Regulation of Inflammasomes by Application of Omega-3 Polyunsaturated Fatty Acids in a Spinal Cord Injury Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedure
2.3. Behavioral Testing
2.4. Fatty Acid Analysis in Blood Samples
2.5. Luxol Fast Blue Staining
2.6. Immunohistochemistry (IHC)
2.7. RNA Extraction and Real-Time PCR
2.8. Enzyme-Linked Immunosorbent Assay
2.9. SDS PAGE and Western Blot
2.10. Data Analysis
3. Results
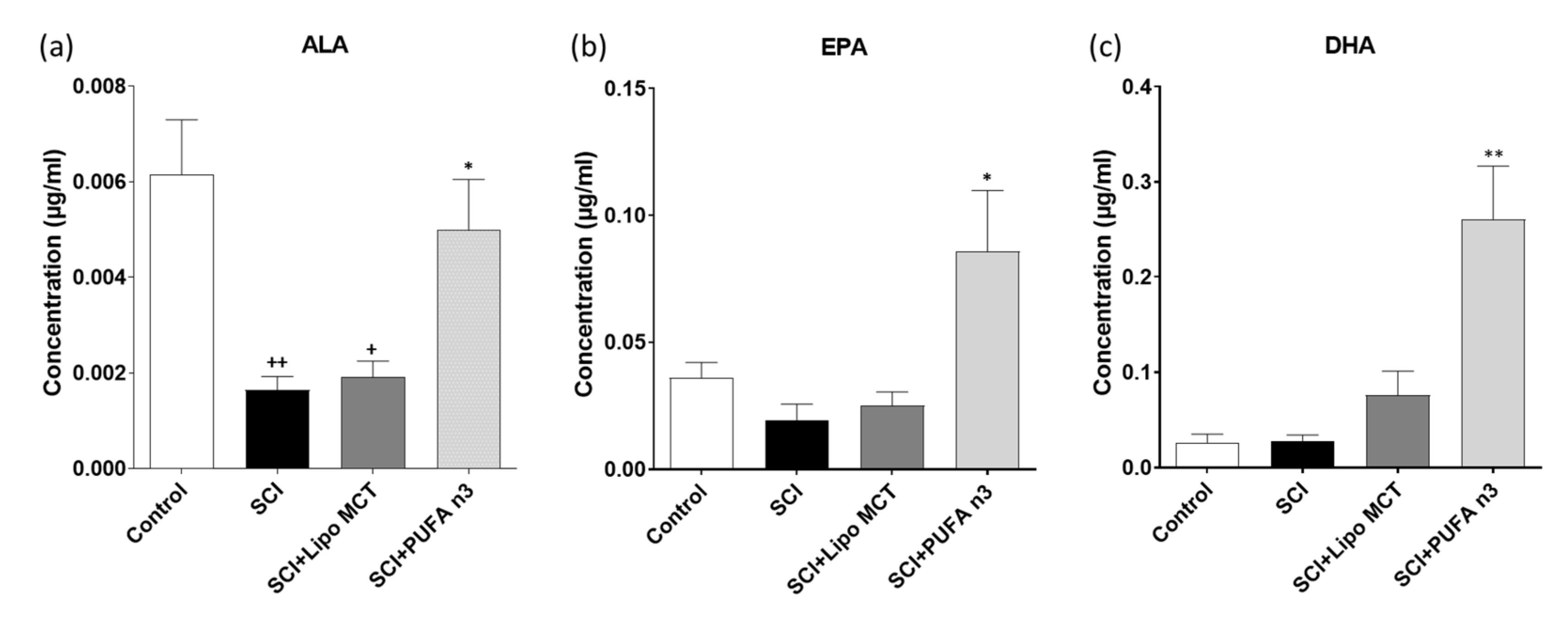
3.1. Plasma Levels of DHA, EPA and ALA
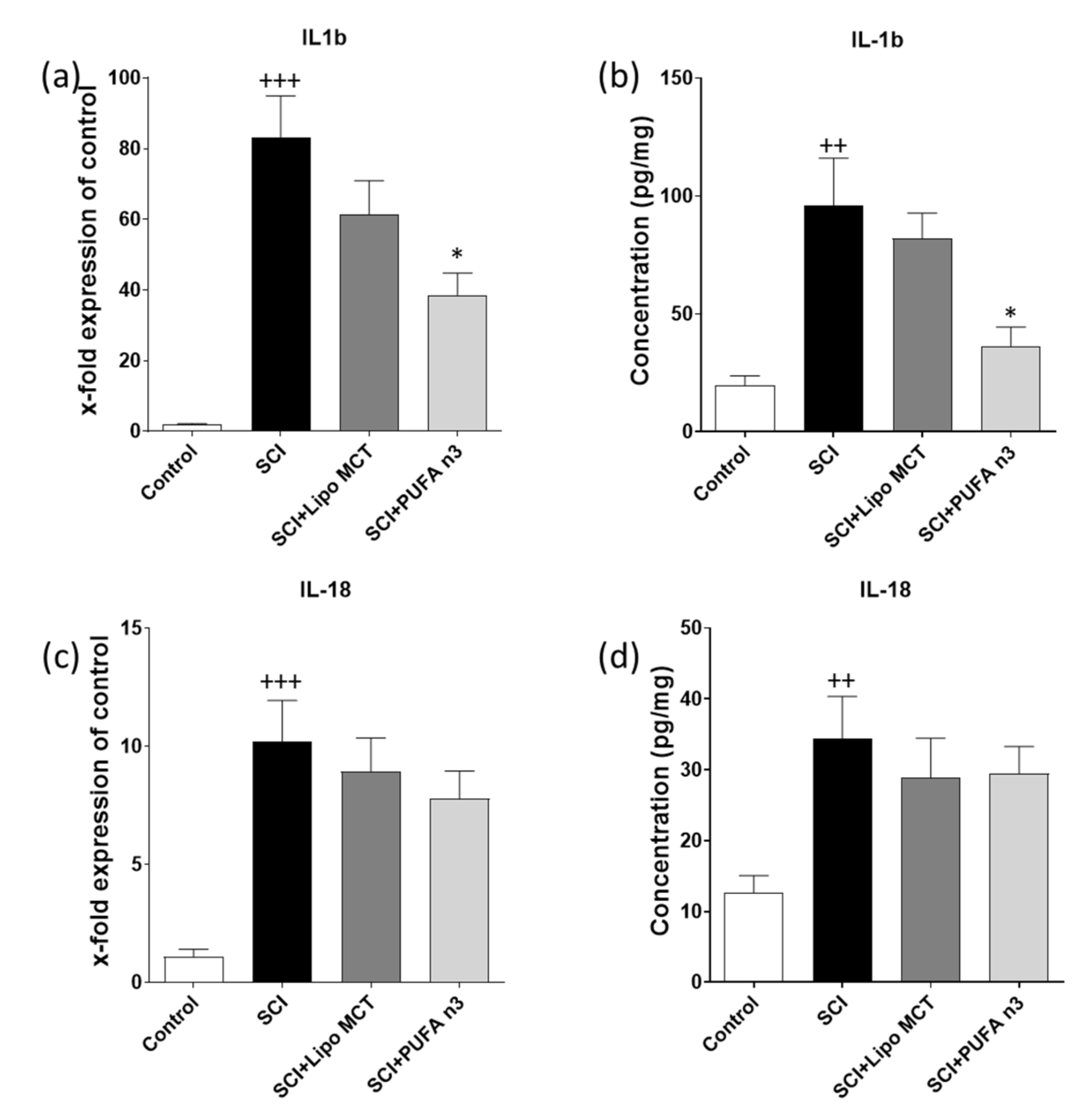
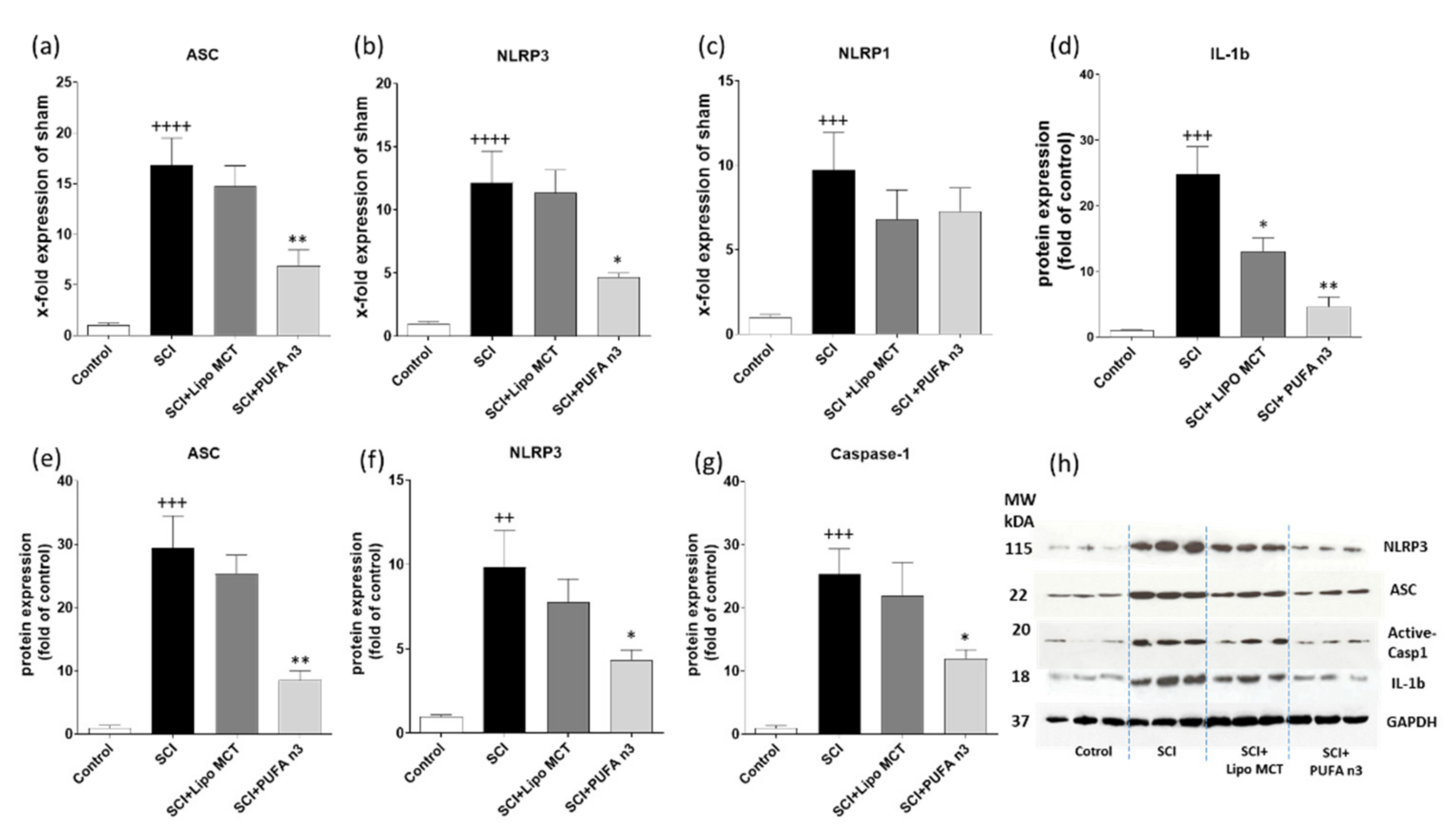
3.2. Effects of PUFA n3 on Inflammatory Cytokines and Inflammasome Components
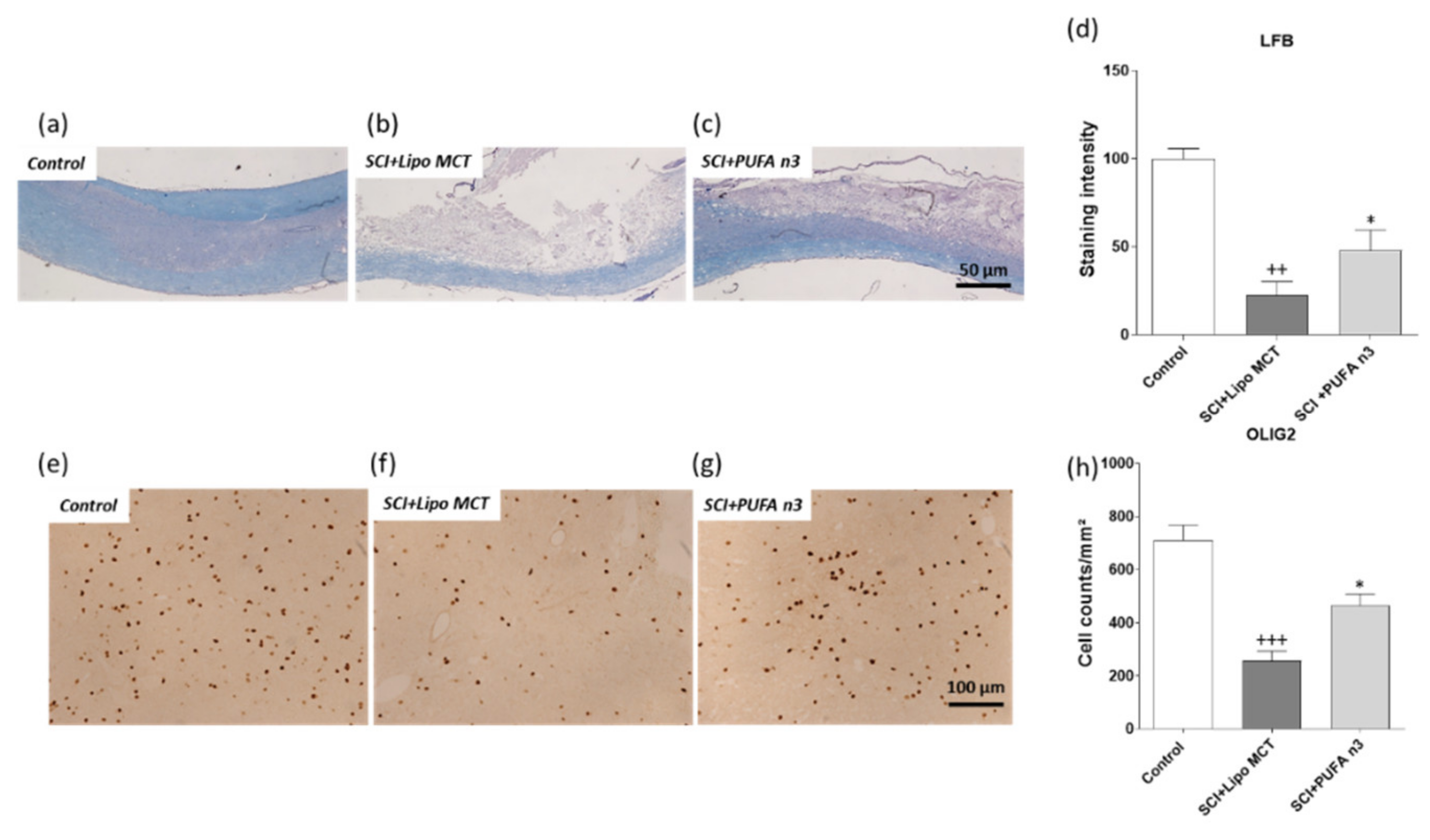
3.3. Effects of PUFA n3 on Remyelination
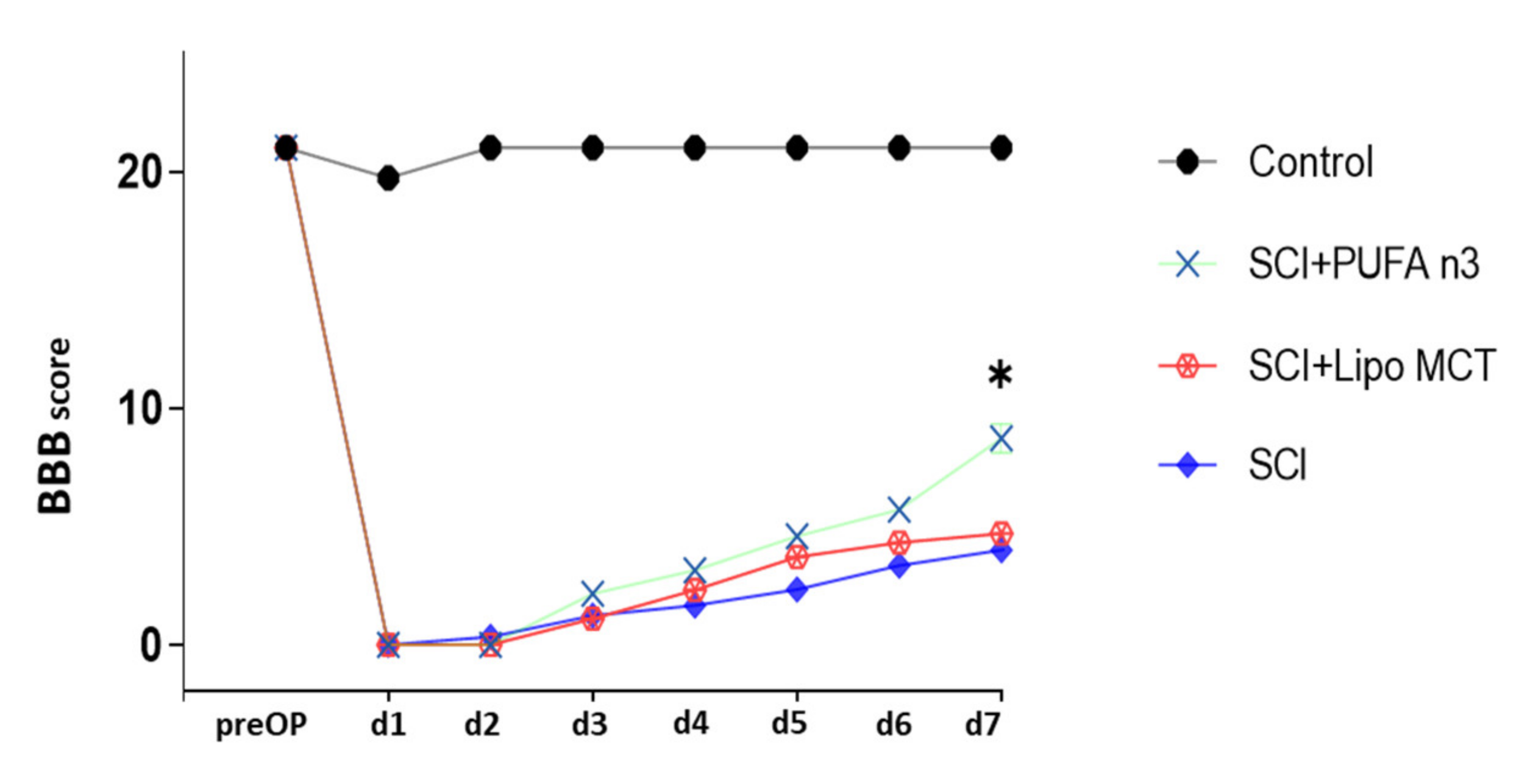
3.4. Effects of PUFA n3 on Glial Cells and Functional Recovery
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehrabi, S.; Eftekhari, S.; Moradi, F.; Delaviz, H.; Pourheidar, B.; Azizi, M.; Zendehdel, A.; Shahbazi, A.; Joghataei, M.T. Cell therapy in spinal cord injury: A mini- reivew. Basic Clin. Neurosci. 2013, 4, 172–176. [Google Scholar] [PubMed]
- De Rivero Vaccari, J.P.; Lotocki, G.; Marcillo, A.E.; Dietrich, W.D.; Keane, R.W. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 3404–3414. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Khanlarkhani, N.; Beyer, C.; Zendedel, A. Inflammasome: Its role in traumatic brain and spinal cord injury. J. Cell Physiol. 2018, 233, 5160–5169. [Google Scholar] [CrossRef] [PubMed]
- De Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 2016, 167, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Johann, S.; Mehrabi, S.; Joghataei, M.T.; Hassanzadeh, G.; Kipp, M.; Beyer, C. Activation and Regulation of NLRP3 Inflammasome by Intrathecal Application of SDF-1a in a Spinal Cord Injury Model. Mol. Neurobiol. 2016, 53, 3063–3075. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Behrmann, D.; Bates, C.M.; Horrocks, L.A. Lipid alterations following impact spinal cord injury in the rat. Mol. Chem. Neuropathol. 1994, 23, 13–26. [Google Scholar] [CrossRef]
- Koyuncu, E.; Nakipoglu Yuzer, G.F.; Yenigun, D.; Ozgirgin, N. The analysis of serum lipid levels in patients with spinal cord injury. J. Spinal Cord Med. 2017, 40, 567–572. [Google Scholar] [CrossRef]
- Laclaustra, M.; Van Den Berg, E.L.; Hurtado-Roca, Y.; Castellote, J.M. Serum lipid profile in subjects with traumatic spinal cord injury. PLoS ONE 2015, 10, e0115522. [Google Scholar] [CrossRef][Green Version]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Brand, A.; Bauer, N.G.; Hallott, A.; Goldbaum, O.; Ghebremeskel, K.; Reifen, R.; Richter-Landsberg, C. Membrane lipid modification by polyunsaturated fatty acids sensitizes oligodendroglial OLN-93 cells against oxidative stress and promotes up-regulation of heme oxygenase-1 (HSP32). J. Neurochem. 2010, 113, 465–476. [Google Scholar] [CrossRef]
- Begum, G.; Yan, H.Q.; Li, L.; Singh, A.; Dixon, C.E.; Sun, D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Habib, P.; Dang, J.; Lammerding, L.; Hoffmann, S.; Beyer, C.; Slowik, A. Omega-3 polyunsaturated fatty acids ameliorate neuroinflammation and mitigate ischemic stroke damage through interactions with astrocytes and microglia. J. Neuroimmunol. 2015, 278, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Bao, Z.; Hou, L.; Liu, Y.; Wang, X.; You, Y.; et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp. Neurol. 2017, 290, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Midgette, Y.; Shah, R. Fish Oil Derived Omega 3 Fatty Acids Suppress Adipose NLRP3 Inflammasome Signaling in Human Obesity. J. Endocr. Soc. 2019, 3, 504–515. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Cordero, K.; Llan, M.S.; De Leon, M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. J. Neurotrauma 2013, 30, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.N.; Gladman, S.J.; Dyall, S.C.; Patel, U.; Virani, N.; Kang, J.X.; Priestley, J.V.; Michael-Titus, A.T. Transgenic mice with high endogenous omega-3 fatty acids are protected from spinal cord injury. Neurobiol. Dis. 2013, 51, 104–112. [Google Scholar] [CrossRef]
- Zendedel, A.; Nobakht, M.; Bakhtiyari, M.; Beyer, C.; Kipp, M.; Baazm, M.; Joghataie, M.T. Stromal cell-derived factor-1 alpha (SDF-1alpha) improves neural recovery after spinal cord contusion in rats. Brain Res. 2012, 1473, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Monnink, F.; Hassanzadeh, G.; Zaminy, A.; Ansar, M.M.; Habib, P.; Slowik, A.; Kipp, M.; Beyer, C. Estrogen Attenuates Local Inflammasome Expression and Activation after Spinal Cord Injury. Mol. Neurobiol. 2018, 55, 1364–1375. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; von Schacky, C. n-3 polyunsaturated fatty acids and human cytokine synthesis. Curr. Opin. Lipidol. 1996, 7, 48–52. [Google Scholar] [CrossRef]
- Sarsilmaz, M.; Songur, A.; Ozyurt, H.; Kus, I.; Ozen, O.A.; Ozyurt, B.; Sogut, S.; Akyol, O. Potential role of dietary omega-3 essential fatty acids on some oxidant/antioxidant parameters in rats’ corpus striatum. Prostaglandins Leukot Essent Fatty Acids 2003, 69, 253–259. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- King, V.R.; Huang, W.L.; Dyall, S.C.; Curran, O.E.; Priestley, J.V.; Michael-Titus, A.T. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 4672–4680. [Google Scholar] [CrossRef]
- Hoffmann, S.; Beyer, C.; Zendedel, A. Comparative analysis of gonadal steroid-mediated neuroprotection after transient focal ischemia in rats: Route of application and substrate composition. J. Mol. Neurosci. 2015, 56, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T., II; Dominguez-Lopez, A.; Miliar-Garcia, A.; Escalona-Cardoso, G.N.; Real-Sandoval, S.A.; Gomez-Alcala, A.; Jaramillo-Flores, M.E. Modulation of the mRNA of the Nlrp3 inflammasome by Morin and PUFAs in an obesity model induced by a high-fat diet. Food Res. Int. 2020, 137, 109706. [Google Scholar] [CrossRef]
- De Boer, A.A.; Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Power, K.A.; Ma, D.W.; Robinson, L.E. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J. Nutr. Biochem. 2016, 34, 61–72. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Yin, H.; Zhang, X.; Li, Y. NLRP3 Inflammasome: A Potential Alternative Therapy Target for Atherosclerosis. Evid Based Complement Alternat Med. 2020, 2020, 1561342. [Google Scholar] [CrossRef]
- Ma, D.; Lu, L.; Boneva, N.B.; Warashina, S.; Kaplamadzhiev, D.B.; Mori, Y.; Nakaya, M.A.; Kikuchi, M.; Tonchev, A.B.; Okano, H.; et al. Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus. Hippocampus 2008, 18, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Cama, V.F.; Marin-Prida, J.; Acosta-Rivero, N.; Acosta, E.F.; Diaz, L.O.; Casadesus, A.V.; Fernandez-Marrero, B.; Gilva-Rodriguez, N.; Cremata-Garcia, D.; Cervantes-Llanos, M.; et al. The microglial NLRP3 inflammasome is involved in human SARS-CoV-2 cerebral pathogenicity: A report of three post-mortem cases. J. Neuroimmunol. 2021, 361, 577728. [Google Scholar] [CrossRef] [PubMed]
- Roosen, K.; Scheld, M.; Mandzhalova, M.; Clarner, T.; Beyer, C.; Zendedel, A. CXCL12 inhibits inflammasome activation in LPS-stimulated BV2 cells. Brain Res. 2021, 1763, 147446. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Chen, W.Y.; Chen, J.B.; Lee, W.C.; Chang, C.C.; Tzeng, H.T.; Huang, C.C.; Chang, Y.J.; Yang, J.L. The AST-120 Recovers Uremic Toxin-Induced Cognitive Deficit via NLRP3 Inflammasome Pathway in Astrocytes and Microglia. Biomedicines 2021, 9, 1252. [Google Scholar] [CrossRef]
- Xu, L.; He, D.; Bai, Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Mol. Neurobiol. 2016, 53, 6709–6715. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhou, G.; Zhuang, J.; Xu, C.; Zhou, H.; Peng, Y.; Cao, Y.; Zeng, H.; Li, J.; Yan, F.; et al. White Matter Injury After Intracerebral Hemorrhage. Front. Neurol. 2021, 12, 562090. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hu, X.; Leak, R.K.; Shi, Y.; An, C.; Suenaga, J.; Chen, J.; Gao, Y. Demyelination as a rational therapeutic target for ischemic or traumatic brain injury. Exp. Neurol. 2015, 272, 17–25. [Google Scholar] [CrossRef] [PubMed]

| Genes | Primer Sequences (5′ to 3′) | |
|---|---|---|
| CycloA | F | GGCAAATGCTGGACCAAACAC |
| R | TTAGAGTTGTCCACAGTCGGAGATG | |
| IL-1b | F | TGGCAACTGTCCCTGAACTC |
| R | GTCGAGATGCTGCTGTGAGA | |
| IL-18 | F | GGACTGGCTGTGACCCTATC |
| R | TGTCCTGGCACACGTTTCTG | |
| NLRP1b | F | GGGGCAGCCAAATCAAGTTC |
| R | TGAGCGGTCATTGCAACTCT | |
| NLRP3 | F | TCTGTTCATTGGCTGCGGAT |
| R | GCCTTTTTCGAACTTGCCGT | |
| ASC | F | GCTGCAGATGGACCCCATAG |
| R | ACATTGTGAGCTCCAAGCCA |
| Antibody | Company | Western Blot | IHC |
|---|---|---|---|
| ASC | Santa Cruz, Dallas, TX, USA | 1:1000 | - |
| OLIG2 | Santa Cruz, Dallas, TX, USA | - | 1:1000 |
| IBA-1 | Abcam plc, Cambridge, UK | - | 1:2500 |
| CASPASE-1 | Santa Cruz, Dallas, TX, USA | 1:1000 | - |
| NLRP3 | Bioss, Woburn, MA, USA | 1:1000 | - |
| GFAP | Abcam plc, Cambridge, UK | - | 1:1000 |
| GAPDH | Sigma Aldrich, Saint Louis, MI, USA | 1:4000 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baazm, M.; Behrens, V.; Beyer, C.; Nikoubashman, O.; Zendedel, A. Regulation of Inflammasomes by Application of Omega-3 Polyunsaturated Fatty Acids in a Spinal Cord Injury Model. Cells 2021, 10, 3147. https://doi.org/10.3390/cells10113147
Baazm M, Behrens V, Beyer C, Nikoubashman O, Zendedel A. Regulation of Inflammasomes by Application of Omega-3 Polyunsaturated Fatty Acids in a Spinal Cord Injury Model. Cells. 2021; 10(11):3147. https://doi.org/10.3390/cells10113147
Chicago/Turabian StyleBaazm, Maryam, Victoria Behrens, Cordian Beyer, Omid Nikoubashman, and Adib Zendedel. 2021. "Regulation of Inflammasomes by Application of Omega-3 Polyunsaturated Fatty Acids in a Spinal Cord Injury Model" Cells 10, no. 11: 3147. https://doi.org/10.3390/cells10113147
APA StyleBaazm, M., Behrens, V., Beyer, C., Nikoubashman, O., & Zendedel, A. (2021). Regulation of Inflammasomes by Application of Omega-3 Polyunsaturated Fatty Acids in a Spinal Cord Injury Model. Cells, 10(11), 3147. https://doi.org/10.3390/cells10113147







