Mechanisms of Plasticity in Subcortical Visual Areas
Abstract
1. Introduction
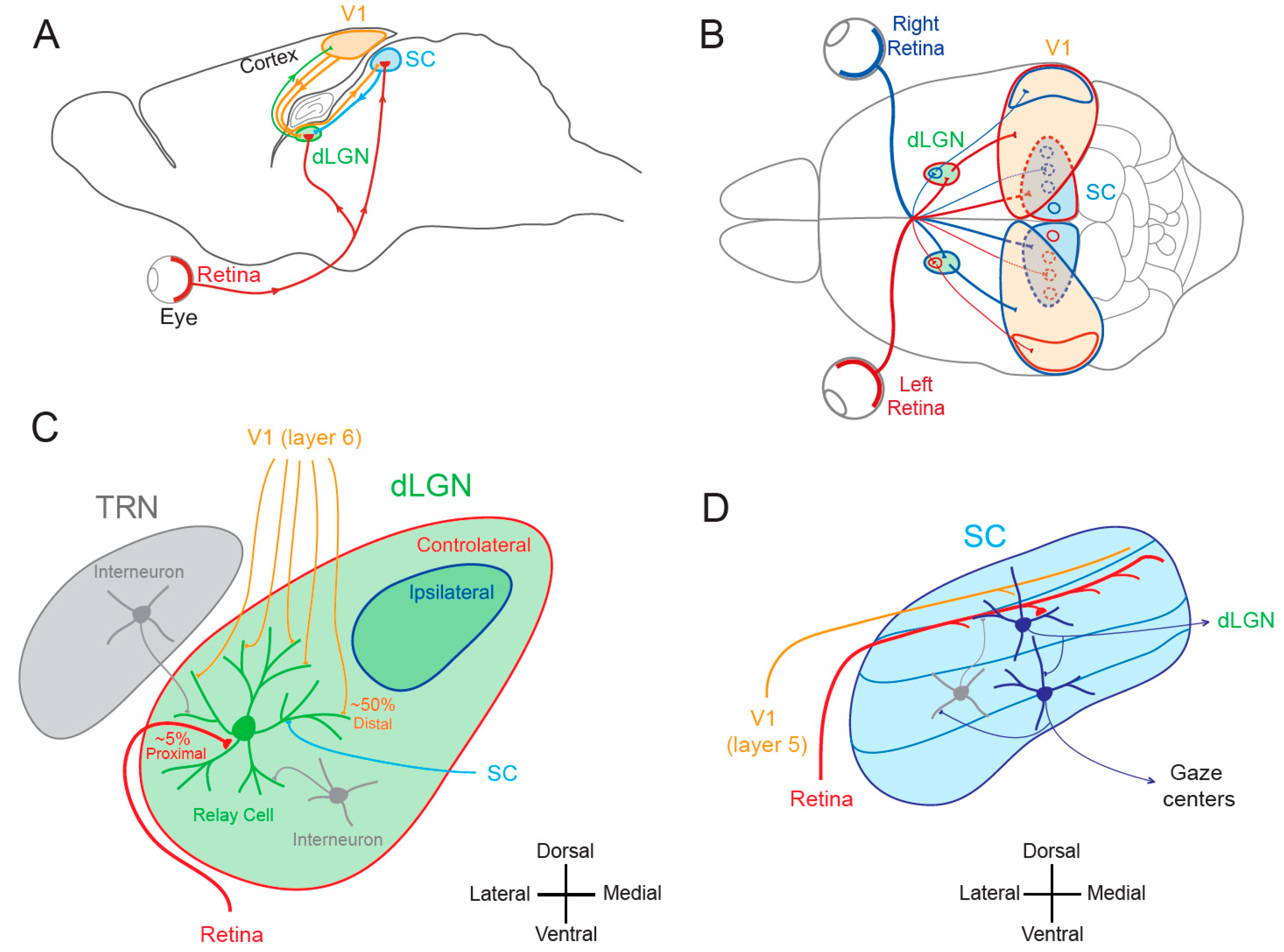
1.1. Lateral Geniculate Nucleus and Superior Colliculus
1.2. Cortical and Subcortical Plasticity
2. Functional Plasticity in Subcortical Visual Areas
2.1. Functional Plasticity in the dLGN
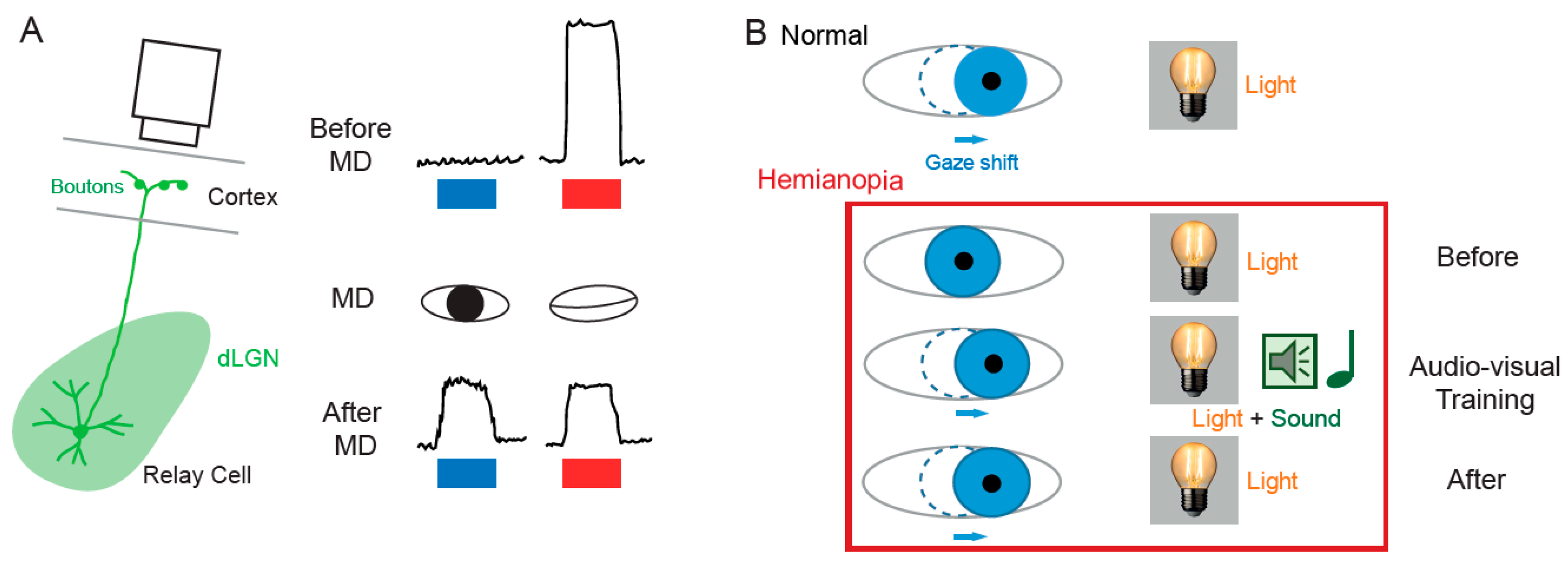
2.2. Functional Plasticity in the SC
3. Structural Plasticity in Subcortical Visual Areas
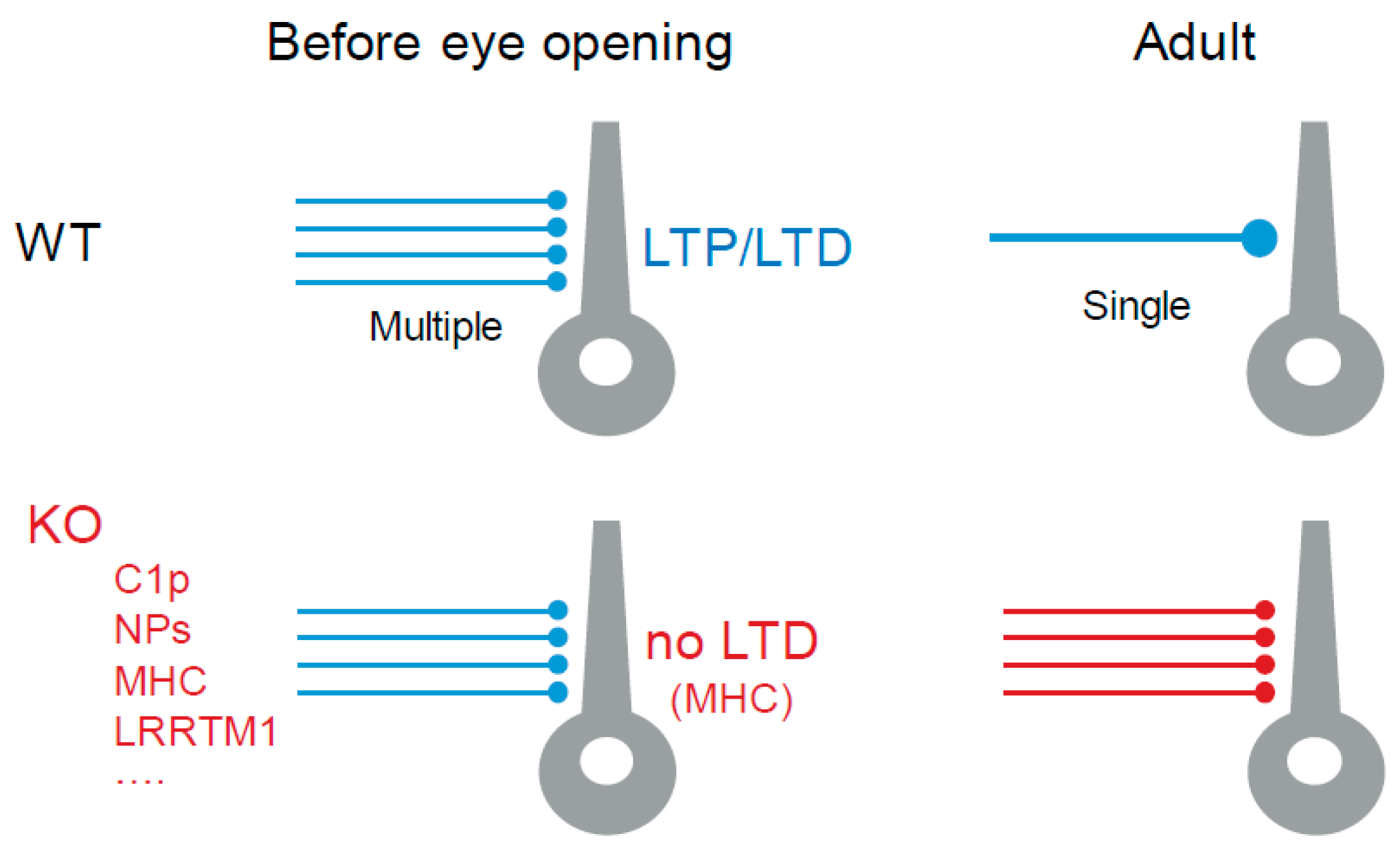
3.1. Structural Plasticity in the dLGN
3.2. Structural Plasticity in the SC
4. Synaptic Plasticity in Subcortical Visual Areas
4.1. Synaptic Plasticity in the dLGN
4.1.1. Hebbian Synaptic Plasticity in the dLGN
4.1.2. Homeostatic Synaptic Plasticity in the dLGN
4.2. Synaptic Plasticity in the SC
4.2.1. Hebbian Synaptic Plasticity in the SC
4.2.2. Homeostatic Synaptic Plasticity in the SC
5. Intrinsic Plasticity in Subcortical Visual Areas
6. Molecular Correlates of Subcortical Plasticity
6.1. Molecular Categorization
6.2. Molecular Actors in Retinogeniculate Synapse Refinement
6.3. Visual Experience-Dependent Maintenance of Retinogeniculate Connections
6.4. Molecular Analysis of Visual Plasticity
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weyand, T.G. The Multifunctional Lateral Geniculate Nucleus. Rev. Neurosci. 2016, 27, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M. Thalamus Plays a Central Role in Ongoing Cortical Functioning. Nat. Neurosci. 2016, 19, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Ghodrati, M.; Khaligh-Razavi, S.-M.; Lehky, S.R. Towards Building a More Complex View of the Lateral Geniculate Nucleus: Recent Advances in Understanding Its Role. Prog. Neurobiol. 2017, 156, 214–255. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.A.; Bickford, M.E.; Cang, J. Unraveling Circuits of Visual Perception and Cognition through the Superior Colliculus. Neuron 2021, 109, 918–937. [Google Scholar] [CrossRef]
- Isa, T.; Marquez-Legorreta, E.; Grillner, S.; Scott, E.K. The Tectum/Superior Colliculus as the Vertebrate Solution for Spatial Sensory Integration and Action. Curr. Biol. 2021, 31, R741–R762. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; McPeek, R.M. Role of the Superior Colliculus in Guiding Movements Not Made by the Eyes. Annu. Rev. Vis Sci 2021. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Feldheim, D.A. The Mouse Superior Colliculus: An Emerging Model for Studying Circuit Formation and Function. Front. Neural Circuits 2018, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Denman, D.J.; Contreras, D. On Parallel Streams through the Mouse Dorsal Lateral Geniculate Nucleus. Front. Neural Circuits 2016, 10, 20. [Google Scholar] [CrossRef]
- Sherman, S.M.; Wilson, J.R.; Kaas, J.H.; Webb, S.V. X- and Y-Cells in the Dorsal Lateral Geniculate Nucleus of the Owl Monkey (Aotus Trivirgatus). Science 1976, 192, 475–477. [Google Scholar] [CrossRef]
- Dreher, B.; Fukada, Y.; Rodieck, R.W. Identification, Classification and Anatomical Segregation of Cells with X-like and Y-like Properties in the Lateral Geniculate Nucleus of Old-World Primates. J. Physiol. 1976, 258, 433–452. [Google Scholar] [CrossRef]
- Lee, P.H.; Sooksawate, T.; Yanagawa, Y.; Isa, K.; Isa, T.; Hall, W.C. Identity of a Pathway for Saccadic Suppression. Proc. Natl. Acad. Sci. USA 2007, 104, 6824–6827. [Google Scholar] [CrossRef]
- Van Horn, S.C.; Erişir, A.; Sherman, S.M. Relative Distribution of Synapses in the A-Laminae of the Lateral Geniculate Nucleus of the Cat. J. Comp. Neurol. 2000, 416, 509–520. [Google Scholar] [CrossRef]
- Sherman, S.M.; Guillery, R.W. On the Actions That One Nerve Cell Can Have on Another: Distinguishing “Drivers” from “Modulators”. Proc. Natl. Acad. Sci. USA 1998, 95, 7121–7126. [Google Scholar] [CrossRef]
- Gale, S.D.; Murphy, G.J. Distinct Cell Types in the Superficial Superior Colliculus Project to the Dorsal Lateral Geniculate and Lateral Posterior Thalamic Nuclei. J. Neurophysiol. 2018, 120, 1286–1292. [Google Scholar] [CrossRef]
- Stein, B.E.; Jiang, W.; Wallace, M.T.; Stanford, T.R. Chapter 10 Nonvisual influences on visual-information processing in the superior colliculus. Prog. Brain Res. 2001, 134, 143–156. [Google Scholar]
- Govindaiah, G.; Campbell, P.W.; Guido, W. Differential Distribution of Ca2+ Channel Subtypes at Retinofugal Synapses. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Ellis, E.M.; Gauvain, G.; Sivyer, B.; Murphy, G.J. Shared and Distinct Retinal Input to the Mouse Superior Colliculus and Dorsal Lateral Geniculate Nucleus. J. Neurophysiol. 2016, 116, 602–610. [Google Scholar] [CrossRef]
- Schiapparelli, L.M.; Shah, S.H.; Ma, Y.; McClatchy, D.B.; Sharma, P.; Li, J.; Yates, J.R.; Goldberg, J.L.; Cline, H.T. The Retinal Ganglion Cell Transportome Identifies Proteins Transported to Axons and Presynaptic Compartments in the Visual System In Vivo. Cell. Rep. 2019, 28, 1935–1947.e5. [Google Scholar] [CrossRef] [PubMed]
- May, P.J. The mammalian superior colliculus: Laminar structure and connections. Prog. Brain Res. 2006, 151, 321–378. [Google Scholar] [PubMed]
- Robinson, D.A. Eye Movements Evoked by Collicular Stimulation in the Alert Monkey. Vis. Res. 1972, 12, 1795–1808. [Google Scholar] [CrossRef]
- Hopp, J.J.; Fuchs, A.F. The Characteristics and Neuronal Substrate of Saccadic Eye Movement Plasticity. Prog. Neurobiol. 2004, 72, 27–53. [Google Scholar] [CrossRef]
- Hooks, B.M.; Chen, C. Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System. Neuron 2020, 106, 21–36. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Hubel, D.H. Effect of Visual Deprivation on Morphology and Physiology of Cells in the Cat’s Lateral Geniculate Body. J. Neurophysiol. 1963, 26, 978–993. [Google Scholar] [CrossRef]
- Derrington, A.M.; Hawken, M.J. Spatial and Temporal Properties of Cat Geniculate Neurones after Prolonged Deprivation. J. Physiol. 1981, 314, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, C.; Vital-Durand, F. Effects of Visual Deprivation on the Development of the Monkey’s Lateral Geniculate Nucleus. J. Physiol. 1986, 380, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J.B.; Schumer, R.A.; Sherman, S.M.; Spear, P.D.; Movshon, J.A. Visual Response Properties of Neurons in the LGN of Normally Reared and Visually Deprived Macaque Monkeys. J. Neurophysiol. 2001, 85, 2111–2129. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Sherman, S.M. Thalamocortical Circuit Motifs: A General Framework. Neuron 2019, 103, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Wright, M.J. Properties of LGN Cells in Kittens Reared with Convergent Squint: A Neurophysiological Demonstration of Amblyopia. Exp. Brain Res. 1976, 25, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.F.; Thompson, B.; Gole, G.; Mullen, K.T. Deficient Responses from the Lateral Geniculate Nucleus in Humans with Amblyopia. Eur. J. Neurosci. 2009, 29, 1064–1070. [Google Scholar] [CrossRef]
- Hammer, S.; Monavarfeshani, A.; Lemon, T.; Su, J.; Fox, M.A. Multiple Retinal Axons Converge onto Relay Cells in the Adult Mouse Thalamus. Cell. Rep. 2015, 12, 1575–1583. [Google Scholar] [CrossRef]
- Morgan, J.L.; Berger, D.R.; Wetzel, A.W.; Lichtman, J.W. The Fuzzy Logic of Network Connectivity in Mouse Visual Thalamus. Cell 2016, 165, 192–206. [Google Scholar] [CrossRef]
- Rompani, S.B.; Müllner, F.E.; Wanner, A.; Zhang, C.; Roth, C.N.; Yonehara, K.; Roska, B. Different Modes of Visual Integration in the Lateral Geniculate Nucleus Revealed by Single-Cell-Initiated Transsynaptic Tracing. Neuron 2017, 93, 767–776.e6. [Google Scholar] [CrossRef] [PubMed]
- Zeater, N.; Cheong, S.K.; Solomon, S.G.; Dreher, B.; Martin, P.R. Binocular Visual Responses in the Primate Lateral Geniculate Nucleus. Curr. Biol. 2015, 25, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Howarth, M.; Walmsley, L.; Brown, T.M. Binocular Integration in the Mouse Lateral Geniculate Nuclei. Curr. Biol. 2014, 24, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.Y.L.; Abdelaal, K.; Salinas, K.J.; Gu, D.; Zeitoun, J.; Figueroa Velez, D.X.; Peach, J.P.; Fowlkes, C.C.; Gandhi, S.P. Long-Term Monocular Deprivation during Juvenile Critical Period Disrupts Binocular Integration in Mouse Visual Thalamus. J. Neurosci. 2020, 40, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Litvina, E.Y.; Chen, C. Functional Convergence at the Retinogeniculate Synapse. Neuron 2017, 96, 330–338.e5. [Google Scholar] [CrossRef]
- Tschetter, W.W.; Govindaiah, G.; Etherington, I.M.; Guido, W.; Niell, C.M. Refinement of Spatial Receptive Fields in the Developing Mouse Lateral Geniculate Nucleus Is Coordinated with Excitatory and Inhibitory Remodeling. J. Neurosci. 2018, 38, 4531–4542. [Google Scholar] [CrossRef]
- Jaepel, J.; Hübener, M.; Bonhoeffer, T.; Rose, T. Lateral Geniculate Neurons Projecting to Primary Visual Cortex Show Ocular Dominance Plasticity in Adult Mice. Nat. Neurosci. 2017, 20, 1708–1714. [Google Scholar] [CrossRef]
- Sommeijer, J.-P.; Ahmadlou, M.; Saiepour, M.H.; Seignette, K.; Min, R.; Heimel, J.A.; Levelt, C.N. Thalamic Inhibition Regulates Critical-Period Plasticity in Visual Cortex and Thalamus. Nat. Neurosci. 2017, 20, 1715–1721. [Google Scholar] [CrossRef]
- Rose, T.; Bonhoeffer, T. Experience-Dependent Plasticity in the Lateral Geniculate Nucleus. Curr. Opin. Neurobiol. 2018, 53, 22–28. [Google Scholar] [CrossRef]
- Bolognini, N.; Rasi, F.; Coccia, M.; Làdavas, E. Visual Search Improvement in Hemianopic Patients after Audio-Visual Stimulation. Brain 2005, 128, 2830–2842. [Google Scholar] [CrossRef]
- Jiang, H.; Stein, B.E.; McHaffie, J.G. Multisensory Training Reverses Midbrain Lesion-Induced Changes and Ameliorates Haemianopia. Nat. Commun. 2015, 6, 7263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.I.; Tao, H.W.; Poo, M. Visual Input Induces Long-Term Potentiation of Developing Retinotectal Synapses. Nat. Neurosci. 2000, 3, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.W.; Zhang, L.I.; Engert, F.; Poo, M. Emergence of Input Specificity of Ltp during Development of Retinotectal Connections In Vivo. Neuron 2001, 31, 569–580. [Google Scholar] [CrossRef]
- Lien, C.-C.; Mu, Y.; Vargas-Caballero, M.; Poo, M. Visual Stimuli-Induced LTD of GABAergic Synapses Mediated by Presynaptic NMDA Receptors. Nat. Neurosci. 2006, 9, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Van Rheede, J.J.; Richards, B.A.; Akerman, C.J. Sensory-Evoked Spiking Behavior Emerges via an Experience-Dependent Plasticity Mechanism. Neuron 2015, 87, 1050–1062. [Google Scholar] [CrossRef]
- Hoffmann, K.P.; Sherman, S.M. Effects of Early Monocular Deprivation on Visual Input to Cat Superior Colliculus. J. Neurophysiol. 1974, 37, 1276–1286. [Google Scholar] [CrossRef]
- Stein, B.E.; Rowland, B.A. Using Superior Colliculus Principles of Multisensory Integration to Reverse Hemianopia. Neuropsychologia 2020, 141, 107413. [Google Scholar] [CrossRef]
- Dakos, A.S.; Jiang, H.; Stein, B.E.; Rowland, B.A. Using the Principles of Multisensory Integration to Reverse Hemianopia. Cereb. Cortex 2020, 30, 2030–2041. [Google Scholar] [CrossRef]
- Yu, L.; Rowland, B.A.; Xu, J.; Stein, B.E. Multisensory Plasticity in Adulthood: Cross-Modal Experience Enhances Neuronal Excitability and Exposes Silent Inputs. J. Neurophysiol. 2013, 109, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.V.; Hamam, B.N.; Fransén, E.; Hasselmo, M.E.; Alonso, A.A. Graded Persistent Activity in Entorhinal Cortex Neurons. Nature 2002, 420, 173–178. [Google Scholar] [CrossRef]
- Connors, B.W. Single-Neuron Mnemonics. Nature 2002, 420, 133–134. [Google Scholar] [CrossRef]
- Frank, L.M.; Brown, E.N. Persistent Activity and Memory in the Entorhinal Cortex. Trends Neurosci. 2003, 26, 400–401. [Google Scholar] [CrossRef]
- Rahmati, M.; DeSimone, K.; Curtis, C.E.; Sreenivasan, K.K. Spatially Specific Working Memory Activity in the Human Superior Colliculus. J. Neurosci. 2020, 40, 9487–9495. [Google Scholar] [CrossRef]
- Soetedjo, R.; Kojima, Y.; Fuchs, A.F. How Cerebellar Motor Learning Keeps Saccades Accurate. J. Neurophysiol. 2019, 121, 2153–2162. [Google Scholar] [CrossRef]
- Kojima, Y.; Soetedjo, R. Elimination of the Error Signal in the Superior Colliculus Impairs Saccade Motor Learning. Proc. Natl. Acad. Sci. USA 2018, 115, E8987–E8995. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Regehr, W.G. Developmental Remodeling of the Retinogeniculate Synapse. Neuron 2000, 28, 955–966. [Google Scholar] [CrossRef]
- Jaubert-Miazza, L.; Green, E.; Lo, F.-S.; Bui, K.; Mills, J.; Guido, W. Structural and Functional Composition of the Developing Retinogeniculate Pathway in the Mouse. Vis. Neurosci. 2005, 22, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Park, S.; Litvina, E.Y.; Morales, J.; Sanes, J.R.; Chen, C. Refinement of the Retinogeniculate Synapse by Bouton Clustering. Neuron 2014, 84, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Krahe, T.E.; El-Danaf, R.N.; Dilger, E.K.; Henderson, S.C.; Guido, W. Morphologically Distinct Classes of Relay Cells Exhibit Regional Preferences in the Dorsal Lateral Geniculate Nucleus of the Mouse. J. Neurosci. 2011, 31, 17437–17448. [Google Scholar] [CrossRef] [PubMed]
- Charalambakis, N.E.; Govindaiah, G.; Campbell, P.W.; Guido, W. Developmental Remodeling of Thalamic Interneurons Requires Retinal Signaling. J. Neurosci. 2019, 39, 3856–3866. [Google Scholar] [CrossRef]
- Butts, D.A.; Kanold, P.O.; Shatz, C.J. A Burst-Based “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS Biol. 2007, 5, e61. [Google Scholar] [CrossRef]
- Krahe, T.E.; Guido, W. Homeostatic Plasticity in the Visual Thalamus by Monocular Deprivation. J. Neurosci. 2011, 31, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Moro, S.S.; Kelly, K.R.; McKetton, L.; Gallie, B.L.; Steeves, J.K.E. Evidence of Multisensory Plasticity: Asymmetrical Medial Geniculate Body in People with One Eye. Neuroimage Clin. 2015, 9, 513–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, D.L.; Salinger, W.L. Loss of X-Cells in Lateral Geniculate Nucleus with Monocular Paralysis: Neural Plasticity in the Adult Cat. Science 1975, 189, 1011–1012. [Google Scholar] [CrossRef]
- Holman, K.D.; Duffy, K.R.; Mitchell, D.E. Short Periods of Darkness Fail to Restore Visual or Neural Plasticity in Adult Cats. Vis. Neurosci. 2018, 35, E002. [Google Scholar] [CrossRef]
- Guillery, R.W. Binocular Competition in the Control of Geniculate Cell Growth. J. Comp. Neurol. 1972, 144, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.L.; Spear, P.D.; Kratz, K.E. Quantitative Studies of Cell Size in the Cat’s Dorsal Lateral Geniculate Nucleus Following Visual Deprivation. J. Comp. Neurol. 1977, 172, 265–281. [Google Scholar] [CrossRef]
- Yücel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Atrophy of Relay Neurons in Magno- and Parvocellular Layers in the Lateral Geniculate Nucleus in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3216–3222. [Google Scholar]
- El-Danaf, R.N.; Krahe, T.E.; Dilger, E.K.; Bickford, M.E.; Fox, M.A.; Guido, W. Developmental Remodeling of Relay Cells in the Dorsal Lateral Geniculate Nucleus in the Absence of Retinal Input. Neural. Dev. 2015, 10, 19. [Google Scholar] [CrossRef]
- Ly, T.; Gupta, N.; Weinreb, R.N.; Kaufman, P.L.; Yücel, Y.H. Dendrite Plasticity in the Lateral Geniculate Nucleus in Primate Glaucoma. Vision Res. 2011, 51, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Smith, J.; Hook, M.J.V. Bilateral Enucleation Induces Homeostatic Plasticity in the Dorsolateral Geniculate Nucleus of Mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mcketton, L.; Kelly, K.R.; Schneider, K.A. Abnormal Lateral Geniculate Nucleus and Optic Chiasm in Human Albinism. J. Comp. Neurol. 2014, 522, 2680–2687. [Google Scholar] [CrossRef]
- Giraldo-Chica, M.; Hegarty, J.P.; Schneider, K.A. Morphological Differences in the Lateral Geniculate Nucleus Associated with Dyslexia. Neuroimage Clin. 2015, 7, 830–836. [Google Scholar] [CrossRef]
- O’Leary, D.D.; Wilkinson, D.G. Eph Receptors and Ephrins in Neural Development. Curr. Opin. Neurobiol. 1999, 9, 65–73. [Google Scholar] [CrossRef]
- Finlay, B.L.; Schneps, S.E.; Schneider, G.E. Orderly Compression of the Retinotectal Projection Following Partial Tectal Ablation in the Newborn Hamster. Nature 1979, 280, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.O.; Schneider, G.E. Plasticity of Retinofugal Projections after Partial Lesions of the Retina in Newborn Syrian Hamsters. J. Comp. Neurol. 1979, 185, 517–567. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; O’Leary, D.D. Responses of Retinal Axons in Vivo and in Vitro to Position-Encoding Molecules in the Embryonic Superior Colliculus. Neuron 1992, 9, 977–989. [Google Scholar] [CrossRef]
- Furman, M.; Crair, M.C. Synapse Maturation Is Enhanced in the Binocular Region of the Retinocollicular Map Prior to Eye Opening. J. Neurophysiol. 2012, 107, 3200–3216. [Google Scholar] [CrossRef][Green Version]
- Lund, R.D.; Cunningham, T.J.; Lund, J.S. Modified Optic Projections after Unilateral Eye Removal in Young Rats. Brain Behav. Evol. 1973, 8, 51–72. [Google Scholar] [CrossRef]
- Torborg, C.L.; Feller, M.B. Spontaneous Patterned Retinal Activity and the Refinement of Retinal Projections. Prog. Neurobiol. 2005, 76, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Bickford, M.E.; Slusarczyk, A.; Dilger, E.K.; Krahe, T.E.; Kucuk, C.; Guido, W. Synaptic Development of the Mouse Dorsal Lateral Geniculate Nucleus. J. Comp. Neurol. 2010, 518, 622–635. [Google Scholar] [CrossRef]
- Hong, Y.K.; Chen, C. Wiring and Rewiring of the Retinogeniculate Synapse. Curr. Opin. Neurobiol. 2011, 21, 228–237. [Google Scholar] [CrossRef]
- Guido, W. Development, Form, and Function of the Mouse Visual Thalamus. J. Neurophysiol. 2018, 120, 211–225. [Google Scholar] [CrossRef]
- Gustafsson, B.; Wigström, H.; Abraham, W.C.; Huang, Y.Y. Long-Term Potentiation in the Hippocampus Using Depolarizing Current Pulses as the Conditioning Stimulus to Single Volley Synaptic Potentials. J. Neurosci. 1987, 7, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Debanne, D.; Gähwiler, B.H.; Thompson, S.M. Asynchronous Pre- and Postsynaptic Activity Induces Associative Long-Term Depression in Area CA1 of the Rat Hippocampus in Vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Danchin, A. Selective Stabilisation of Developing Synapses as a Mechanism for the Specification of Neuronal Networks. Nature 1976, 264, 705–712. [Google Scholar] [CrossRef]
- Corriveau, R.A.; Huh, G.S.; Shatz, C.J. Regulation of Class I MHC Gene Expression in the Developing and Mature CNS by Neural Activity. Neuron 1998, 21, 505–520. [Google Scholar] [CrossRef]
- Huh, G.S.; Boulanger, L.M.; Du, H.; Riquelme, P.A.; Brotz, T.M.; Shatz, C.J. Functional Requirement for Class I MHC in CNS Development and Plasticity. Science 2000, 290, 2155–2159. [Google Scholar] [CrossRef]
- Goddard, C.A.; Butts, D.A.; Shatz, C.J. Regulation of CNS Synapses by Neuronal MHC Class, I. Proc. Natl. Acad. Sci. USA 2007, 104, 6828–6833. [Google Scholar] [CrossRef]
- Needleman, L.A.; Liu, X.-B.; El-Sabeawy, F.; Jones, E.G.; McAllister, A.K. MHC Class I Molecules Are Present Both Pre- and Postsynaptically in the Visual Cortex during Postnatal Development and in Adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 16999–17004. [Google Scholar] [CrossRef]
- Elmer, B.M.; McAllister, A.K. Major Histocompatibility Complex Class I Proteins in Brain Development and Plasticity. Trends Neurosci. 2012, 35, 660–670. [Google Scholar] [CrossRef]
- Lee, H.; Brott, B.K.; Kirkby, L.A.; Adelson, J.D.; Cheng, S.; Feller, M.B.; Datwani, A.; Shatz, C.J. Synapse Elimination and Learning Rules Co-Regulated by MHC Class I H2-Db. Nature 2014, 509, 195–200. [Google Scholar] [CrossRef]
- Zhang, L.I.; Tao, H.W.; Holt, C.E.; Harris, W.A.; Poo, M. A Critical Window for Cooperation and Competition among Developing Retinotectal Synapses. Nature 1998, 395, 37–44. [Google Scholar] [CrossRef]
- Vislay-Meltzer, R.L.; Kampff, A.R.; Engert, F. Spatiotemporal Specificity of Neuronal Activity Directs the Modification of Receptive Fields in the Developing Retinotectal System. Neuron 2006, 50, 101–114. [Google Scholar] [CrossRef]
- Okada, Y.; Miyamoto, T. Formation of Long-Term Potentiation in Superior Colliculus Slices from the Guinea Pig. Neurosci. Lett. 1989, 96, 108–113. [Google Scholar] [CrossRef]
- Okada, Y. The Properties of the Long-Term Potentiation (LTP) in the Superior Colliculus. Prog. Brain Res. 1993, 95, 287–296. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Phillips, M.A.; Constantine-Paton, M. Long-Term Potentiation in the Juvenile Superior Colliculus Requires Simultaneous Activation of NMDA Receptors and L-Type Ca2+ Channels and Reflects Addition of Newly Functional Synapses. J. Neurosci. 2006, 26, 12647–12655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, W.; Constantine-Paton, M. Eye Opening Rapidly Induces Synaptic Potentiation and Refinement. Neuron 2004, 43, 237–249. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Murata, Y.; Constantine-Paton, M. Eye Opening and PSD95 Are Required for Long-Term Potentiation in Developing Superior Colliculus. Proc. Natl. Acad. Sci. USA 2013, 110, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Lo, F.-S.; Mize, R.R. Properties of LTD and LTP of Retinocollicular Synaptic Transmission in the Developing Rat Superior Colliculus. Eur. J. Neurosci. 2002, 15, 1421–1432. [Google Scholar] [CrossRef]
- Van Keuren-Jensen, K.; Cline, H.T. Visual Experience Regulates Metabotropic Glutamate Receptor-Mediated Plasticity of AMPA Receptor Synaptic Transmission by Homer1a Induction. J. Neurosci. 2006, 26, 7575–7580. [Google Scholar] [CrossRef]
- Deeg, K.E.; Aizenman, C.D. Sensory Modality-Specific Homeostatic Plasticity in the Developing Optic Tectum. Nat. Neurosci. 2011, 14, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Debanne, D.; Inglebert, Y.; Russier, M. Plasticity of Intrinsic Neuronal Excitability. Curr. Opin. Neurobiol. 2019, 54, 73–82. [Google Scholar] [CrossRef]
- Debanne, D.; Russier, M. The Contribution of Ion Channels in Input-Output Plasticity. Neurobiol. Learn Mem. 2019, 166, 107095. [Google Scholar] [CrossRef] [PubMed]
- Daoudal, G.; Debanne, D. Long-Term Plasticity of Intrinsic Excitability: Learning Rules and Mechanisms. Learn. Mem. 2003, 10, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Titley, H.K.; Brunel, N.; Hansel, C. Toward a Neurocentric View of Learning. Neuron 2017, 95, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fricker, D.; Brager, D.H.; Chen, X.; Lu, H.-C.; Chitwood, R.A.; Johnston, D. Activity-Dependent Decrease of Excitability in Rat Hippocampal Neurons through Increases in I(h). Nat. Neurosci. 2005, 8, 1542–1551. [Google Scholar] [CrossRef]
- Brager, D.H.; Johnston, D. Plasticity of Intrinsic Excitability during Long-Term Depression Is Mediated through MGluR-Dependent Changes in I(h) in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2007, 27, 13926–13937. [Google Scholar] [CrossRef]
- Campanac, E.; Daoudal, G.; Ankri, N.; Debanne, D. Downregulation of Dendritic I(h) in CA1 Pyramidal Neurons after LTP. J. Neurosci. 2008, 28, 8635–8643. [Google Scholar] [CrossRef]
- Gasselin, C.; Inglebert, Y.; Ankri, N.; Debanne, D. Plasticity of Intrinsic Excitability during LTD Is Mediated by Bidirectional Changes in H-Channel Activity. Sci. Rep. 2017, 7, 14418. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kang, N.; Jiang, L.; Nedergaard, M.; Kang, J. Activity-Dependent Long-Term Potentiation of Intrinsic Excitability in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2005, 25, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Campanac, E.; Gasselin, C.; Baude, A.; Rama, S.; Ankri, N.; Debanne, D. Enhanced Intrinsic Excitability in Basket Cells Maintains Excitatory-Inhibitory Balance in Hippocampal Circuits. Neuron 2013, 77, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Incontro, S.; Sammari, M.; Azzaz, F.; Inglebert, Y.; Ankri, N.; Russier, M.; Fantini, J.; Debanne, D. Endocannabinoids Tune Intrinsic Excitability in O-LM Interneurons by Direct Modulation of Post-Synaptic Kv7 Channels. J. Neurosci. 2021. [Google Scholar] [CrossRef]
- Desai, N.S.; Rutherford, L.C.; Turrigiano, G.G. Plasticity in the Intrinsic Excitability of Cortical Pyramidal Neurons. Nat. Neurosci. 1999, 2, 515–520. [Google Scholar] [CrossRef]
- Cudmore, R.H.; Turrigiano, G.G. Long-Term Potentiation of Intrinsic Excitability in LV Visual Cortical Neurons. J. Neurophysiol. 2004, 92, 341–348. [Google Scholar] [CrossRef]
- Nataraj, K.; Le Roux, N.; Nahmani, M.; Lefort, S.; Turrigiano, G. Visual Deprivation Suppresses L5 Pyramidal Neuron Excitability by Preventing the Induction of Intrinsic Plasticity. Neuron 2010, 68, 750–762. [Google Scholar] [CrossRef]
- Nataraj, K.; Turrigiano, G. Regional and Temporal Specificity of Intrinsic Plasticity Mechanisms in Rodent Primary Visual Cortex. J. Neurosci. 2011, 31, 17932–17940. [Google Scholar] [CrossRef]
- Lambo, M.E.; Turrigiano, G.G. Synaptic and Intrinsic Homeostatic Mechanisms Cooperate to Increase L2/3 Pyramidal Neuron Excitability during a Late Phase of Critical Period Plasticity. J. Neurosci. 2013, 33, 8810–8819. [Google Scholar] [CrossRef]
- Aizenman, C.D.; Akerman, C.J.; Jensen, K.R.; Cline, H.T. Visually Driven Regulation of Intrinsic Neuronal Excitability Improves Stimulus Detection In Vivo. Neuron 2003, 39, 831–842. [Google Scholar] [CrossRef]
- Pratt, K.G.; Aizenman, C.D. Homeostatic Regulation of Intrinsic Excitability and Synaptic Transmission in a Developing Visual Circuit. J. Neurosci. 2007, 27, 8268–8277. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.E.; Khakhalin, A.S. Intrinsic Temporal Tuning of Neurons in the Optic Tectum Is Shaped by Multisensory Experience. J. Neurophysiol. 2019, 122, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Van Hook, M.J.; Monaco, C.; Bierlein, E.R.; Smith, J.C. Neuronal and Synaptic Plasticity in the Visual Thalamus in Mouse Models of Glaucoma. Front. Cell. Neurosci. 2020, 14, 626056. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Cheadle, L.; Hrvatin, S.; Nagy, M.A.; Rivera, S.; Crow, M.; Gillis, J.; Kirchner, R.; Greenberg, M.E. Single-Cell Transcriptomics of the Developing Lateral Geniculate Nucleus Reveals Insights into Circuit Assembly and Refinement. Proc. Natl. Acad. Sci. USA 2018, 115, E1051–E1060. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, L.; Tzeng, C.P.; Kalish, B.T.; Harmin, D.A.; Rivera, S.; Ling, E.; Nagy, M.A.; Hrvatin, S.; Hu, L.; Stroud, H.; et al. Visual Experience-Dependent Expression of Fn14 Is Required for Retinogeniculate Refinement. Neuron 2018, 99, 525–539.e10. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, U.; Monavarfeshani, A.; Su, K.; Zabet-Moghadam, M.; Cole, J.; Carnival, E.; Su, J.; Mirzaei, M.; Gupta, V.; Salekdeh, G.H.; et al. Distribution and Development of Molecularly Distinct Perineuronal Nets in Visual Thalamus. J. Neurochem. 2018, 147, 626–646. [Google Scholar] [CrossRef]
- Phillips, J.W.; Schulmann, A.; Hara, E.; Winnubst, J.; Liu, C.; Valakh, V.; Wang, L.; Shields, B.C.; Korff, W.; Chandrashekar, J.; et al. A Repeated Molecular Architecture across Thalamic Pathways. Nat. Neurosci. 2019, 22, 1925–1935. [Google Scholar] [CrossRef]
- Bakken, T.E.; van Velthoven, C.T.; Menon, V.; Hodge, R.D.; Yao, Z.; Nguyen, T.N.; Graybuck, L.T.; Horwitz, G.D.; Bertagnolli, D.; Goldy, J.; et al. Single-Cell and Single-Nucleus RNA-Seq Uncovers Shared and Distinct Axes of Variation in Dorsal LGN Neurons in Mice, Non-Human Primates, and Humans. Elife 2021, 10, e64875. [Google Scholar] [CrossRef]
- Liu, H.-H.; McClatchy, D.B.; Schiapparelli, L.; Shen, W.; Yates, J.R.; Cline, H.T. Role of the Visual Experience-Dependent Nascent Proteome in Neuronal Plasticity. Elife 2018, 7, e33420. [Google Scholar] [CrossRef]
- Chen, X.; Aslam, M.; Gollisch, T.; Allen, K.; von Engelhardt, J. CKAMP44 Modulates Integration of Visual Inputs in the Lateral Geniculate Nucleus. Nat. Commun. 2018, 9, 261. [Google Scholar] [CrossRef]
- Stephany, C.-É.; Ma, X.; Dorton, H.M.; Wu, J.; Solomon, A.M.; Frantz, M.G.; Qiu, S.; McGee, A.W. Distinct Circuits for Recovery of Eye Dominance and Acuity in Murine Amblyopia. Curr. Biol. 2018, 28, 1914–1923.e5. [Google Scholar] [CrossRef] [PubMed]
- Mudd, D.B.; Balmer, T.S.; Kim, S.Y.; Machhour, N.; Pallas, S.L. TrkB Activation during a Critical Period Mimics the Protective Effects of Early Visual Experience on Perception and the Stability of Receptive Fields in Adult Superior Colliculus. J. Neurosci. 2019, 39, 4475–4488. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Andoh, M.; Koyama, R. Microglia Regulate Synaptic Development and Plasticity. Dev. Neurobiol. 2021, 81, 568–590. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Scott-Hewitt, N.; Perrucci, F.; Morini, R.; Erreni, M.; Mahoney, M.; Witkowska, A.; Carey, A.; Faggiani, E.; Schuetz, L.T.; Mason, S.; et al. Local Externalization of Phosphatidylserine Mediates Developmental Synaptic Pruning by Microglia. EMBO J. 2020, 39, e105380. [Google Scholar] [CrossRef]
- Yuzaki, M. Two Classes of Secreted Synaptic Organizers in the Central Nervous System. Annu. Rev. Physiol. 2018, 80, 243–262. [Google Scholar] [CrossRef]
- Bjartmar, L.; Huberman, A.D.; Ullian, E.M.; Rentería, R.C.; Liu, X.; Xu, W.; Prezioso, J.; Susman, M.W.; Stellwagen, D.; Stokes, C.C.; et al. Neuronal Pentraxins Mediate Synaptic Refinement in the Developing Visual System. J. Neurosci. 2006, 26, 6269–6281. [Google Scholar] [CrossRef]
- Suzuki, K.; Elegheert, J.; Song, I.; Sasakura, H.; Senkov, O.; Matsuda, K.; Kakegawa, W.; Clayton, A.J.; Chang, V.T.; Ferrer-Ferrer, M.; et al. A Synthetic Synaptic Organizer Protein Restores Glutamatergic Neuronal Circuits. Science 2020, 369, eabb4853. [Google Scholar] [CrossRef]
- Linhoff, M.W.; Laurén, J.; Cassidy, R.M.; Dobie, F.A.; Takahashi, H.; Nygaard, H.B.; Airaksinen, M.S.; Strittmatter, S.M.; Craig, A.M. An Unbiased Expression Screen for Synaptogenic Proteins Identifies the LRRTM Protein Family as Synaptic Organizers. Neuron 2009, 61, 734–749. [Google Scholar] [CrossRef]
- Monavarfeshani, A.; Stanton, G.; Van Name, J.; Su, K.; Mills, W.A.; Swilling, K.; Kerr, A.; Huebschman, N.A.; Su, J.; Fox, M.A. LRRTM1 Underlies Synaptic Convergence in Visual Thalamus. Elife 2018, 7, e33498. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, U.; Matsuzaki, H.; Yamamoto, T. Protein Kinase C Delta (PKC Delta): Activation Mechanisms and Functions. J. Biochem. 2002, 132, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Hooks, B.M.; Chen, C. Critical Periods in the Visual System: Changing Views for a Model of Experience-Dependent Plasticity. Neuron 2007, 56, 312–326. [Google Scholar] [CrossRef]
- Kielland, A.; Bochorishvili, G.; Corson, J.; Zhang, L.; Rosin, D.L.; Heggelund, P.; Zhu, J.J. Activity Patterns Govern Synapse-Specific AMPA Receptor Trafficking between Deliverable and Synaptic Pools. Neuron 2009, 62, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Kielland, A.; Heggelund, P. AMPA and NMDA Currents Show Different Short-Term Depression in the Dorsal Lateral Geniculate Nucleus of the Rat. J. Physiol. 2002, 542, 99–106. [Google Scholar] [CrossRef]
- Noutel, J.; Hong, Y.K.; Leu, B.; Kang, E.; Chen, C. Experience-Dependent Retinogeniculate Synapse Remodeling Is Abnormal in MeCP2-Deficient Mice. Neuron 2011, 70, 35–42. [Google Scholar] [CrossRef]
- Cheadle, L.; Rivera, S.A.; Phelps, J.S.; Ennis, K.A.; Stevens, B.; Burkly, L.C.; Lee, W.-C.A.; Greenberg, M.E. Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron 2020, 108, 451–468.e9. [Google Scholar] [CrossRef]
- McGee, A.W.; Yang, Y.; Fischer, Q.S.; Daw, N.W.; Strittmatter, S.M. Experience-Driven Plasticity of Visual Cortex Limited by Myelin and Nogo Receptor. Science 2005, 309, 2222–2226. [Google Scholar] [CrossRef]
- Stephany, C.-É.; Ikrar, T.; Nguyen, C.; Xu, X.; McGee, A.W. Nogo Receptor 1 Confines a Disinhibitory Microcircuit to the Critical Period in Visual Cortex. J. Neurosci. 2016, 36, 11006–11012. [Google Scholar] [CrossRef]
- Pigeat, R.; Chausson, P.; Dreyfus, F.M.; Leresche, N.; Lambert, R.C. Sleep Slow Wave-Related Homo and Heterosynaptic LTD of Intrathalamic GABAAergic Synapses: Involvement of T-Type Ca2+ Channels and Metabotropic Glutamate Receptors. J. Neurosci. 2015, 35, 64–73. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duménieu, M.; Marquèze-Pouey, B.; Russier, M.; Debanne, D. Mechanisms of Plasticity in Subcortical Visual Areas. Cells 2021, 10, 3162. https://doi.org/10.3390/cells10113162
Duménieu M, Marquèze-Pouey B, Russier M, Debanne D. Mechanisms of Plasticity in Subcortical Visual Areas. Cells. 2021; 10(11):3162. https://doi.org/10.3390/cells10113162
Chicago/Turabian StyleDuménieu, Maël, Béatrice Marquèze-Pouey, Michaël Russier, and Dominique Debanne. 2021. "Mechanisms of Plasticity in Subcortical Visual Areas" Cells 10, no. 11: 3162. https://doi.org/10.3390/cells10113162
APA StyleDuménieu, M., Marquèze-Pouey, B., Russier, M., & Debanne, D. (2021). Mechanisms of Plasticity in Subcortical Visual Areas. Cells, 10(11), 3162. https://doi.org/10.3390/cells10113162






