Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Chemicals
2.3. Animal Treatment and Preparation of Brain and Liver Microsomes
2.4. CYP2D Enzyme Activity in Brain and Liver Microsomes
2.5. Evaluation of CYP2D Protein in Brain and Liver Microsomes
2.6. Examination of the Expression of Genes Coding for CYP2D Enzymes in the Brain and Liver
2.7. Data Analysis
3. Results
3.1. Inhibition of CYP2D Activity by Iloperidone in Control Brain and Liver Microsomes (Experiment I)
3.2. The Effect of Chronic Iloperidone Treatment on the CYP2D Activity in the Brain and Liver Microsomes (Experiment II)
3.3. The Effect of Chronic Iloperidone Treatment on the CYP2D Protein Level in Microsomes Derived from the Brain and Liver (Experiment II)
3.4. The Effect of Chronic Iloperidone Treatment on the CYP2D Gene Expression in the Brain and Liver (Experiment II)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2019, 64, 5–17. [Google Scholar] [CrossRef]
- Caccia, S.; Pasina, L.; Nobili, A. New Atypical Antipsychotics for Schizophrenia: Iloperidone. Drug Des. Devel. Ther. 2010, 4, 33–48. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Stahl, S.M. Iloperidone, Asenapine and Lurasidone: A Primer on Their Current Status. Expert Opin. Pharmacother. 2012, 13, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Hertel, P.; Fagerquist, M.V.; Svensson, T.H. Enhanced Cortical Dopamine Output and Antipsychotic-like Effects of Raclopride by Alpha2 Adrenoceptor Blockade. Science 1999, 286, 105–107. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Li, Z.; Kaneda, Y.; Ichikawa, J. Serotonin Receptors: Their Key Role in Drugs to Treat Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Patteet, L.; Morrens, M.; Maudens, K.E.; Niemegeers, P.; Sabbe, B.; Neels, H. Therapeutic Drug Monitoring of Common Antipsychotics. Ther. Drug Monit. 2012, 34, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L. A Review of the Pharmacology, Efficacy and Tolerability of Recently Approved and Upcoming Oral Antipsychotics: An Evidence-Based Medicine Approach. CNS Drugs 2013, 27, 879–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Han, C.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Asenapine, Blonanserin, Iloperidone, Lurasidone, and Sertindole: Distinctive Clinical Characteristics of 5 Novel Atypical Antipsychotics. Clin. Neuropharmacol. 2013, 36, 223–238. [Google Scholar] [CrossRef]
- Scott, L.J. Iloperidone: In Schizophrenia. CNS Drugs 2009, 23, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. A History of the Roles of Cytochrome P450 Enzymes in the Toxicity of Drugs. Toxicol. Res. 2020, 37, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.W.; McLean, K.J.; Grant, J.L.; Makris, T.M. Structure and Function of the Cytochrome P450 Peroxygenase Enzymes. Biochem. Soc. Trans. 2018, 46, 183–196. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Tyndale, R.F. Cytochrome P450-Mediated Drug Metabolism in the Brain. J. Psychiatry Neurosci. 2013, 38, 152–163. [Google Scholar] [CrossRef]
- Toselli, F.; Dodd, P.R.; Gillam, E.M.J. Emerging Roles for Brain Drug-Metabolizing Cytochrome P450 Enzymes in Neuropsychiatric Conditions and Responses to Drugs. Drug Metab. Rev. 2016, 48, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Tolledo, C.; Stocco, M.R.; Miksys, S.; Gonzalez, F.J.; Tyndale, R.F. Human CYP2D6 Is Functional in Brain In Vivo: Evidence from Humanized CYP2D6 Transgenic Mice. Mol. Neurobiol. 2020, 57, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Miksys, S.; Rao, Y.; Sellers, E.M.; Kwan, M.; Mendis, D.; Tyndale, R.F. Regional and Cellular Distribution of CYP2D Subfamily Members in Rat Brain. Xenobiotica 2000, 30, 547–564. [Google Scholar] [CrossRef]
- Hiroi, T.; Chow, T.; Imaoka, S.; Funae, Y. Catalytic Specificity of CYP2D Isoforms in Rat and Human. Drug Metab. Dispos. 2002, 30, 970–976. [Google Scholar] [CrossRef]
- Hiroi, T.; Imaoka, S.; Chow, T.; Funae, Y. Tissue Distributions of CYP2D1, 2D2, 2D3 and 2D4 MRNA in Rats Detected by RT-PCR. Biochim. Biophys. Acta 1998, 1380, 305–312. [Google Scholar] [CrossRef]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the Brain. Drug Metab. Pharmacokinet. 2003, 18, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Zeldin, D.C.; Hoffman, S.M.G.; Maltais, L.J.; Wain, H.M.; Nebert, D.W. Comparison of Cytochrome P450 (CYP) Genes from the Mouse and Human Genomes, Including Nomenclature Recommendations for Genes, Pseudogenes and Alternative-Splice Variants. Pharmacogenetics 2004, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Miksys, S.L.; Gaedigk, A.; Kish, S.J.; Mash, D.C.; Tyndale, R.F. The Neuroprotective Enzyme CYP2D6 Increases in the Brain with Age and Is Lower in Parkinson’s Disease Patients. Neurobiol. Aging 2012, 33, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Haduch, A.; Pukło, R.; Alenina, N.; Nikiforuk, A.; Popik, P.; Bader, M.; Daniel, W.A. The Effect of Ageing and Cerebral Serotonin Deficit on the Activity of Cytochrome P450 2D (CYP2D) in the Brain and Liver of Male Rats. Neurochem. Int. 2020, 141, 104884. [Google Scholar] [CrossRef]
- Miksys, S.; Tyndale, R.F. The Unique Regulation of Brain Cytochrome P450 2 (CYP2) Family Enzymes by Drugs and Genetics. Drug Metab. Rev. 2004, 36, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Bromek, E.; Haduch, A.; Daniel, W.A. The Ability of Cytochrome P450 2D Isoforms to Synthesize Dopamine in the Brain: An in Vitro Study. Eur. J. Pharmacol. 2010, 626, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Haduch, A.; Bromek, E.; Kot, M.; Kamińska, K.; Gołembiowska, K.; Daniel, W.A. The Cytochrome P450 2D-Mediated Formation of Serotonin from 5-Methoxytryptamine in the Brain in Vivo: A Microdialysis Study. J. Neurochem. 2015, 133, 83–92. [Google Scholar] [CrossRef]
- Haduch, A.; Daniel, W.A. The Engagement of Brain Cytochrome P450 in the Metabolism of Endogenous Neuroactive Substrates: A Possible Role in Mental Disorders. Drug Metab. Rev. 2018, 50, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, W.; Hiroi, T.; Shiraishi, M.; Osada, M.; Imaoka, S.; Kominami, S.; Igarashi, T.; Funae, Y. Cytochrome P450 2D Catalyze Steroid 21-Hydroxylation in the Brain. Endocrinology 2004, 145, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Capdevila, J.H.; Strobel, H.W. Recombinant Cytochrome P450 2D18 Metabolism of Dopamine and Arachidonic Acid. J. Pharmacol. Exp. Ther. 2000, 294, 1120–1130. [Google Scholar]
- Laux-Biehlmann, A.; Mouheiche, J.; Vérièpe, J.; Goumon, Y. Endogenous Morphine and Its Metabolites in Mammals: History, Synthesis, Localization and Perspectives. Neuroscience 2013, 233, 95–117. [Google Scholar] [CrossRef]
- Danek, P.J.; Wójcikowski, J.; Daniel, W.A. The Atypical Neuroleptics Iloperidone and Lurasidone Inhibit Human Cytochrome P450 Enzymes in Vitro. Evaluation of Potential Metabolic Interactions. Pharmacol. Rep. 2020, 72, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Danek, P.J.; Wójcikowski, J.; Daniel, W.A. Asenapine and Iloperidone Decrease the Expression of Major Cytochrome P450 Enzymes CYP1A2 and CYP3A4 in Human Hepatocytes. A Significance for Drug-Drug Interactions during Combined Therapy. Toxicol. Appl. Pharmacol. 2020, 406, 115239. [Google Scholar] [CrossRef] [PubMed]
- Boyda, H.N.; Procyshyn, R.M.; Pang, C.C.Y.; Hawkes, E.; Wong, D.; Jin, C.H.; Honer, W.G.; Barr, A.M. Metabolic Side-Effects of the Novel Second-Generation Antipsychotic Drugs Asenapine and Iloperidone: A Comparison with Olanzapine. PLoS ONE 2013, 8, e53459. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Tarazi, F.I. Long-Term Effects of Iloperidone on Cerebral Serotonin and Adrenoceptor Subtypes. J. Mol. Neurosci 2018, 66, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Strupczewski, J.T.; Bordeau, K.J.; Chiang, Y.; Glamkowski, E.J.; Conway, P.G.; Corbett, R.; Hartman, H.B.; Szewczak, M.R.; Wilmot, C.A.; Helsley, G.C. 3-[[(Aryloxy)Alkyl]Piperidinyl]-1,2-Benzisoxazoles as D2/5-HT2 Antagonists with Potential Atypical Antipsychotic Activity: Antipsychotic Profile of Iloperidone (HP 873). J. Med. Chem. 1995, 38, 1119–1131. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2007. [Google Scholar]
- Hiroi, T.; Kishimoto, W.; Chow, T.; Imaoka, S.; Igarashi, T.; Funae, Y. Progesterone Oxidation by Cytochrome P450 2D Isoforms in the Brain. Endocrinology 2001, 142, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Haduch, A.; Bromek, E.; Daniel, W.A. The Effect of Psychotropic Drugs on Cytochrome P450 2D (CYP2D) in Rat Brain. Eur. J. Pharmacol. 2011, 651, 51–58. [Google Scholar] [CrossRef]
- Haduch, A.; Rysz, M.; Papp, M.; Daniel, W.A. The Activity of Brain and Liver Cytochrome P450 2D (CYP2D) Is Differently Affected by Antidepressants in the Chronic Mild Stress (CMS) Model of Depression in the Rat. Biochem. Pharmacol. 2018, 156, 398–405. [Google Scholar] [CrossRef]
- Wójcikowski, J.; Danek, P.J.; Basińska-Ziobroń, A.; Pukło, R.; Daniel, W.A. In Vitro Inhibition of Human Cytochrome P450 Enzymes by the Novel Atypical Antipsychotic Drug Asenapine: A Prediction of Possible Drug-Drug Interactions. Pharmacol. Rep. 2020, 72, 612–621. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bromek, E.; Haduch, A.; Gołembiowska, K.; Daniel, W.A. Cytochrome P450 Mediates Dopamine Formation in the Brain in Vivo. J. Neurochem. 2011, 118, 806–815. [Google Scholar] [CrossRef]
- Danek, P.J.; Bromek, E.; Daniel, W.A. The Influence of Long-Term Treatment with Asenapine on Liver Cytochrome P450 Expression and Activity in the Rat. The Involvement of Different Mechanisms. Pharmaceuticals 2021, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Danek, P.J.; Kuban, W.; Daniel, W.A. The Effect of Chronic Iloperidone Treatment on Cytochrome P450 Expression and Activity in the Rat Liver: Involvement of Neuroendocrine Mechanisms. Int. J. Mol. Sci. 2021, 22, 8447. [Google Scholar] [CrossRef] [PubMed]
- Rysz, M.; Bromek, E.; Haduch, A.; Liskova, B.; Wójcikowski, J.; Daniel, W.A. The Reverse Role of the Hypothalamic Paraventricular (PVN) and Arcuate (ARC) Nuclei in the Central Serotonergic Regulation of the Liver Cytochrome P450 Isoform CYP2C11. Biochem. Pharmacol. 2016, 112, 82–89. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Approval Package: Fanapt (Iloperidone) NDA 022192. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022192s000TOC.cfm (accessed on 27 November 2019).
- Danek, P.J.; Bromek, E.; Haduch, A.; Daniel, W.A. Chronic Treatment with Asenapine Affects Cytochrome P450 2D (CYP2D) in Rat Brain and Liver. Pharmacological Aspects. Neurochem. Int. 2021, 151, 105209. [Google Scholar] [CrossRef]
- Gemperle, A.Y.; Enz, A.; Pozza, M.F.; Lüthi, A.; Olpe, H.R. Effects of Clozapine, Haloperidol and Iloperidone on Neurotransmission and Synaptic Plasticity in Prefrontal Cortex and Their Accumulation in Brain Tissue: An in Vitro Study. Neuroscience 2003, 117, 681–695. [Google Scholar] [CrossRef]
- Daniel, W.A. Mechanisms of Cellular Distribution of Psychotropic Drugs. Significance for Drug Action and Interactions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 65–73. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Daniel, W.A. Role of Brain Cytochrome P450 (CYP2D) in the Metabolism of Monoaminergic Neurotransmitters. Pharmacol Rep. 2013, 65, 1519–1528. [Google Scholar] [CrossRef]
- Hedlund, E.; Wyss, A.; Kainu, T.; Backlund, M.; Köhler, C.; Pelto-Huikko, M.; Gustafsson, J.A.; Warner, M. Cytochrome P4502D4 in the Brain: Specific Neuronal Regulation by Clozapine and Toluene. Mol. Pharmacol 1996, 50, 342–350. [Google Scholar] [PubMed]
- Miller, R.T.; Miksys, S.; Hoffmann, E.; Tyndale, R.F. Ethanol Self-Administration and Nicotine Treatment Increase Brain Levels of CYP2D in African Green Monkeys. Br. J. Pharmacol. 2014, 171, 3077–3088. [Google Scholar] [CrossRef]
- Yue, J.; Miksys, S.; Hoffmann, E.; Tyndale, R.F. Chronic Nicotine Treatment Induces Rat CYP2D in the Brain but Not in the Liver: An Investigation of Induction and Time Course. J. Psychiatry Neurosci. 2008, 33, 54–63. [Google Scholar]
- Kuban, W.; Daniel, W.A. Cytochrome P450 Expression and Regulation in the Brain. Drug Metab. Rev. 2020, 53, 1–29. [Google Scholar] [CrossRef]
- Citrome, L. Asenapine Review, Part I: Chemistry, Receptor Affinity Profile, Pharmacokinetics and Metabolism. Expert Opin. Drug Metab. Toxicol. 2014, 10, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Delcourte, S.; Abrial, E.; Etiévant, A.; Rovera, R.; Arnt, J.; Didriksen, M.; Haddjeri, N. Asenapine Modulates Mood-Related Behaviors and 5-HT1A/7 Receptors-Mediated Neurotransmission. CNS Neurosci. Ther. 2017, 23, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, E.J.; Pickel, V.M. Ultrastructure of Serotonin-Immunoreactive Terminals in the Core and Shell of the Rat Nucleus Accumbens: Cellular Substrates for Interactions with Catecholamine Afferents. J. Comp. Neurol. 1993, 334, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Prensa, L.; Richard, S.; Parent, A. Chemical Anatomy of the Human Ventral Striatum and Adjacent Basal Forebrain Structures. J. Comp. Neurol. 2003, 460, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, Y.; Chaki, S. Neurochemistry of the Nucleus Accumbens and Its Relevance to Depression and Antidepressant Action in Rodents. Curr. Neuropharmacol. 2006, 4, 277–291. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Fan, G.; Li, A.; Yuan, J.; Xu, T. Cell-Type-Specific Afferent Innervation of the Nucleus Accumbens Core and Shell. Front. Neuroanat. 2018, 12, 84. [Google Scholar] [CrossRef]
- Marcus, M.M.; Nomikos, G.G.; Svensson, T.H. Differential Actions of Typical and Atypical Antipsychotic Drugs on Dopamine Release in the Core and Shell of the Nucleus Accumbens. Eur. Neuropsychopharmacol. 1996, 6, 29–38. [Google Scholar] [CrossRef]
- Deutch, A.Y.; Lee, M.C.; Iadarola, M.J. Regionally Specific Effects of Atypical Antipsychotic Drugs on Striatal Fos Expression: The Nucleus Accumbens Shell as a Locus of Antipsychotic Action. Mol. Cell Neurosci. 1992, 3, 332–341. [Google Scholar] [CrossRef]
- Deutch, A.Y.; Cameron, D.S. Pharmacological Characterization of Dopamine Systems in the Nucleus Accumbens Core and Shell. Neuroscience 1992, 46, 49–56. [Google Scholar] [CrossRef]
- McMillan, D.M.; Tyndale, R.F. CYP-Mediated Drug Metabolism in the Brain Impacts Drug Response. Pharmacol. Ther. 2018, 184, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Na, S.; Wu, J.; Yang, Z.; Xie, X.; Wan, Y.; Li, K.; Yue, J. The Involvement of PPARs in the Selective Regulation of Brain CYP2D by Growth Hormone. Neuroscience 2018, 379, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.N.; Emtner, M.; Roos, P.; Nyberg, F. Characterization of Putative Growth Hormone Receptors in Human Choroid Plexus. Brain Res. 1991, 546, 222–226. [Google Scholar] [CrossRef]
- Nyberg, F.; Burman, P. Growth Hormone and Its Receptors in the Central Nervous System—Location and Functional Significance. Horm. Res. 1996, 45, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cadet, P.; Baggerman, G.; Mantione, K.J.; Stefano, G.B. Human White Blood Cells Synthesize Morphine: CYP2D6 Modulation. J. Immunol. 2005, 175, 7357–7362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Stefano, G.B. Comparative Aspects of Endogenous Morphine Synthesis and Signaling in Animals. Ann. N. Y. Acad. Sci. 2009, 1163, 330–339. [Google Scholar] [CrossRef]
- Stefano, G.B.; Kream, R.M.; Mantione, K.J.; Sheehan, M.; Cadet, P.; Zhu, W.; Bilfinger, T.V.; Esch, T. Endogenous Morphine/Nitric Oxide-Coupled Regulation of Cellular Physiology and Gene Expression: Implications for Cancer Biology. Semin. Cancer Biol. 2008, 18, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Khatsenko, O.G.; Gross, S.S.; Rifkind, A.B.; Vane, J.R. Nitric Oxide Is a Mediator of the Decrease in Cytochrome P450-Dependent Metabolism Caused by Immunostimulants. Proc. Natl. Acad. Sci. USA 1993, 90, 11147–11151. [Google Scholar] [CrossRef] [PubMed]
- Mantione, K.J.; Cadet, P.; Zhu, W.; Kream, R.M.; Sheehan, M.; Fricchione, G.L.; Goumon, Y.; Esch, T.; Stefano, G.B. Endogenous Morphine Signaling via Nitric Oxide Regulates the Expression of CYP2D6 and COMT: Autocrine/Paracrine Feedback Inhibition. Addict. Biol. 2008, 13, 118–123. [Google Scholar] [CrossRef]
- Stefano, G.B.; Goumon, Y.; Casares, F.; Cadet, P.; Fricchione, G.L.; Rialas, C.; Peter, D.; Sonetti, D.; Guarna, M.; Welters, I.D.; et al. Endogenous Morphine. Trends Neurosci. 2000, 23, 436–442. [Google Scholar] [CrossRef]
- Niznik, H.B.; Tyndale, R.F.; Sallee, F.R.; Gonzalez, F.J.; Hardwick, J.P.; Inaba, T.; Kalow, W. The Dopamine Transporter and Cytochrome P45OIID1 (Debrisoquine 4-Hydroxylase) in Brain: Resolution and Identification of Two Distinct [3H]GBR-12935 Binding Proteins. Arch. Biochem. Biophys. 1990, 276, 424–432. [Google Scholar] [CrossRef]
- Niwa, T.; Okada, K.; Hiroi, T.; Imaoka, S.; Narimatsu, S.; Funae, Y. Effect of Psychotropic Drugs on the 21-Hydroxylation of Neurosteroids, Progesterone and Allopregnanolone, Catalyzed by Rat CYP2D4 and Human CYP2D6 in the Brain. Biol. Pharmaceutical. Bull. 2008, 31, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Cao, T.; Zhou, X.; Yao, J.K. Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Front. Psychiatry 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
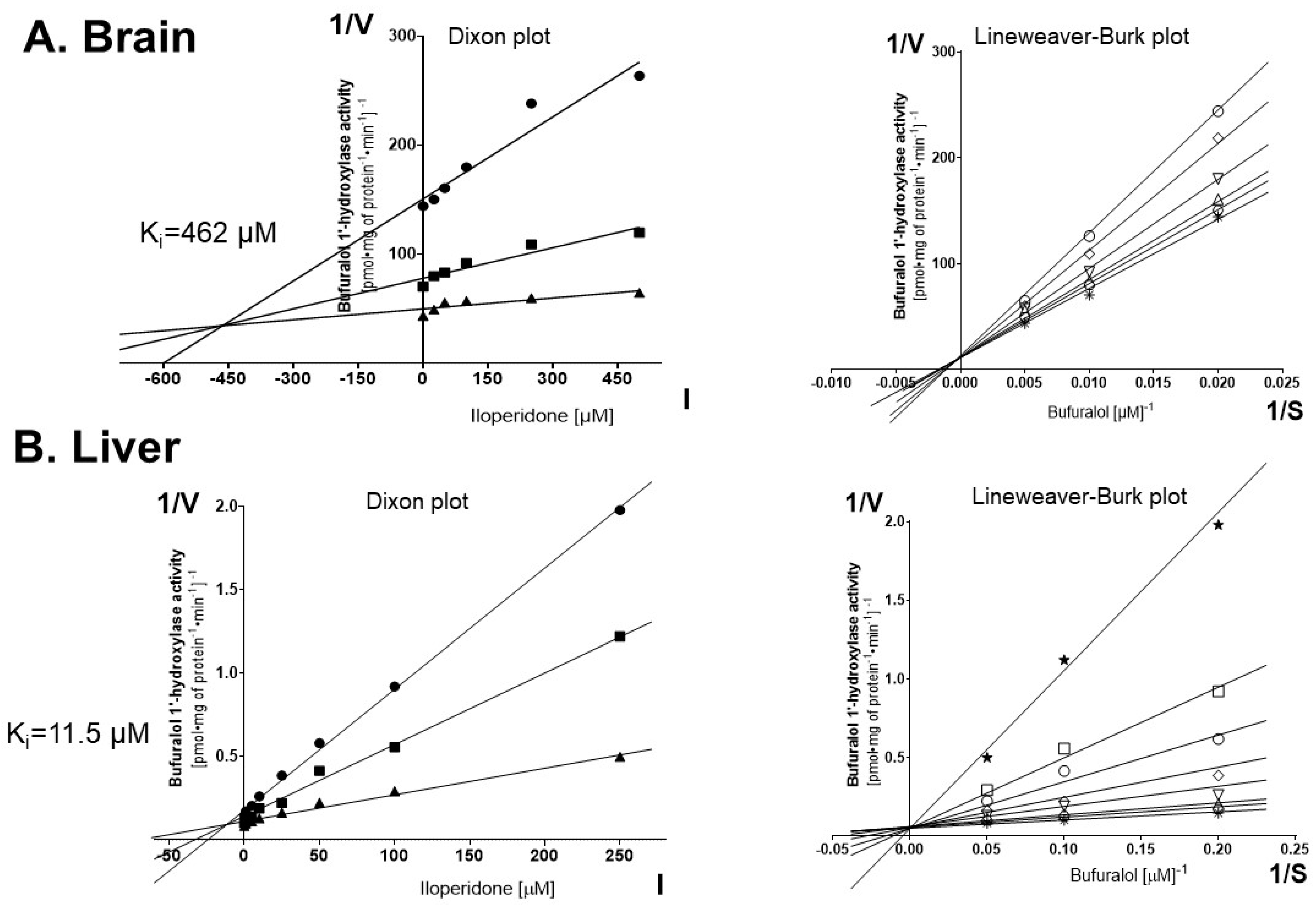

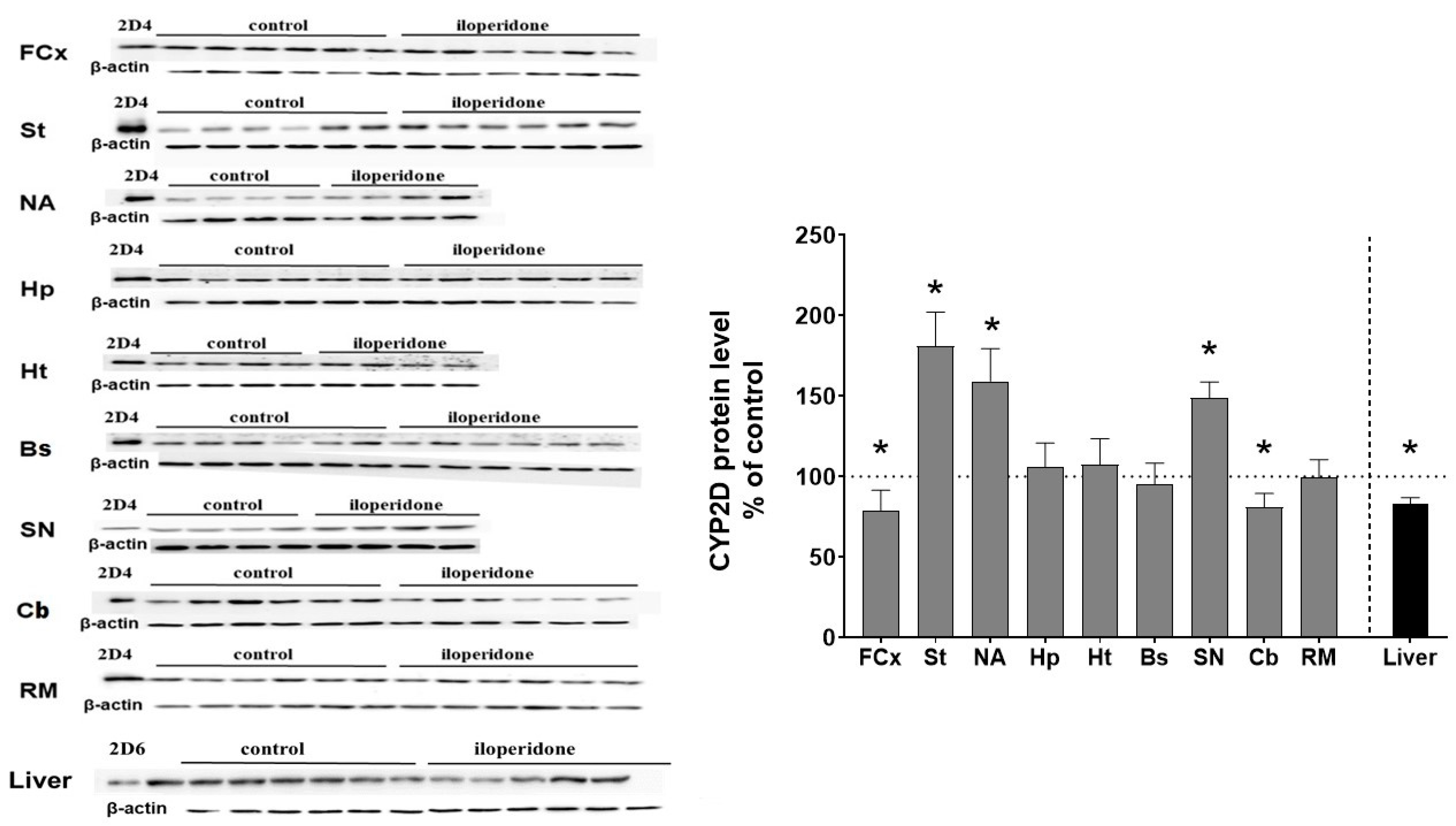
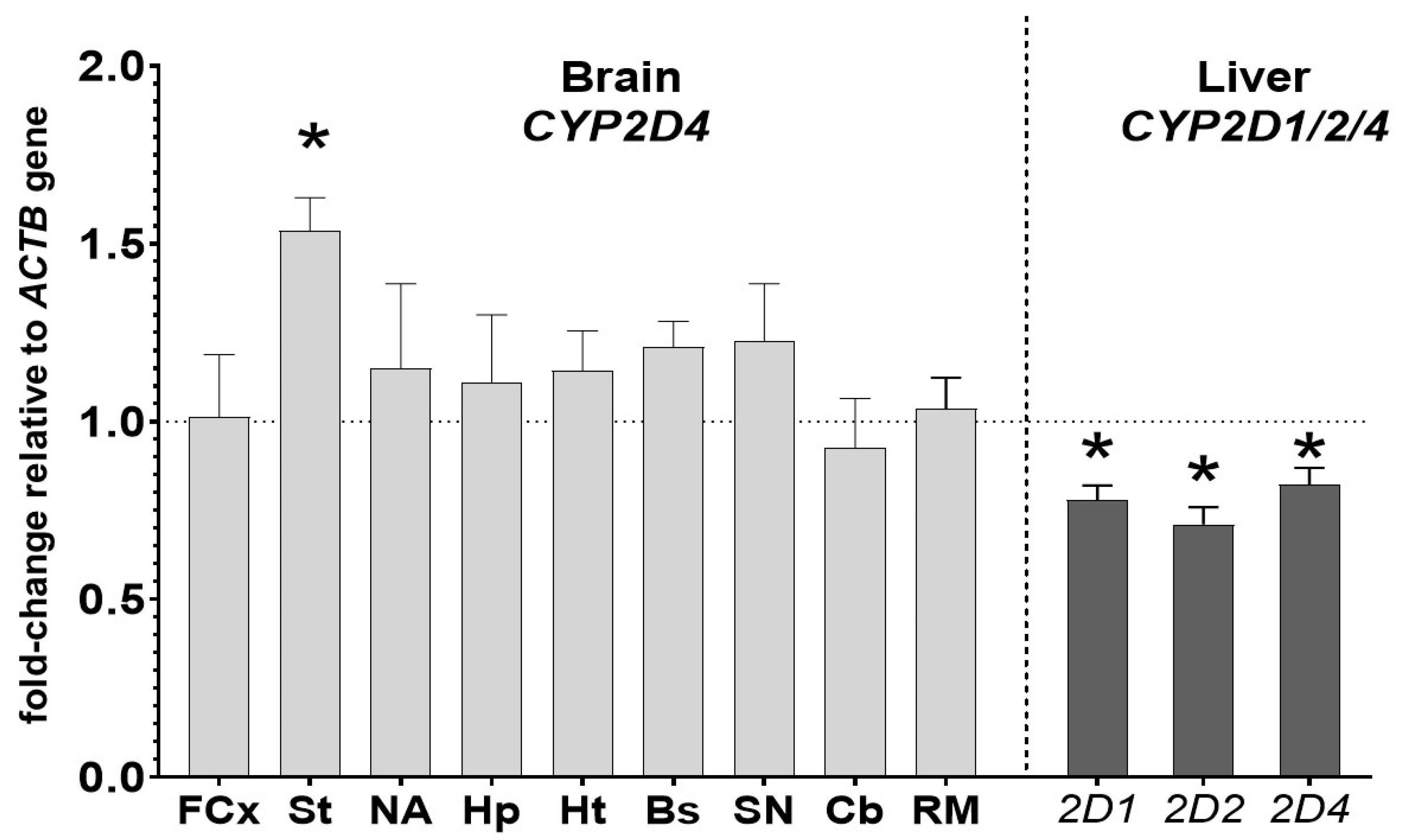
| Tissue | CYP2D Activity (% of Control) | CYP2D Protein Level (% of Control) | CYP2D mRNA Level (% of Control) | |||
|---|---|---|---|---|---|---|
| CYP2D1 | CYP2D2 | CYP2D4 | ||||
| Brain structures | FCx | 67 ± 2.7 ↓ * | 78 ± 12.4 ↓ * | n.t. | n.t. | 101 ± 17.5 |
| St | 124 ± 9.5 ↑ * | 181 ± 21.1 ↑ * | n.t. | n.t. | 153 ± 9.4 ↑ * | |
| NA | 136 ± 5.1 ↑ * | 159 ± 20.3 ↑ * | n.t. | n.t. | 115 ± 23.8 | |
| Hp | 99 ± 13.6 | 105 ± 14.9 | n.t. | n.t. | 111 ± 18.9 | |
| Ht | 93 ± 4.7 | 107 ± 15.9 | n.t. | n.t. | 114 ± 11.2 | |
| Bs | 90 ± 8.1 | 95 ± 12.8 | n.t. | n.t. | 121 ± 7.1 | |
| SN | 126 ± 13.7 ↑ * | 148 ± 9.9 ↑ * | n.t. | n.t. | 123 ± 16 | |
| Cb | 79 ± 8 ↓ * | 80 ± 8.5 ↓ * | n.t. | n.t. | 93 ± 13.8 | |
| RM | 97 ± 8.7 | 99 ± 10.8 | n.t. | n.t. | 103 ± 8.6 | |
| Liver | 67 ± 5.3 ↓ * | 82 ± 4.1 ↓ * | 78 ± 4.1 ↓ * | 71 ± 4.8 ↓ * | 82 ± 4.7 ↓ * | |
| Drug | Liver | Brain Structures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FCx | St | NA | Hp | Ht | Bs | SN | Cb | RM | ||
| Iloperidone (D2, D3, 5-HT2A, α1, α2) |  |  |  |  |  |  |  |  |  |  |
| Asenapine (D1,D2, D3, D4, 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, 5-HT6, 5-HT7, α1, α2, H1) |  |  |  |  |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danek, P.J.; Daniel, W.A. Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms. Cells 2021, 10, 3472. https://doi.org/10.3390/cells10123472
Danek PJ, Daniel WA. Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms. Cells. 2021; 10(12):3472. https://doi.org/10.3390/cells10123472
Chicago/Turabian StyleDanek, Przemysław J., and Władysława A. Daniel. 2021. "Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms" Cells 10, no. 12: 3472. https://doi.org/10.3390/cells10123472
APA StyleDanek, P. J., & Daniel, W. A. (2021). Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms. Cells, 10(12), 3472. https://doi.org/10.3390/cells10123472








