Alterations in Immune Response Profile of Tumor-Draining Lymph Nodes after High-Intensity Focused Ultrasound Ablation of Breast Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HIFU Treatment
2.3. Surgery
2.4. Histomorphological Analysis
2.5. Immunohistochemical Staining
2.6. Statistical Analysis
3. Results
3.1. Clinical and Pathological Characteristics of the Patients with Breast Cancer
3.2. Alterations in the Immune Response of TDLNs after HIFU Treatment
3.3. HIFU Increases Profile of Immune Cells in TDLNs
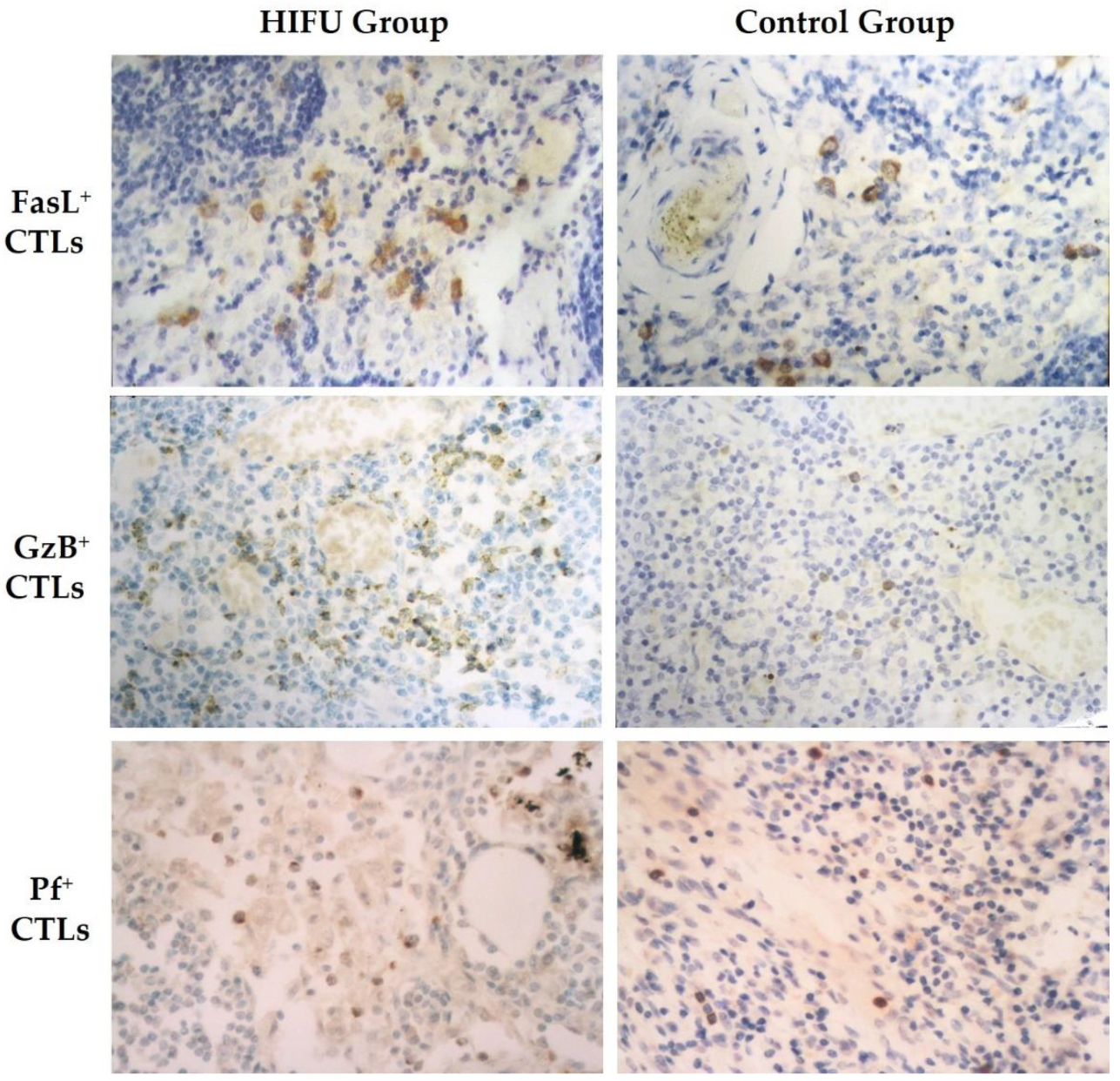
3.4. HIFU Activates Cytotoxic T Lymphocytes in TDLNs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferris, R.L.; Lotze, M.T.; Leong, S.P.L.; Hoon, D.S.B.; Morton, D.L. Lymphatics, lymph nodes and the immune system: Barriers and gateways for cancer spread. Clin. Exp. Metastasis 2012, 29, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; King, M.R. Microenvironment of tumor-draining lymph nodes: Opportunities for liposome-based targeted therapy. Int. J. Mol. Sci. 2014, 15, 20209–20239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giorgio, A.; Mingazzini, P.; Sammartino, P.; Canavese, A.; Arnone, P.; Scarpini, M. Host defense and survival in patients with lung carcinoma. Cancer 2000, 89, 2038–2045. [Google Scholar] [CrossRef]
- Tsakraklides, V.; Anastassiades, O.T.; Kersey, J.H.; Good, R.A. Prognostic significance of the regional lymph node histology in cancer of the breast. Cancer 1974, 34, 1259–1267. [Google Scholar] [CrossRef]
- Cottier, H.; Turk, J.; Sobin, L. A proposal for a standardized system of reporting human lymph node morphology in relation to immunological function. Bull. World Health Organ. 1972, 47, 375–417. [Google Scholar] [CrossRef] [PubMed]
- Check, I.J.; Cobb, M.; Hunter, R.L. The relationship between cytotoxicity and prognostically significant histologic changes in lymph nodes from patients with cancer of the breast. Am. J. Pathol. 1980, 98, 325–338. [Google Scholar]
- Wu, F.; Zhou, L.; Chen, W.R. Host antitumour immune responses to HIFU ablation. Int. J. Hyperth. 2007, 23, 165–171. [Google Scholar] [CrossRef]
- Mauri, G.; Nicosia, L.; Xu, Z.; Di Pietro, S.; Monfardini, L.; Bonomo, G.; Varano, G.M.; Prada, F.; Della Vigna, P.; Orsi, F. Focused ultrasound: Tumour ablation and its potential to enhance immunological therapy to cancer. Br. J. Radiol. 2018, 91, 20170641. [Google Scholar] [CrossRef]
- Wu, F. Heat-based tumor ablation: Role of the immune response. Adv. Exp. Med. Biol. 2016, 880, 131–153. [Google Scholar]
- van den Bijgaart, R.J.; Eikelenboom, D.C.; Hoogenboom, M.; Fütterer, J.J.; den Brok, M.H.; Adema, G.J. Thermal and mechanical high-intensity focused ultrasound: Perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol. Immunother. 2017, 66, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Adnan, A.; Muñoz, N.M.; Prakash, P.; Habibollahi, P.; Cressman, E.N.K.; Sheth, R.A. Hyperthermia and tumor immunity. Cancers 2021, 13, 2507. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Hode, T.; Lam, S.S.K.; Chen, W.R. Novel immune stimulant amplifies direct tumoricidal effect of cancer ablation therapies and their systemic antitumor immune efficacy. Cells 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Shen, Y.; Xie, J.; Meng, Z. Immunomodulatory effects of ablation therapy on tumors: Potentials for combination with immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188385. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Zhu, X.Q.; Lu, P.; Zhou, Q.; Zhang, J.; Wu, F. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med. Biol. 2009, 35, 50–57. [Google Scholar] [CrossRef]
- Lu, P.; Zhu, X.Q.; Xu, Z.L.; Zhou, Q.; Zhang, J.; Wu, F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery 2009, 145, 286–293. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Cao, Y.D.; Chen, W.Z.; Bai, J.; Zou, J.Z.; Zhu, H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br. J. Cancer 2003, 89, 2227–2233. [Google Scholar] [CrossRef]
- Zheng, R.; Shu, S. Immune response to cancer and its regulation in regional lymph nodes. J. Surg. Oncol. 2011, 103, 550–554. [Google Scholar] [CrossRef]
- Grant, S.M.; Lou, M.; Yao, L.; Germain, R.N.; Radtke, A.J. The lymph node at a glance—How spatial organization optimizes the immune response. J. Cell Sci. 2020, 133, jcs241828. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Muscarella, R.A., Jr.; Jones, D. The multifaceted effects of breast cancer on tumor-draining lymph nodes. Am. J. Pathol. 2021, 191, 1353–1363. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 2006, 213, 146–158. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Arenas-Del Angel, M.C.; Zenteno, E.; Chávez, R.; Lascurain, R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol. Immunol. 2009, 6, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Weigelin, B.; Krause, M.; Friedl, P. Cytotoxic T lymphocyte migration and effector function in the tumor microenvironment. Immunol. Lett. 2011, 138, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [Green Version]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Cochran, A.J.; Huang, R.-R.; Lee, J.; Itakura, E.; Leong, S.P.L.; Essner, R. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 2006, 6, 659–670. [Google Scholar] [CrossRef]
- Núñez, N.G.; Tosello Boari, J.; Ramos, R.N.; Richer, W.; Cagnard, N.; Anderfuhren, C.D.; Niborski, L.L.; Bigot, J.; Meseure, D.; De La Rochere, P.; et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat. Commun. 2020, 11, 3272. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- van Pul, K.M.; Fransen, M.F.; van de Ven, R.; de Gruijl, T.D. Immunotherapy goes local: The central role of lymph nodes in driving tumor infiltration and efficacy. Front. Immunol. 2021, 12, 643291. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef] [Green Version]
- Sheybani, N.D.; Witter, A.R.; Thim, E.A.; Yagita, H.; Bullock, T.N.J.; Price, R.J. Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity. J. Immunother. Cancer 2020, 8, e001008. [Google Scholar] [CrossRef] [PubMed]
- Fite, B.Z.; Wang, J.; Kare, A.J.; Ilovitsh, A.; Chavez, M.; Ilovitsh, T.; Zhang, N.; Chen, W.; Robinson, E.; Zhang, H.; et al. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci. Rep. 2021, 11, 927. [Google Scholar] [CrossRef] [PubMed]



| TDLNs Immunoreactivity Pattern | Control Group (n = 25) | HIFU Group (n = 23) |
|---|---|---|
| No immune response # | 9 (36%) | 0 (0%) * |
| Immune response | 16 (64%) | 23 (100%) * |
| Cellular & humoral immune response | 2 (8%) | 18 (78.3%) * |
| Cellular immune response | 9 (36%) | 3 (13%) * |
| Humoral immune response | 5 (20%) | 2 (8.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.-Q.; Lu, P.; Xu, Z.-L.; Zhou, Q.; Zhang, J.; Wang, Z.-B.; Wu, F. Alterations in Immune Response Profile of Tumor-Draining Lymph Nodes after High-Intensity Focused Ultrasound Ablation of Breast Cancer Patients. Cells 2021, 10, 3346. https://doi.org/10.3390/cells10123346
Zhu X-Q, Lu P, Xu Z-L, Zhou Q, Zhang J, Wang Z-B, Wu F. Alterations in Immune Response Profile of Tumor-Draining Lymph Nodes after High-Intensity Focused Ultrasound Ablation of Breast Cancer Patients. Cells. 2021; 10(12):3346. https://doi.org/10.3390/cells10123346
Chicago/Turabian StyleZhu, Xue-Qiang, Pei Lu, Zhong-Lin Xu, Qiang Zhou, Jun Zhang, Zhi-Biao Wang, and Feng Wu. 2021. "Alterations in Immune Response Profile of Tumor-Draining Lymph Nodes after High-Intensity Focused Ultrasound Ablation of Breast Cancer Patients" Cells 10, no. 12: 3346. https://doi.org/10.3390/cells10123346
APA StyleZhu, X.-Q., Lu, P., Xu, Z.-L., Zhou, Q., Zhang, J., Wang, Z.-B., & Wu, F. (2021). Alterations in Immune Response Profile of Tumor-Draining Lymph Nodes after High-Intensity Focused Ultrasound Ablation of Breast Cancer Patients. Cells, 10(12), 3346. https://doi.org/10.3390/cells10123346





