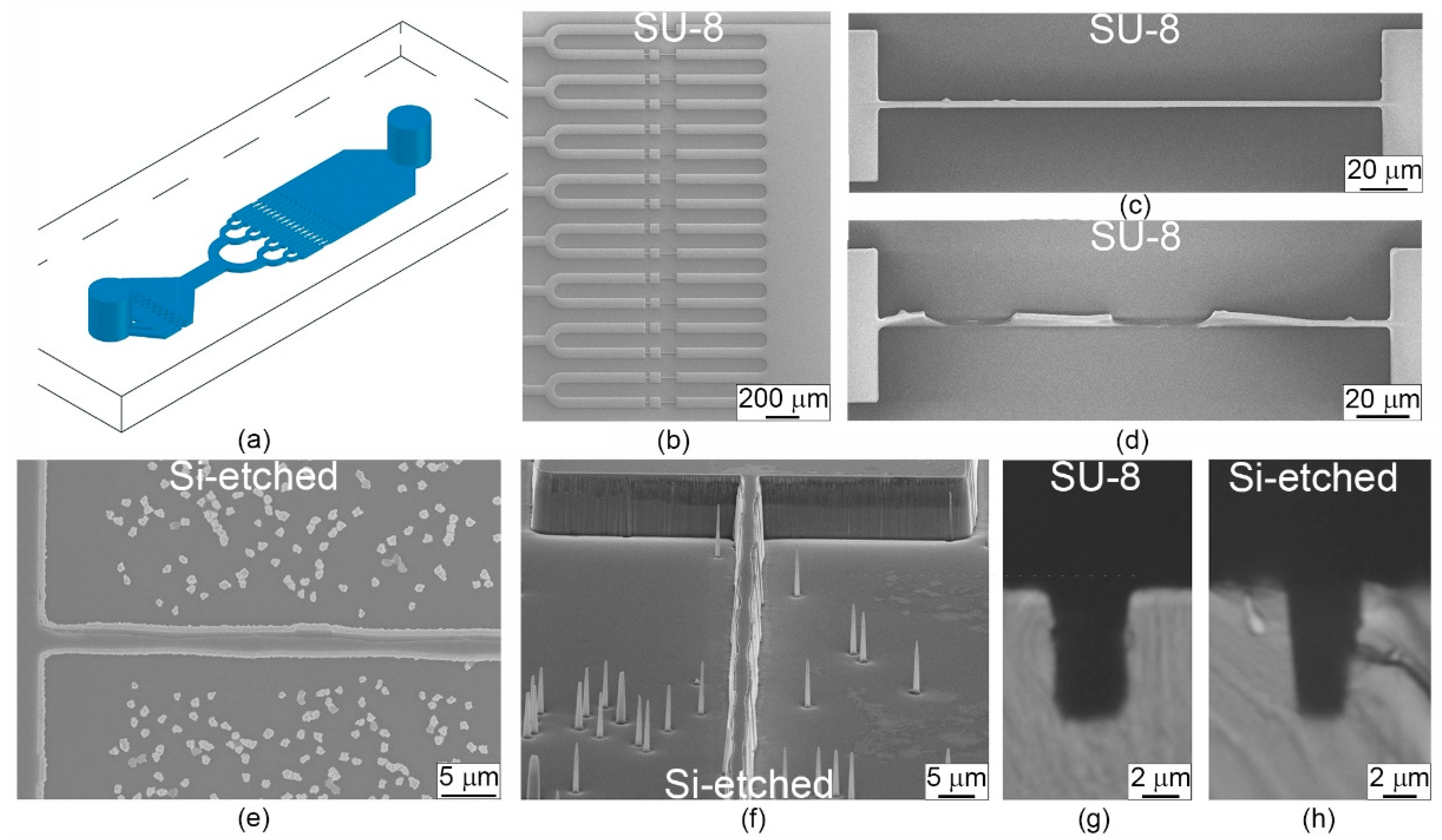
2.2. Fabrication of Microfluidic Devices
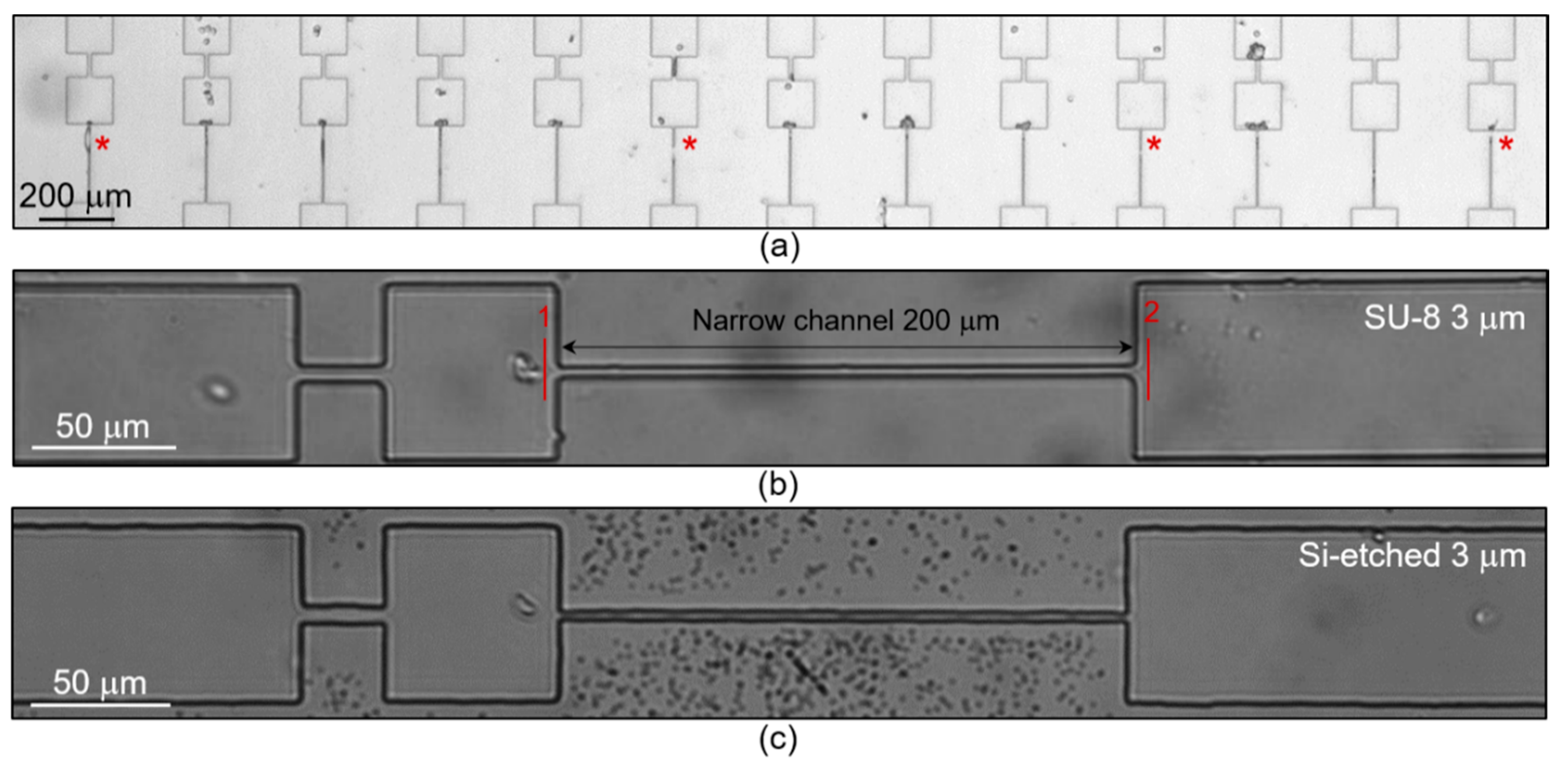
2.3. Microfluidic Experimental Procedure and Image Analysis
2.4. Osmotic Fragility Test
2.5. Statistics
3. Results and Discussion
3.1. Design of Microfluidic Chip
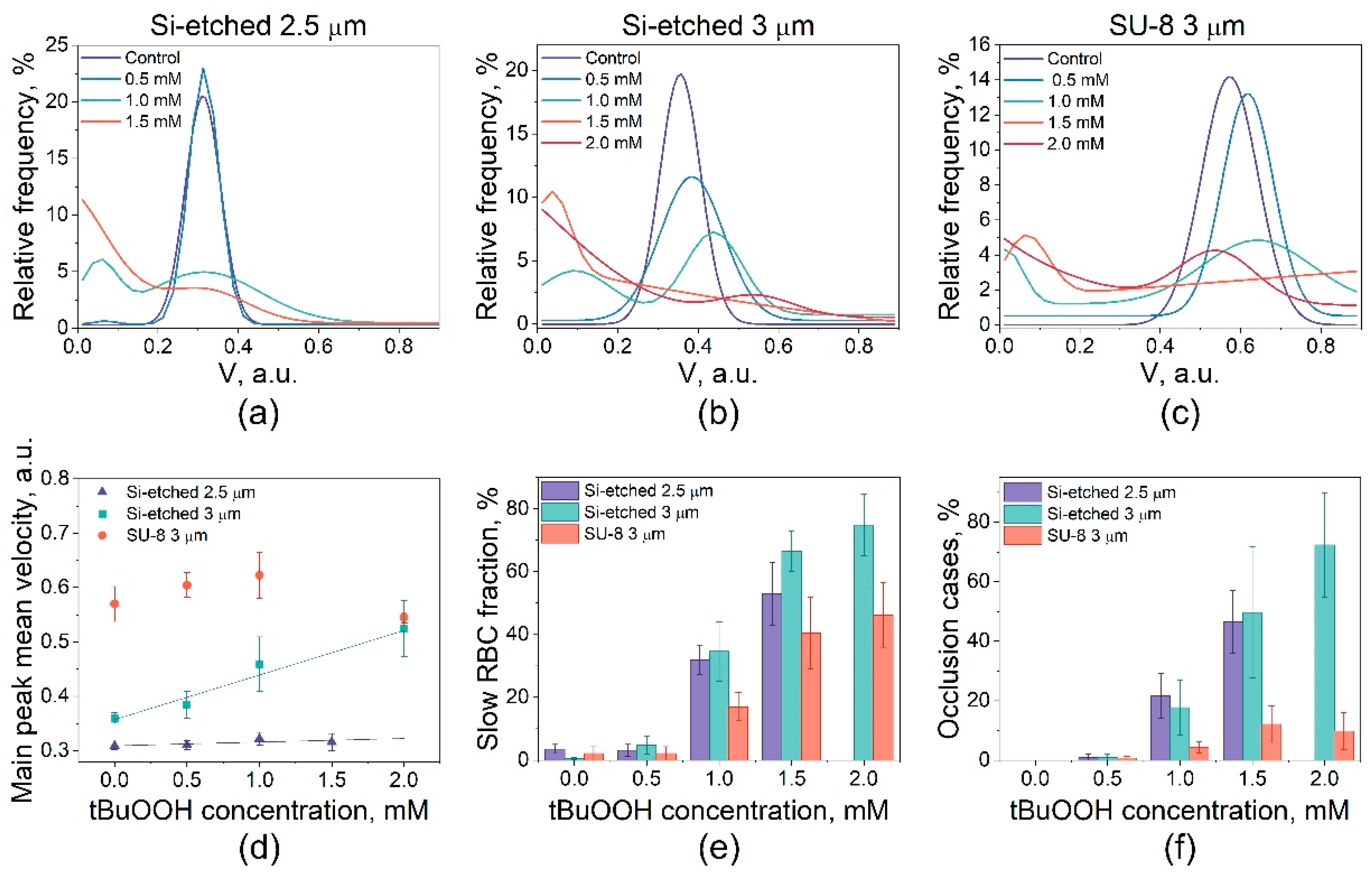
3.2. Study of the RBCs Transport in Microchannels under Oxidative Stress
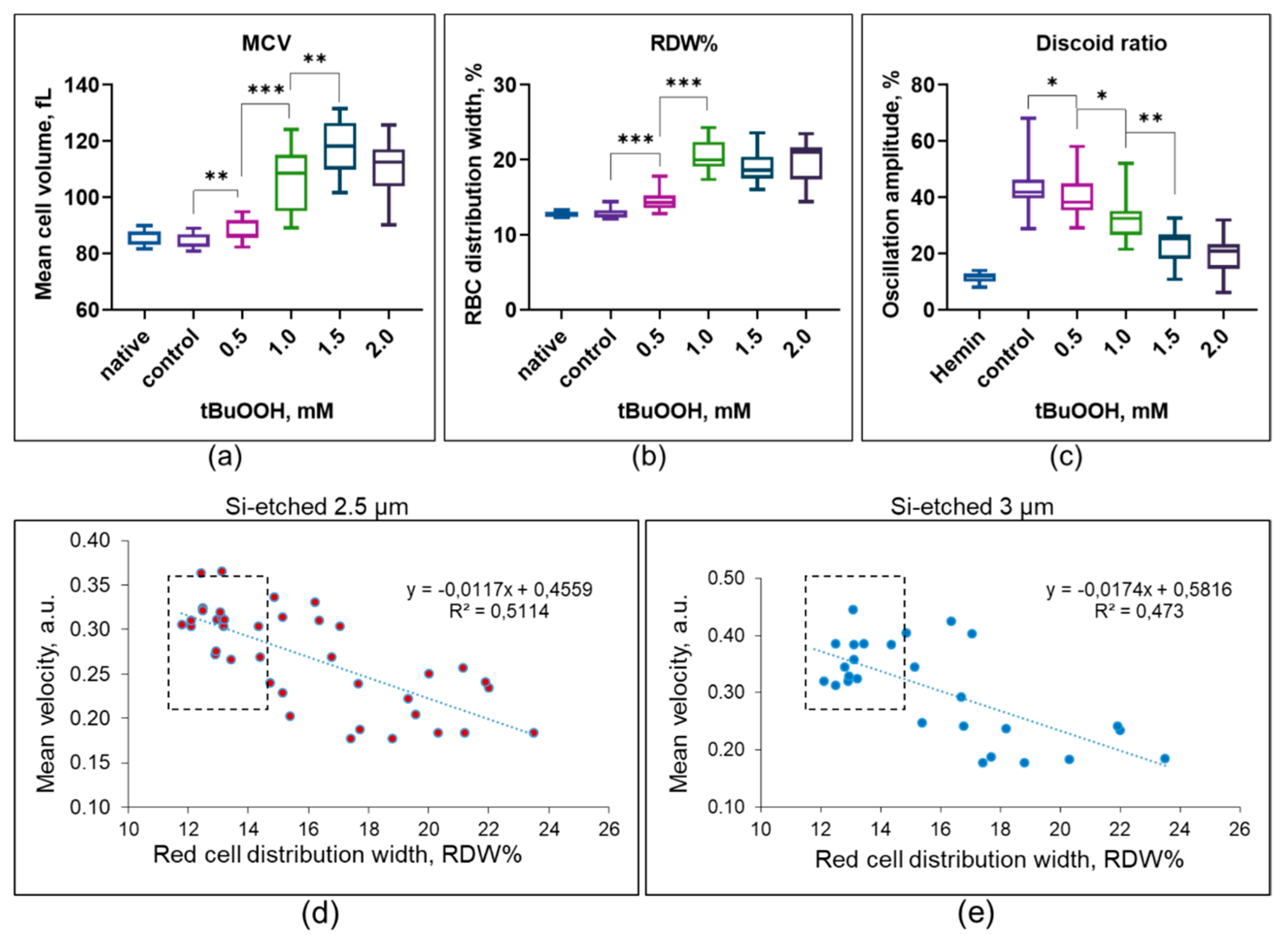
3.3. Hematological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in Organ-on-a-Chip Engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-Chip: Recent Breakthroughs and Future Prospects. BioMed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/Body-on-a-Chip Based on Microfluidic Technology for Drug Discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Geiser, T.; et al. Second-Generation Lung-on-a-Chip with an Array of Stretchable Alveoli Made with a Biological Membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]
- Huang, D.; Liu, T.; Liao, J.; Maharjan, S.; Xie, X.; Pérez, M.; Anaya, I.; Wang, S.; Tirado Mayer, A.; Kang, Z.; et al. Reversed-Engineered Human Alveolar Lung-on-a-Chip Model. Proc. Natl. Acad. Sci. USA 2021, 118, e2016146118. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of Human Organs-on-Chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef]
- Wilmer, M.J.; Ng, C.P.; Lanz, H.L.; Vulto, P.; Suter-Dick, L.; Masereeuw, R. Kidney-on-a-Chip Technology for Drug-Induced Nephrotoxicity Screening. Trends Biotechnol. 2016, 34, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S. Kidney-on-a-Chip: A New Technology for Predicting Drug Efficacy, Interactions, and Drug-Induced Nephrotoxicity. CDM 2018, 19, 577–583. [Google Scholar] [CrossRef]
- Yin, L.; Du, G.; Zhang, B.; Zhang, H.; Yin, R.; Zhang, W.; Yang, S.-M. Efficient Drug Screening and Nephrotoxicity Assessment on Co-Culture Microfluidic Kidney Chip. Sci. Rep. 2020, 10, 6568. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Jeong, S.; Choi, N.; Kim, H.N. Brain-on-a-Chip: A History of Development and Future Perspective. Biomicrofluidics 2019, 13, 051301. [Google Scholar] [CrossRef] [PubMed]
- Cluitmans, J.C.A.; Hardeman, M.R. Red Blood Cell Deformability during Storage: Towards Functional Proteomics and Metabolomics in the Blood Bank. Blood Transfus. 2012, 10, s12–s18. [Google Scholar] [PubMed]
- Barshtein, G.; Pajic-Lijakovic, I.; Gural, A. Deformability of Stored Red Blood Cells. Front. Physiol. 2021, 12, 722896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Shin, S. Advances in the Measurement of Red Blood Cell Deformability: A Brief Review. JCB 2015, 1, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A Comparison of Methods to Assess Cell Mechanical Properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, M. Studying the Mechanics of Cellular Processes by Atomic Force Microscopy. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 83, pp. 347–372. ISBN 978-0-12-370500-6. [Google Scholar]
- González-Bermúdez, B.; Guinea, G.V.; Plaza, G.R. Advances in Micropipette Aspiration: Applications in Cell Biomechanics, Models, and Extended Studies. Biophys. J. 2019, 116, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Käs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef] [Green Version]
- Urbanska, M.; Muñoz, H.E.; Shaw Bagnall, J.; Otto, O.; Manalis, S.R.; Di Carlo, D.; Guck, J. A Comparison of Microfluidic Methods for High-Throughput Cell Deformability Measurements. Nat. Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Rosenbluth, M.J.; Lam, W.A.; Fletcher, D.A. Analyzing Cell Mechanics in Hematologic Diseases with Microfluidic Biophysical Flow Cytometry. Lab Chip 2008, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Son, S.; Amodei, D.; Cermak, N.; Shaw, J.; Kang, J.H.; Hecht, V.C.; Winslow, M.M.; Jacks, T.; Mallick, P.; et al. Characterizing Deformability and Surface Friction of Cancer Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7580–7585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, J.R.; Steinwachs, J.; Kolb, T.; Lautscham, L.A.; Harder, I.; Whyte, G.; Fabry, B. Microconstriction Arrays for High-Throughput Quantitative Measurements of Cell Mechanical Properties. Biophys. J. 2015, 109, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyberg, K.D.; Hu, K.H.; Kleinman, S.H.; Khismatullin, D.B.; Butte, M.J.; Rowat, A.C. Quantitative Deformability Cytometry: Rapid, Calibrated Measurements of Cell Mechanical Properties. Biophys. J. 2017, 113, 1574–1584. [Google Scholar] [CrossRef] [Green Version]
- Otto, O.; Rosendahl, P.; Mietke, A.; Golfier, S.; Herold, C.; Klaue, D.; Girardo, S.; Pagliara, S.; Ekpenyong, A.; Jacobi, A.; et al. Real-Time Deformability Cytometry: On-the-Fly Cell Mechanical Phenotyping. Nat. Methods 2015, 12, 199–202. [Google Scholar] [CrossRef]
- Gossett, D.R.; Tse, H.T.K.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Di Carlo, D. Hydrodynamic Stretching of Single Cells for Large Population Mechanical Phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [Green Version]
- Barshtein, G.; Gural, A.; Zelig, O.; Arbell, D.; Yedgar, S. Unit-to-Unit Variability in the Deformability of Red Blood Cells. Transfus. Apher. Sci. 2020, 59, 102876. [Google Scholar] [CrossRef]
- Yalcin, O.; Ortiz, D.; Tsai, A.G.; Johnson, P.C.; Cabrales, P. Microhemodynamic Aberrations Created by Transfusion of Stored Blood: Storage Lesions Effects on Cell Dynamics. Transfusion 2014, 54, 1015–1027. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.W.; Bhagat, A.A.S.; Lin Chong, A.G.; Mao, P.; Wei Tan, K.S.; Han, J.; Lim, C.T. Deformability Based Cell Margination—A Simple Microfluidic Design for Malaria-Infected Erythrocyte Separation. Lab Chip 2010, 10, 2605. [Google Scholar] [CrossRef]
- Man, Y.; Kucukal, E.; An, R.; Watson, Q.D.; Bosch, J.; Zimmerman, P.A.; Little, J.A.; Gurkan, U.A. Microfluidic Assessment of Red Blood Cell Mediated Microvascular Occlusion. Lab Chip 2020, 20, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Aich, A.; Lamarre, Y.; Sacomani, D.P.; Kashima, S.; Covas, D.T.; de la Torre, L.G. Microfluidics in Sickle Cell Disease Research: State of the Art and a Perspective Beyond the Flow Problem. Front. Mol. Biosci. 2021, 7, 558982. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.K.; Won, D.I.; Shin, S.; Suh, J.S. Assessment of Hemorheological Deformability of Human Red Cells Exposed to Tert -Butyl Hydroperoxide, Verapamil and Ascorbate by Ektacytometer. Ann. Lab. Med. 2008, 28, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skverchinskaya, E.A.; Tapinova, O.D.; Filatov, N.A.; Besedina, N.A.; Mindukshev, I.V.; Bukatin, A.S. Investigation of Erythrocyte Transport through Microchannels After the Induction of Oxidative Stress with Tert-Butyl Peroxide. Tech. Phys. 2020, 65, 1491–1496. [Google Scholar] [CrossRef]
- Arashiki, N.; Kimata, N.; Manno, S.; Mohandas, N.; Takakuwa, Y. Membrane Peroxidation and Methemoglobin Formation Are Both Necessary for Band 3 Clustering: Mechanistic Insights into Human Erythrocyte Senescence. Biochemistry 2013, 52, 5760–5769. [Google Scholar] [CrossRef] [Green Version]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.P.; Winlove, C.P.; Petrov, P.G. Effect of Hydroperoxides on Red Blood Cell Membrane Mechanical Properties. Biophys. J. 2011, 101, 1921–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft Lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Bukatin, A.S.; Mukhin, I.S.; Malyshev, E.I.; Kukhtevich, I.V.; Evstrapov, A.A.; Dubina, M.V. Fabrication of High-Aspect-Ratio Microstructures in Polymer Microfluid Chips for in Vitro Single-Cell Analysis. Tech. Phys. 2016, 61, 1566–1571. [Google Scholar] [CrossRef]
- Mindukshev, I.V.; Kudryavtsev, I.V.; Serebriakova, M.K.; Trulioff, A.S.; Gambaryan, S.P.; Sudnitsyna, J.S.; Avdonin, P.V.; Jenkins, R.O.; Goncharov, N.V. Flow Cytometry and Light-Scattering Techniques in Evaluation of Nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 379–393. ISBN 978-0-12-821038-3. [Google Scholar]
- Mindukshev, I.; Gambaryan, S.; Kehrer, L.; Schuetz, C.; Kobsar, A.; Rukoyatkina, N.; Nikolaev, V.O.; Krivchenko, A.; Watson, S.P.; Walter, U.; et al. Low Angle Light Scattering Analysis: A Novel Quantitative Method for Functional Characterization of Human and Murine Platelet Receptors. Clin. Chem. Lab. Med. 2012, 50, 1253–1262. [Google Scholar] [CrossRef]
- Tsai, C.-H.D.; Sakuma, S.; Arai, F.; Taniguchi, T.; Ohtani, T.; Sakata, Y.; Kaneko, M. Geometrical Alignment for Improving Cell Evaluation in a Microchannel with Application on Multiple Myeloma Red Blood Cells. RSC Adv. 2014, 4, 45050–45058. [Google Scholar] [CrossRef]
- Abgrall, P.; Conedera, V.; Camon, H.; Gue, A.-M.; Nguyen, N.-T. SU-8 as a Structural Material for Labs-on-Chips and Microelectromechanical Systems. Electrophoresis 2007, 28, 4539–4551. [Google Scholar] [CrossRef] [PubMed]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.-T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull. 2010, 35, 382–388. [Google Scholar] [CrossRef]
- Jeong, J.H.; Sugii, Y.; Minamiyama, M.; Okamoto, K. Measurement of RBC Deformation and Velocity in Capillaries in Vivo. Microvasc. Res. 2006, 71, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, E.; Chernysh, A.; Moroz, V.; Gudkova, O.; Sergunova, V.; Kuzovlev, A. Transformation of Membrane Nanosurface of Red Blood Cells under Hemin Action. Sci. Rep. 2015, 4, 6033. [Google Scholar] [CrossRef] [Green Version]
- Bogdanova, A.; Kaestner, L.; Simionato, G.; Wickrema, A.; Makhro, A. Heterogeneity of Red Blood Cells: Causes and Consequences. Front. Physiol. 2020, 11, 392. [Google Scholar] [CrossRef]
- Sinha, A.; Chu, T.T.T.; Dao, M.; Chandramohanadas, R. Single-Cell Evaluation of Red Blood Cell Bio-Mechanical and Nano-Structural Alterations upon Chemically Induced Oxidative Stress. Sci. Rep. 2015, 5, 9768. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, C.; Guo, K.; Huang, C.; Mo, L. Prognostic Role of Red Blood Cell Distribution Width in Patients with Sepsis: A Systematic Review and Meta-Analysis. BMC Immunol. 2020, 21, 40. [Google Scholar] [CrossRef]
- Luo, R.; Hu, J.; Jiang, L.; Zhang, M. Prognostic Value of Red Blood Cell Distribution Width in Non-Cardiovascular Critically or Acutely Patients: A Systematic Review. PLoS ONE 2016, 11, e0167000. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Benoit, J.L.; Benoit, S.; Pulvino, C.; Berger, B.A.; de Olivera, M.H.S.; Crutchfield, C.A.; Lippi, G. Red Blood Cell Distribution Width (RDW) Predicts COVID-19 Severity: A Prospective, Observational Study from the Cincinnati SARS-CoV-2 Emergency Department Cohort. Diagnostics 2020, 10, 618. [Google Scholar] [CrossRef]
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical Phenotype of Blood Cells Is Altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Faivre, M.; Renoux, C.; Bessaa, A.; Costa, L.D.; Joly, P.; Gauthier, A.; Connes, P. Mechanical Signature of Red Blood Cells Flowing Out of a Microfluidic Constriction Is Impacted by Membrane Elasticity, Cell Surface-to-Volume Ratio and Diseases. Front. Physiol. 2020, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in Cardiovascular Disease Research: State of the Art and Future Outlook. Microsyst. Nanoeng. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
| Channels Size | Number of Donors | Control | tBuOOH | Total | |||
|---|---|---|---|---|---|---|---|
| 0.5 mM | 1 mM | 1.5 mM | 2.0 mM | ||||
| 1.5 µm (SU-8) | 3 | 516 | 427 | - | - | - | 943 |
| 2.5 µm (Si-etched) | 5 | 10,834 | 8450 | 4438 | 3194 | 184 | 27,100 |
| 3 µm (Si-etched) | 3 | 5188 | 4886 | 3915 | 1446 | 674 | 16,109 |
| 3 µm (SU-8) | 3 | 1451 | 1771 | 1575 | 736 | 294 | 5827 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besedina, N.A.; Skverchinskaya, E.A.; Ivanov, A.S.; Kotlyar, K.P.; Morozov, I.A.; Filatov, N.A.; Mindukshev, I.V.; Bukatin, A.S. Microfluidic Characterization of Red Blood Cells Microcirculation under Oxidative Stress. Cells 2021, 10, 3552. https://doi.org/10.3390/cells10123552
Besedina NA, Skverchinskaya EA, Ivanov AS, Kotlyar KP, Morozov IA, Filatov NA, Mindukshev IV, Bukatin AS. Microfluidic Characterization of Red Blood Cells Microcirculation under Oxidative Stress. Cells. 2021; 10(12):3552. https://doi.org/10.3390/cells10123552
Chicago/Turabian StyleBesedina, Nadezhda A., Elisaveta A. Skverchinskaya, Alexander S. Ivanov, Konstantin P. Kotlyar, Ivan A. Morozov, Nikita A. Filatov, Igor V. Mindukshev, and Anton S. Bukatin. 2021. "Microfluidic Characterization of Red Blood Cells Microcirculation under Oxidative Stress" Cells 10, no. 12: 3552. https://doi.org/10.3390/cells10123552
APA StyleBesedina, N. A., Skverchinskaya, E. A., Ivanov, A. S., Kotlyar, K. P., Morozov, I. A., Filatov, N. A., Mindukshev, I. V., & Bukatin, A. S. (2021). Microfluidic Characterization of Red Blood Cells Microcirculation under Oxidative Stress. Cells, 10(12), 3552. https://doi.org/10.3390/cells10123552








