Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity
Abstract
1. Pathophysiology of Spinal Cord Injury
2. Detrimental Consequences after Spinal Cord Injury and Axonal Regeneration Failure
3. Therapeutic Approaches to Overcome Obstacles in the Lesion Core and Promote Regeneration
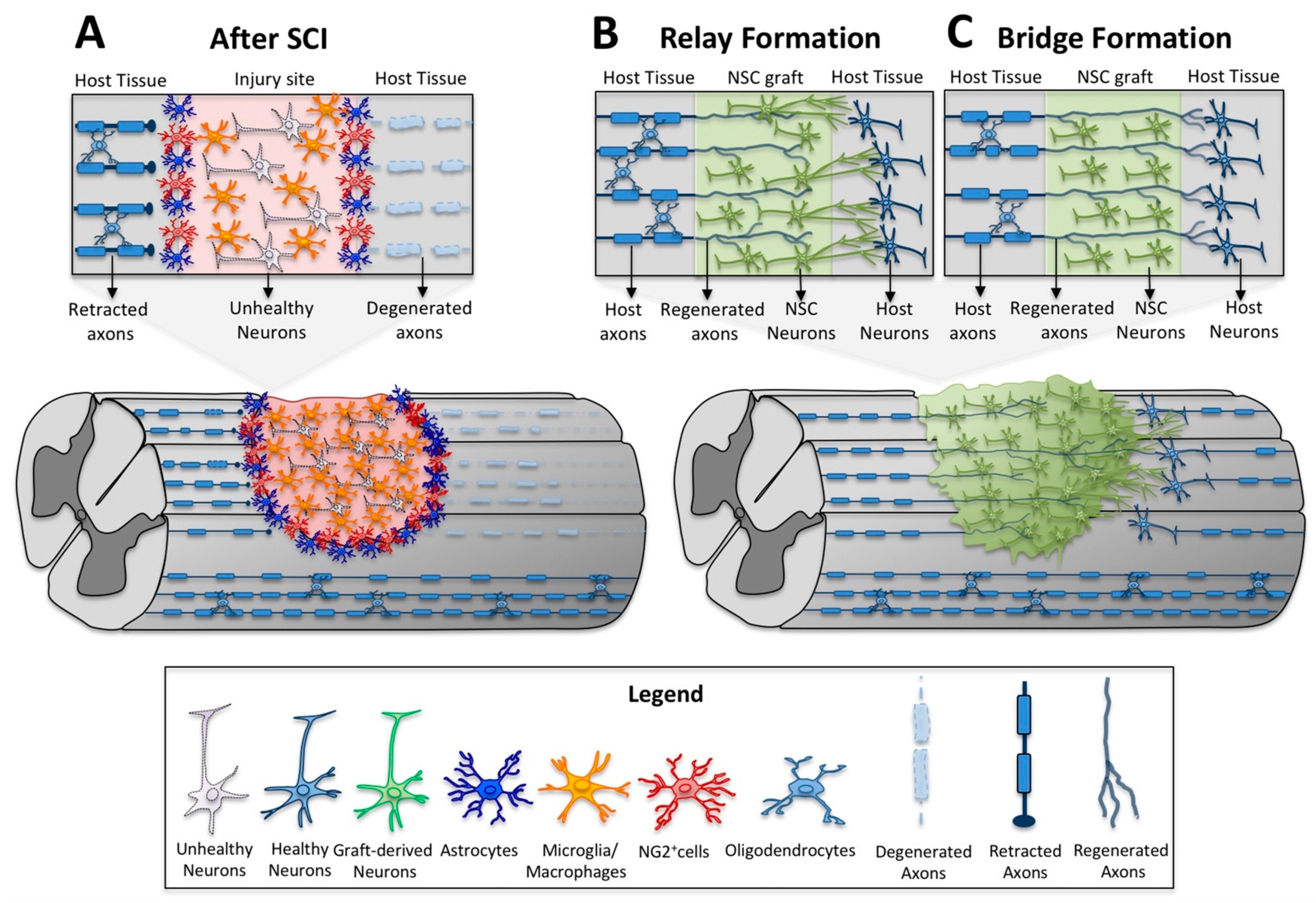
4. Neural Stem Cell Transplants as a Relay for Spinal Cord Connectivity
5. Emerging Therapeutic Interventions Using Human ESCs and iPSCs
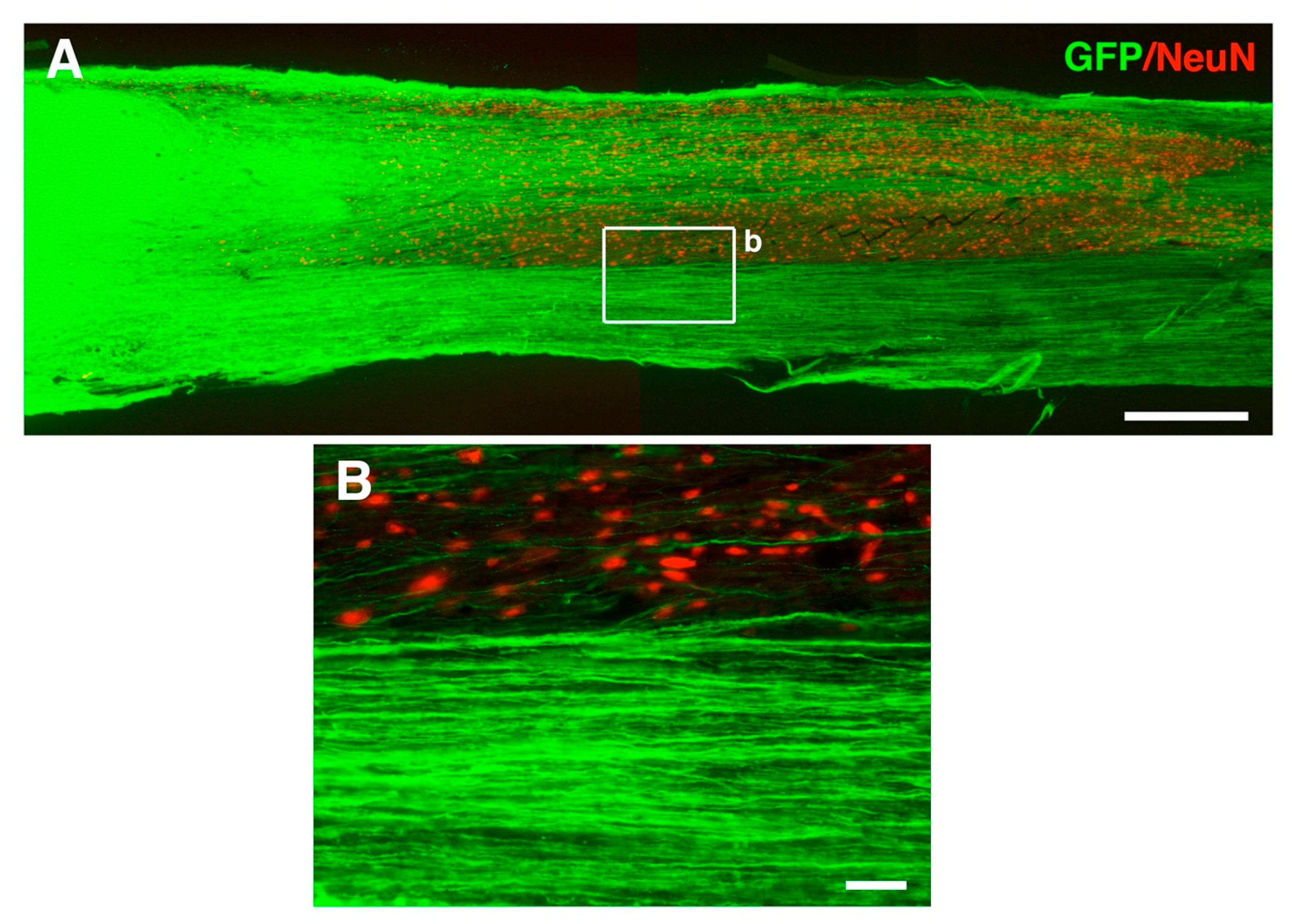
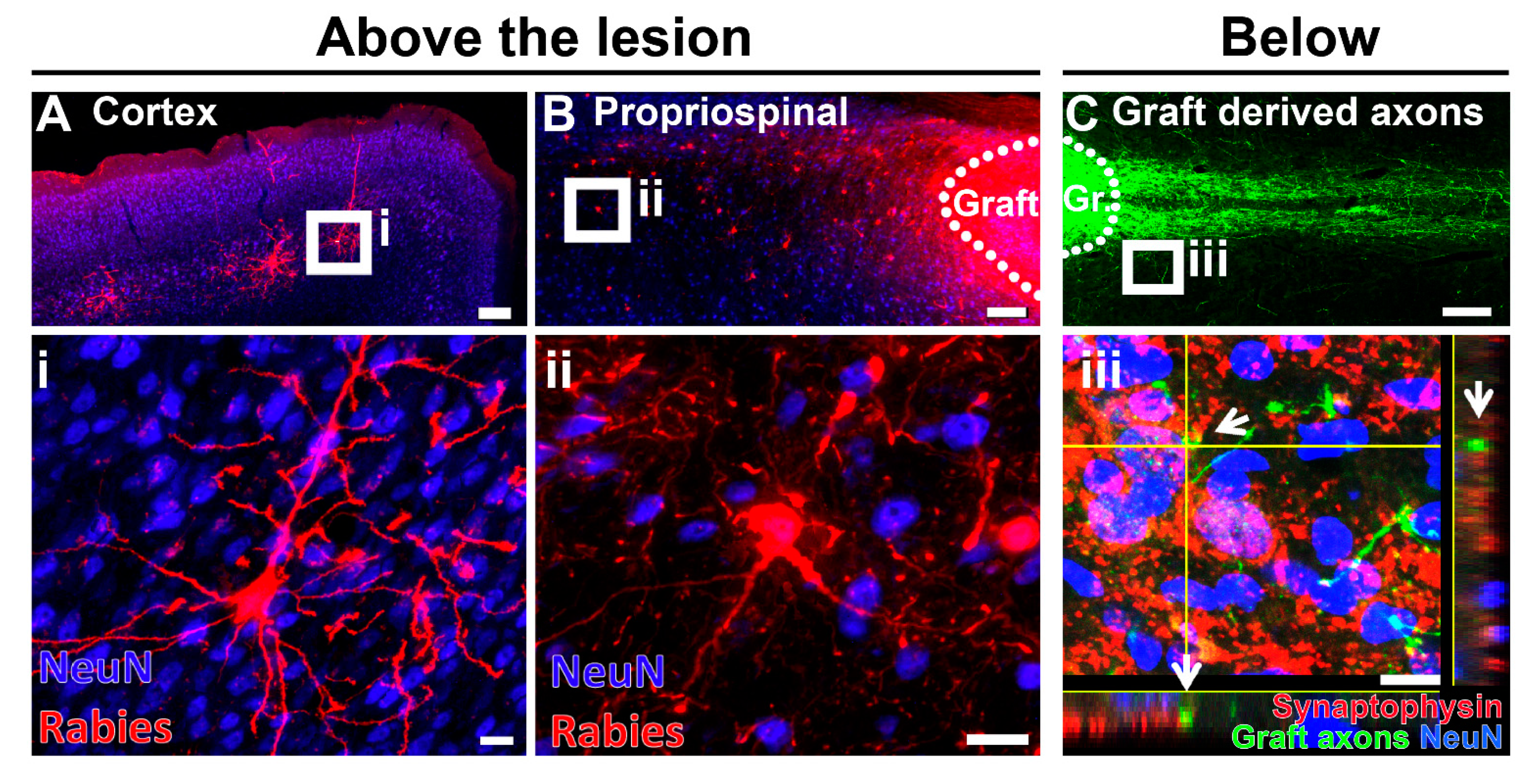
6. Motor Axon Regeneration and Relays in Neural Stem Cell Transplantation
7. Sensory Axon Regeneration and Relays in Neural Stem Cell Transplantation
8. Stem-Cell-Mediated Modulation of Pain after SCI
9. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018–17021. [Google Scholar] [CrossRef]
- Wilson, J.R.; Forgione, N.; Fehlings, M.G. Emerging therapies for acute traumatic spinal cord injury. CMAJ 2013, 185, 485–492. [Google Scholar] [CrossRef]
- Tator, C.H. Acute spinal cord injury: A review of recent studies of treatment and pathophysiology. Can. Med. Assoc. J. 1972, 107, 143–145. [Google Scholar] [PubMed]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal. Cord. 2003, 41, 369–378. [Google Scholar] [CrossRef]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef]
- Emery, E.; Aldana, P.; Bunge, M.B.; Puckett, W.; Srinivasan, A.; Keane, R.W.; Bethea, J.; Levi, A.D. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 1998, 89, 911–920. [Google Scholar] [CrossRef]
- Nashmi, R.; Fehlings, M.G. Mechanisms of axonal dysfunction after spinal cord injury: With an emphasis on the role of voltage-gated potassium channels. Brain Res. Rev. 2001, 38, 165–191. [Google Scholar] [CrossRef]
- Sun, X.; Jones, Z.B.; Chen, X.-M.; Zhou, L.; So, K.-F.; Ren, Y. Multiple organ dysfunction and systemic inflammation after spinal cord injury: A complex relationship. J. Neuroinflamm. 2016, 13, 260. [Google Scholar] [CrossRef]
- Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006, 494, 578–594. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef]
- Popovich, P.G.; Longbrake, E.E. Can the immune system be harnessed to repair the CNS? Nat. Rev. Neurosci. 2008, 9, 481–493. [Google Scholar] [CrossRef]
- Elkabes, S.; DiCicco-Bloom, E.M.; Black, I.B. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 1996, 16, 2508–2521. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nitta, A.; Fukumitsu, H.; Nomoto, H.; Shen, L.; Furukawa, S. Involvement of glial cell line-derived neurotrophic factor in activation processes of rodent macrophages. J. Neurosci. Res. 2005, 79, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Kotter, M.R.; Setzu, A.; Sim, F.J.; van Rooijen, N.; Franklin, R.J. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 2001, 35, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Henzl, M.T.; Lorber, B.; Nakazawa, T.; Thomas, T.T.; Jiang, F.; Langer, R.; Benowitz, L.I. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006, 9, 843–852. [Google Scholar] [CrossRef]
- Freria, C.M.; Hall, J.C.E.; Wei, P.; Guan, Z.; McTigue, D.M.; Popovich, P.G. Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting and synaptogenesis after spinal cord injury in mice. J. Neurosci. 2017, 37, 3568–3587. [Google Scholar] [CrossRef]
- Kubo, M.; Hanada, T.; Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003, 4, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Wu, D.; Zhang, Y.; Bo, X.; Subang, M.C.; Wang, P.; Richardson, P.M. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J. Neurosci. 2006, 26, 9512–9519. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Nakamura, S.; Guan, Z.; van Rooijen, N.; Ankeny, D.P.; Popovich, P.G. Macrophages promote axon regeneration with concurrent neurotoxicity. J. Neurosci. 2009, 29, 3956–3968. [Google Scholar] [CrossRef]
- Tedeschi, A.; Bradke, F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr. Opin. Neurobiol. 2017, 42, 118–127. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Verhaagen, J. Intrinsic Determinants of Axon Regeneration. Dev. Neurobiol. 2018, 78, 890–897. [Google Scholar] [CrossRef]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Popovich, P.G. Inflammation and axon regeneration. Curr. Opin. Neurol. 2011, 24, 577–583. [Google Scholar] [CrossRef]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Jones, L.L.; Yamaguchi, Y.; Stallcup, W.B.; Tuszynski, M.H. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 2002, 22, 2792–2803. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Schnell, L.; Schwab, M.E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 1990, 343, 269–272. [Google Scholar] [CrossRef]
- Schwab, M.E.; Caroni, P. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron 2008, 60, 404–405. [Google Scholar] [CrossRef] [PubMed]
- GrandPré, T.; Li, S.; Strittmatter, S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 2002, 417, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, W.B.J.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shubayev, V.I. Matrix metalloproteinase-9 controls proliferation of NG2+ progenitor cells immediately after spinal cord injury. Exp. Neurol. 2011, 231, 236–246. [Google Scholar] [CrossRef]
- Zhang, Y.; Tohyama, K.; Winterbottom, J.K.; Haque, N.S.; Schachner, M.; Lieberman, A.R.; Anderson, P.N. Correlation between putative inhibitory molecules at the dorsal root entry zone and failure of dorsal root axonal regeneration. Mol. Cell. Neurosci. 2001, 17, 444–459. [Google Scholar] [CrossRef]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A pericyte origin of spinal cord scar tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef]
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, I.; Tator, C.H. Effect of a single huge dose of methylprednisolone on blood flow, evoked potentials, and histology after acute spinal cord injury in the rat. Neurol. Res. 1997, 19, 289–299. [Google Scholar] [CrossRef]
- Bresnahan, J.C. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J. Neurol. Sci. 1978, 37, 59–82. [Google Scholar] [CrossRef]
- Rooney, G.E.; Endo, T.; Ameenuddin, S.; Chen, B.; Vaishya, S.; Gross, L.; Schiefer, T.K.; Currier, B.L.; Spinner, R.J.; Yaszemski, M.J.; et al. Importance of the vasculature in cyst formation after spinal cord injury. J. Neurosurg. Spine 2009, 11, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Horn, K.P.; Busch, S.A.; Hawthorne, A.L.; van Rooijen, N.; Silver, J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 2008, 28, 9330–9341. [Google Scholar] [CrossRef]
- Busch, S.A.; Horn, K.P.; Silver, D.J.; Silver, J. Overcoming macrophage-mediated axonal dieback following CNS injury. J. Neurosci. 2009, 29, 9967–9976. [Google Scholar] [CrossRef]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. Demyelination in spinal cord injury. J. Neurol. Sci. 1989, 91, 1–14. [Google Scholar] [CrossRef]
- Steward, O.; Sharp, K.; Yee, K.M.; Hofstadter, M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp. Neurol. 2008, 209, 446–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Williams, P.R.; He, Z. SOCS3: A common target for neuronal protection and axon regeneration after spinal cord injury. Exp. Neurol. 2015, 263, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Zukor, K.; Belin, S.; Wang, C.; Keelan, N.; Wang, X.; He, Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J. Neurosci. 2013, 33, 15350–15361. [Google Scholar] [CrossRef]
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010, 13, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.E.A.; Hurlbert, R.J.; Fehlings, M.G.; Yong, V.W. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain 2003, 126, 1628–1637. [Google Scholar] [CrossRef]
- Jorge, A.; Taylor, T.; Agarwal, N.; Hamilton, D.K. Current Agents and Related Therapeutic Targets for Inflammation after Acute Traumatic Spinal Cord Injury. World Neurosurg. 2019, 132, 138–147. [Google Scholar] [CrossRef]
- Hall, E.D.; Braughler, J.M. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na++ K+-ATPase activity. Dose-response analysis during 1st hour after contusion injury in the cat. J. Neurosurg. 1982, 57, 247–253. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H.; Zhang, M.; Liu, Z.; Lao, L.; Deng, C.; Chen, J.; Zhong, G. An Agonist of the Protective Factor SIRT1 Improves Functional Recovery and Promotes Neuronal Survival by Attenuating Inflammation after Spinal Cord Injury. J. Neurosci. 2017, 37, 2916–2930. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qu, Y.; Stewart, T.J.; Howard, M.J.; Chakrabortty, S.; Holekamp, T.F.; McDonald, J.W. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl. Acad. Sci. USA 2000, 97, 6126–6131. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.A.; Wilcox, J.T.; Fehlings, M.G. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp. Neurol. 2012, 235, 78–90. [Google Scholar] [CrossRef]
- Nori, S.; Nakamura, M.; Okano, H. Plasticity and regeneration in the injured spinal cord after cell transplantation therapy. Prog. Brain Res. 2017, 231, 33–56. [Google Scholar]
- Reier, P.J. Neural tissue grafts and repair of the injured spinal cord. Neuropathol. Appl. Neurobiol. 1985, 11, 81–104. [Google Scholar] [CrossRef]
- Stokes, B.T.; Reier, P.J. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp. Neurol. 1992, 116, 1–12. [Google Scholar] [CrossRef]
- Bregman, B.S.; Kunkel-Bagden, E.; Reier, P.J.; Dai, H.N.; McAtee, M.; Gao, D. Recovery of function after spinal cord injury: Mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp. Neurol. 1993, 123, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Das, G.D. Neural transplantation in the spinal cord of adult rats. Conditions, survival, cytology and connectivity of the transplants. J. Neurol. Sci. 1983, 62, 191–210. [Google Scholar] [CrossRef]
- Kao, C.C.; Chang, L.W.; Bloodworth, J.M. Axonal regeneration across transected mammalian spinal cords: An electron microscopic study of delayed microsurgical nerve grafting. Exp. Neurol. 1977, 54, 591–615. [Google Scholar] [CrossRef]
- Kao, C.C. Comparison of healing process in transected spinal cords grafted with autogenous brain tissue, sciatic nerve, and nodose ganglion. Exp. Neurol. 1974, 44, 424–439. [Google Scholar] [CrossRef]
- Aguayo, A.J.; Björklund, A.; Stenevi, U.; Carlstedt, T. Fetal mesencephalic neurons survive and extend long axons across peripheral nervous system grafts inserted into the adult rat striatum. Neurosci. Lett. 1984, 45, 53–58. [Google Scholar] [CrossRef]
- Aguayo, A.J.; Benfey, M.; David, S. A potential for axonal regeneration in neurons of the adult mammalian nervous system. Birth Defects Orig. Artic. Ser. 1983, 19, 327–340. [Google Scholar]
- Bregman, B.S.; Bernstein-Goral, H.; Kunkel-Bagden, E. CNS transplants promote anatomical plasticity and recovery of function after spinal cord injury. Restor. Neurol. Neurosci. 1991, 2, 327–338. [Google Scholar] [CrossRef]
- White, T.E.; Lane, M.A.; Sandhu, M.S.; O’Steen, B.E.; Fuller, D.D.; Reier, P.J. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp. Neurol. 2010, 225, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Z.; Lane, M.A.; Dougherty, B.J.; Mercier, L.M.; Sandhu, M.S.; Sanchez, J.C.; Reier, P.J.; Fuller, D.D. Intraspinal transplantation and modulation of donor neuron electrophysiological activity. Exp. Neurol. 2014, 251, 47–57. [Google Scholar] [CrossRef][Green Version]
- Bonner, J.F.; Connors, T.M.; Silverman, W.F.; Kowalski, D.P.; Lemay, M.A.; Fischer, I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J. Neurosci. 2011, 31, 4675–4686. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Kadoya, K.; Tuszynski, M.H. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr. Opin. Neurobiol. 2014, 27, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef]
- Adler, A.F.; Lee-Kubli, C.; Kumamaru, H.; Kadoya, K.; Tuszynski, M.H. Comprehensive Monosynaptic Rabies Virus Mapping of Host Connectivity with Neural Progenitor Grafts after Spinal Cord Injury. Stem Cell Rep. 2017, 8, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Brock, J.H.; Lu, P.; Kumamaru, H.; Salegio, E.A.; Kadoya, K.; Weber, J.L.; Liang, J.J.; Moseanko, R.; Hawbecker, S.; et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018, 24, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Alto, L.T.; Havton, L.A.; Conner, J.M.; Hollis, E.R.; Blesch, A.; Tuszynski, M.H. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat. Neurosci. 2009, 12, 1106–1113. [Google Scholar] [CrossRef]
- Lu, P.; Blesch, A.; Graham, L.; Wang, Y.; Samara, R.; Banos, K.; Haringer, V.; Havton, L.; Weishaupt, N.; Bennett, D.; et al. Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 2012, 32, 8208–8218. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Eidelberg, E.; Story, J.L.; Walden, J.G.; Meyer, B.L. Anatomical correlates of return of locomotor function after partial spinal cord lesions in cats. Exp. Brain Res. 1981, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Tator, C.H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 1995, 132, 220–228. [Google Scholar] [CrossRef]
- Cajal, S.R.Y.; DeFelipe, J.; Jones, E.G. CaJal’s Degeneration and Regeneration of the Nervous System; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Lu, P.; Ceto, S.; Wang, Y.; Graham, L.; Wu, D.; Kumamaru, H.; Staufenberg, E.; Tuszynski, M.H. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J. Clin. Investig. 2017, 127, 3287–3299. [Google Scholar] [CrossRef]
- Johnson, P.J.; Tatara, A.; Shiu, A.; Sakiyama-Elbert, S.E. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transpl. 2010, 19, 89–101. [Google Scholar] [CrossRef]
- Robinson, J.; Lu, P. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp. Neurol. 2017, 291, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef]
- Zavvarian, M.-M.; Hong, J.; Fehlings, M.G. The Functional Role of Spinal Interneurons Following Traumatic Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Y.; Bin, Y.; Zhang, Z.; Brommer, B.; Williams, P.R.; Liu, Y.; Hegarty, S.V.; Zhou, S.; Zhu, J.; et al. Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations. Cell 2018, 174, 1599. [Google Scholar] [CrossRef] [PubMed]
- Butts, J.C.; Iyer, N.; White, N.; Thompson, R.; Sakiyama-Elbert, S.; McDevitt, T.C. V2a interneuron differentiation from mouse and human pluripotent stem cells. Nat. Protoc. 2019, 14, 3033–3058. [Google Scholar] [CrossRef]
- Zholudeva, L.V.; Iyer, N.; Qiang, L.; Spruance, V.M.; Randelman, M.L.; White, N.W.; Bezdudnaya, T.; Fischer, I.; Sakiyama-Elbert, S.E.; Lane, M.A. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2883–2903. [Google Scholar] [CrossRef]
- Coumans, J.V.; Lin, T.T.; Dai, H.N.; MacArthur, L.; McAtee, M.; Nash, C.; Bregman, B.S. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J. Neurosci. 2001, 21, 9334–9344. [Google Scholar] [CrossRef]
- Martello, G.; Smith, A. The nature of embryonic stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 647–675. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Kumamaru, H.; Lu, P.; Rosenzweig, E.S.; Tuszynski, M.H. Activation of Intrinsic Growth State Enhances Host Axonal Regeneration into Neural Progenitor Cell Grafts. Stem Cell Rep. 2018, 11, 861–868. [Google Scholar] [CrossRef]
- Javali, A.; Misra, A.; Leonavicius, K.; Acharyya, D.; Vyas, B.; Sambasivan, R. Co-expression of Tbx6 and Sox2 identifies a novel transient neuromesoderm progenitor cell state. Development 2017, 144, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Jungverdorben, J.; Wiethoff, H.; Lin, Q.; Flitsch, L.J.; Eckert, D.; Hebisch, M.; Fischer, J.; Kesavan, J.; Weykopf, B.; et al. A stably self-renewing adult blood-derived induced neural stem cell exhibiting patternability and epigenetic rejuvenation. Nat. Commun. 2018, 9, 4047. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Knutsen, A.K.; Peruzzotti-Jametti, L.; Korotcov, A.; Bosomtwi, A.; Dardzinski, B.J.; Bernstock, J.D.; Rizzi, S.; Edenhofer, F.; Pluchino, S.; et al. Transplantation of induced neural stem cells (iNSCs) into chronically demyelinated corpus callosum ameliorates motor deficits. Acta Neuropathol. Commun. 2020, 8, 84. [Google Scholar] [CrossRef]
- Kuypers, H.G. A new look at the organization of the motor system. Prog. Brain Res. 1982, 57, 381–403. [Google Scholar]
- Bortoff, G.A.; Strick, P.L. Corticospinal terminations in two new-world primates: Further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J. Neurosci. 1993, 13, 5105–5118. [Google Scholar] [CrossRef]
- Savio, T.; Schwab, M.E. Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc. Natl. Acad. Sci. USA 1990, 87, 4130–4133. [Google Scholar] [CrossRef]
- Sicotte, M.; Tsatas, O.; Jeong, S.Y.; Cai, C.-Q.; He, Z.; David, S. Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol. Cell. Neurosci. 2003, 23, 251–263. [Google Scholar] [CrossRef]
- Fouad, K.; Klusman, I.; Schwab, M.E. Regenerating corticospinal fibers in the Marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur. J. Neurosci. 2004, 20, 2479–2482. [Google Scholar] [CrossRef] [PubMed]
- Schnell, L.; Schneider, R.; Kolbeck, R.; Barde, Y.A.; Schwab, M.E. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 1994, 367, 170–173. [Google Scholar] [CrossRef]
- Grill, R.; Murai, K.; Blesch, A.; Gage, F.H.; Tuszynski, M.H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 1997, 17, 5560–5572. [Google Scholar] [CrossRef]
- Pfeifer, K.; Vroemen, M.; Blesch, A.; Weidner, N. Adult neural progenitor cells provide a permissive guiding substrate for corticospinal axon growth following spinal cord injury. Eur. J. Neurosci. 2004, 20, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Oudega, M.; Bates, M.L.; Wood, P.M.; Kleitman, N.; Bunge, M.B. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 2002, 22, 6670–6681. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, Y.; Sun, F.; Wang, X.; Liu, X.; He, Z. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun. 2015, 6, 1611. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, M.G.; Wang, Z.; Lerch, J.K.; Motti, D.; Zhang, Y.P.; Shields, C.B.; Lee, J.K.; Goldberg, J.L.; Lemmon, V.P.; Bixby, J.L. Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc. Natl. Acad. Sci. USA 2012, 109, 7517–7522. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Park, K.K.; Belin, S.; Wang, D.; Lu, T.; Chen, G.; Zhang, K.; Yeung, C.; Feng, G.; Yankner, B.A.; et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 2011, 480, 372–375. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008, 322, 963–966. [Google Scholar] [CrossRef]
- Kumamaru, H.; Kadoya, K.; Adler, A.F.; Takashima, Y.; Graham, L.; Coppola, G.; Tuszynski, M.H. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 2018, 15, 723–731. [Google Scholar] [CrossRef]
- Kumamaru, H.; Lu, P.; Rosenzweig, E.S.; Kadoya, K.; Tuszynski, M.H. Regenerating Corticospinal Axons Innervate Phenotypically Appropriate Neurons within Neural Stem Cell Grafts. Cell Rep. 2019, 26, 2329–2339.e4. [Google Scholar] [CrossRef]
- Ceto, S.; Sekiguchi, K.J.; Takashima, Y.; Nimmerjahn, A.; Tuszynski, M.H. Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020, 27, 430–440.e5. [Google Scholar] [CrossRef]
- Dulin, J.N.; Adler, A.F.; Kumamaru, H.; Poplawski, G.H.D.; Lee-Kubli, C.; Strobl, H.; Gibbs, D.; Kadoya, K.; Fawcett, J.W.; Lu, P.; et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat. Commun. 2018, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, G.H.D.; Kawaguchi, R.; Van Niekerk, E.; Lu, P.; Mehta, N.; Canete, P.; Lie, R.; Dragatsis, I.; Meves, J.M.; Zheng, B.; et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature 2020, 581, 77–82. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and Cellular Mechanisms of Axonal Regeneration After Spinal Cord Injury. Mol. Cell Proteomics 2016, 15, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Bradley, P.M.; Jacobi, A.; Kerschensteiner, M.; Bareyre, F.M. STAT3 promotes corticospinal remodelling and functional recovery after spinal cord injury. EMBO Rep. 2013, 14, 931–937. [Google Scholar] [CrossRef]
- Wang, Z.; Reynolds, A.; Kirry, A.; Nienhaus, C.; Blackmore, M.G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 2015, 35, 3139–3145. [Google Scholar] [CrossRef]
- Lawrence, D.G.; Hopkins, D.A. The development of motor control in the rhesus monkey: Evidence concerning the role of corticomotoneuronal connections. Brain 1976, 99, 235–254. [Google Scholar] [CrossRef]
- Costello, M.B.; Fragaszy, D.M. Prehension in Cebus and Saimiri: I. Grip type and hand preference. Am. J. Primatol. 1988, 15, 235–245. [Google Scholar] [CrossRef]
- Fragaszy, D.M.; Mason, W.A. Comparisons of feeding behavior in captive squirrel and titi monkeys (Saimiri sciureus and Callicebus moloch). J. Comp. Psychol. 1983, 97, 310–326. [Google Scholar] [CrossRef]
- Finnerup, N.B. Pain in patients with spinal cord injury. Pain 2013, 154 (Suppl. S1), S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Zeilig, G.; Enosh, S.; Rubin-Asher, D.; Lehr, B.; Defrin, R. The nature and course of sensory changes following spinal cord injury: Predictive properties and implications on the mechanism of central pain. Brain 2012, 135, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Blesch, A.; Lu, P.; Tsukada, S.; Alto, L.T.; Roet, K.; Coppola, G.; Geschwind, D.; Tuszynski, M.H. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: Superiority to camp-mediated effects. Exp. Neurol. 2012, 235, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.M.; Issa, V.M. Peripheral injury enhances central regeneration of primary sensory neurones. Nature 1984, 309, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Bráz, J.M.; Sharif-Naeini, R.; Vogt, D.; Kriegstein, A.; Alvarez-Buylla, A.; Rubenstein, J.L.; Basbaum, A.I. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 2012, 74, 663–675. [Google Scholar] [CrossRef]
- Fandel, T.M.; Trivedi, A.; Nicholas, C.R.; Zhang, H.; Chen, J.; Martinez, A.F.; Noble-Haeusslein, L.J.; Kriegstein, A.R. Transplanted Human Stem Cell-Derived Interneuron Precursors Mitigate Mouse Bladder Dysfunction and Central Neuropathic Pain after Spinal Cord Injury. Cell Stem Cell 2016, 19, 544–557. [Google Scholar] [CrossRef]
- Hwang, I.; Hahm, S.-C.; Choi, K.-A.; Park, S.-H.; Jeong, H.; Yea, J.-H.; Kim, J.; Hong, S. Intrathecal Transplantation of Embryonic Stem Cell-Derived Spinal GABAergic Neural Precursor Cells Attenuates Neuropathic Pain in a Spinal Cord Injury Rat Model. Cell Transpl. 2016, 25, 593–607. [Google Scholar] [CrossRef]
- Dugan, E.A.; Jergova, S.; Sagen, J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020, 327, 113208. [Google Scholar] [CrossRef]
- Brock, J.H.; Graham, L.; Staufenberg, E.; Im, S.; Tuszynski, M.H. Rodent Neural Progenitor Cells Support Functional Recovery after Cervical Spinal Cord Contusion. J. Neurotrauma 2018, 35, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Y.; Baussi, O.; Levine, A.; Chen, Y.; Schacher, S. Persistent long-term synaptic plasticity requires activation of a new signaling pathway by additional stimuli. J. Neurosci. 2011, 31, 8841–8850. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Conner, J.M.; Nagahara, A.H.; Tuszynski, M.H. Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets. Proc. Natl. Acad. Sci. USA 2016, 113, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Allegra Mascaro, A.L.; Conti, E.; Lai, S.; Di Giovanna, A.P.; Spalletti, C.; Alia, C.; Panarese, A.; Scaglione, A.; Sacconi, L.; Micera, S.; et al. Combined Rehabilitation Promotes the Recovery of Structural and Functional Features of Healthy Neuronal Networks after Stroke. Cell Rep. 2019, 28, 3474–3485.e6. [Google Scholar] [CrossRef]
- Hurd, C.; Weishaupt, N.; Fouad, K. Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp. Neurol. 2013, 247, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, C.; Carey, M.; Zheng, Y.; He, X.; Cao, Q.L. Reaching and Grasping Training Improves Functional Recovery after Chronic Cervical Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- García-Alías, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef]
- Dai, H.; MacArthur, L.; McAtee, M.; Hockenbury, N.; Tidwell, J.L.; McHugh, B.; Mansfield, K.; Finn, T.; Hamers, F.P.T.; Bregman, B.S. Activity-based therapies to promote forelimb use after a cervical spinal cord injury. J. Neurotrauma 2009, 26, 1719–1732. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freria, C.M.; Van Niekerk, E.; Blesch, A.; Lu, P. Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity. Cells 2021, 10, 3296. https://doi.org/10.3390/cells10123296
de Freria CM, Van Niekerk E, Blesch A, Lu P. Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity. Cells. 2021; 10(12):3296. https://doi.org/10.3390/cells10123296
Chicago/Turabian Stylede Freria, Camila Marques, Erna Van Niekerk, Armin Blesch, and Paul Lu. 2021. "Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity" Cells 10, no. 12: 3296. https://doi.org/10.3390/cells10123296
APA Stylede Freria, C. M., Van Niekerk, E., Blesch, A., & Lu, P. (2021). Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity. Cells, 10(12), 3296. https://doi.org/10.3390/cells10123296





