Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response
Abstract
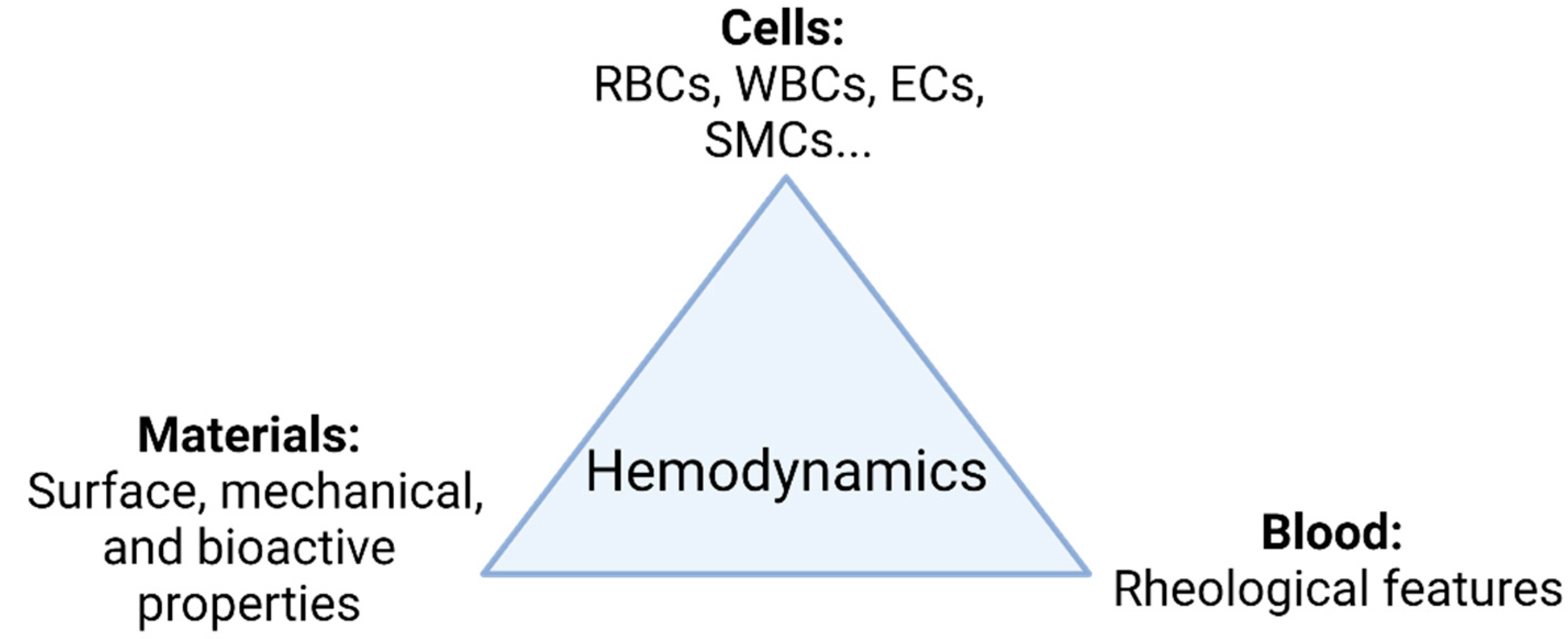
1. Introduction
2. Materials and Methods
3. Phases of the Vascular Graft Response
4. Phase I of Vascular Graft Response
4.1. Peri-Implantation Period Conditions
4.2. CASE A: The Vascular Graft Withstands Implantation, but Occlusion Occurs Rapidly
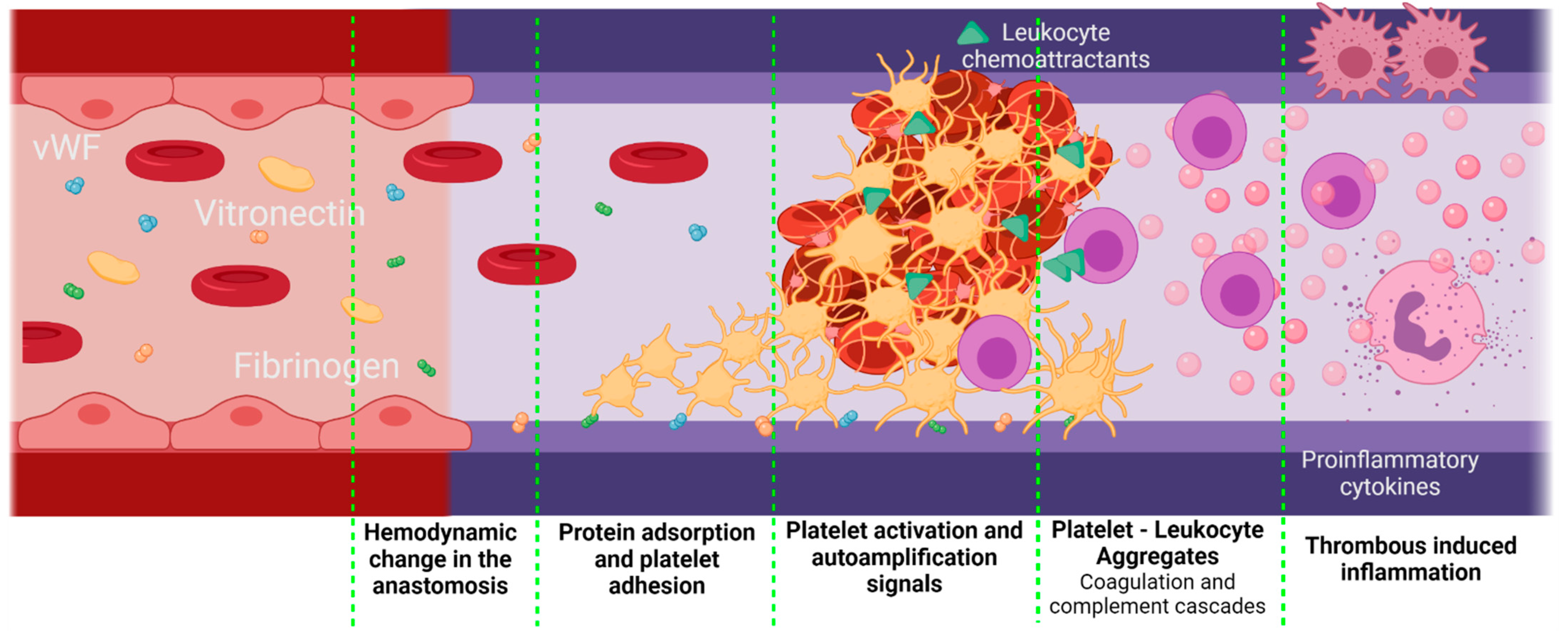
Coagulation Cascade in Non-Inflammatory Conditions but Response to Contact with Surfaces and Non-Stable Hemodynamic Conditions
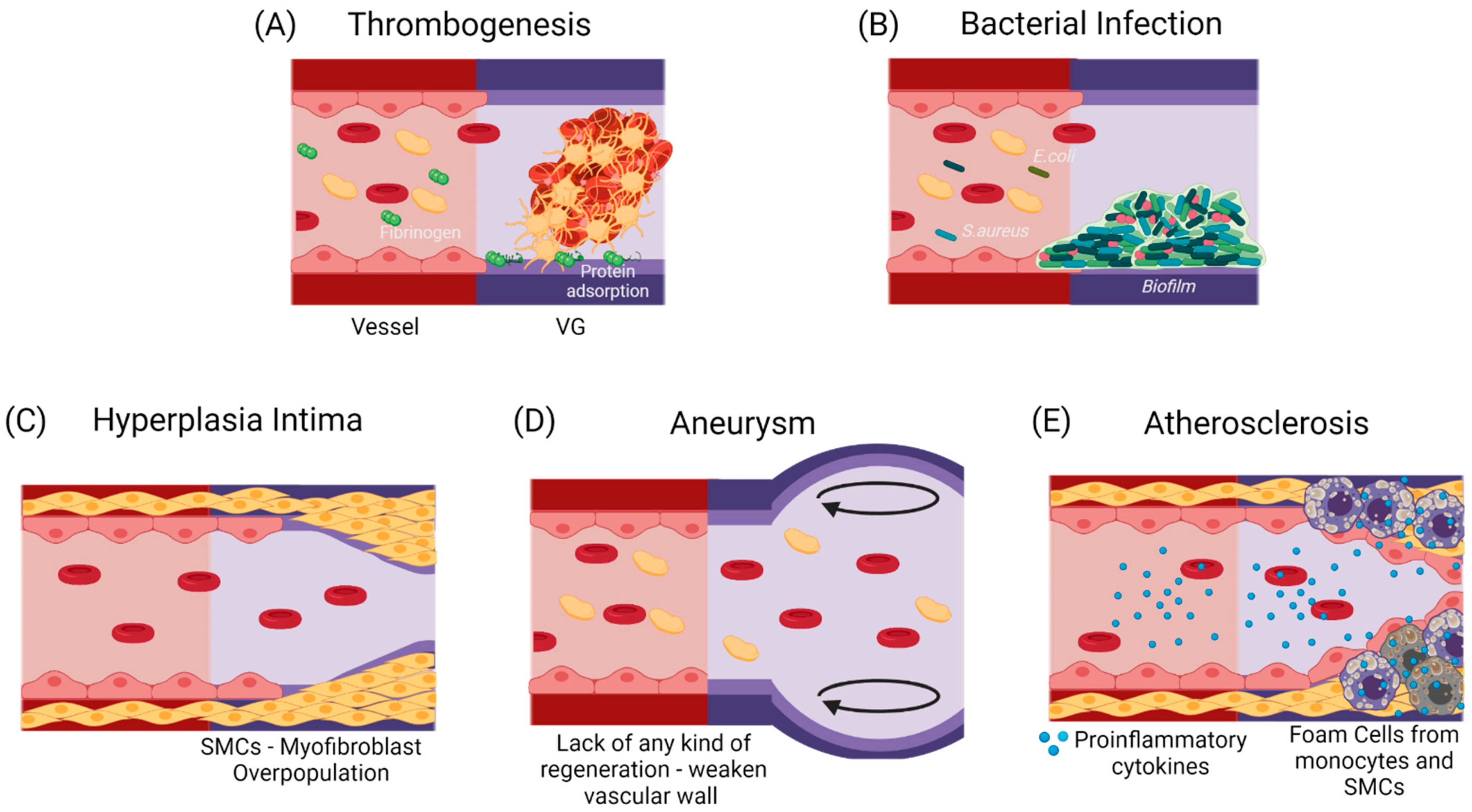
4.3. CASE B: The Vascular Graft Fails Immediately due to Complications Related to a Strong Foreign Body Response
4.3.1. Thrombogenesis-Mediated Inflammation
4.3.2. Complement Cascade
4.3.3. Cytokines and Degradation Products
4.3.4. Bacterial Infection
4.4. CASE C: The Graft Is Successfully Implanted at the Intervention Site and the Inflammatory-Remodeling Processes Begin
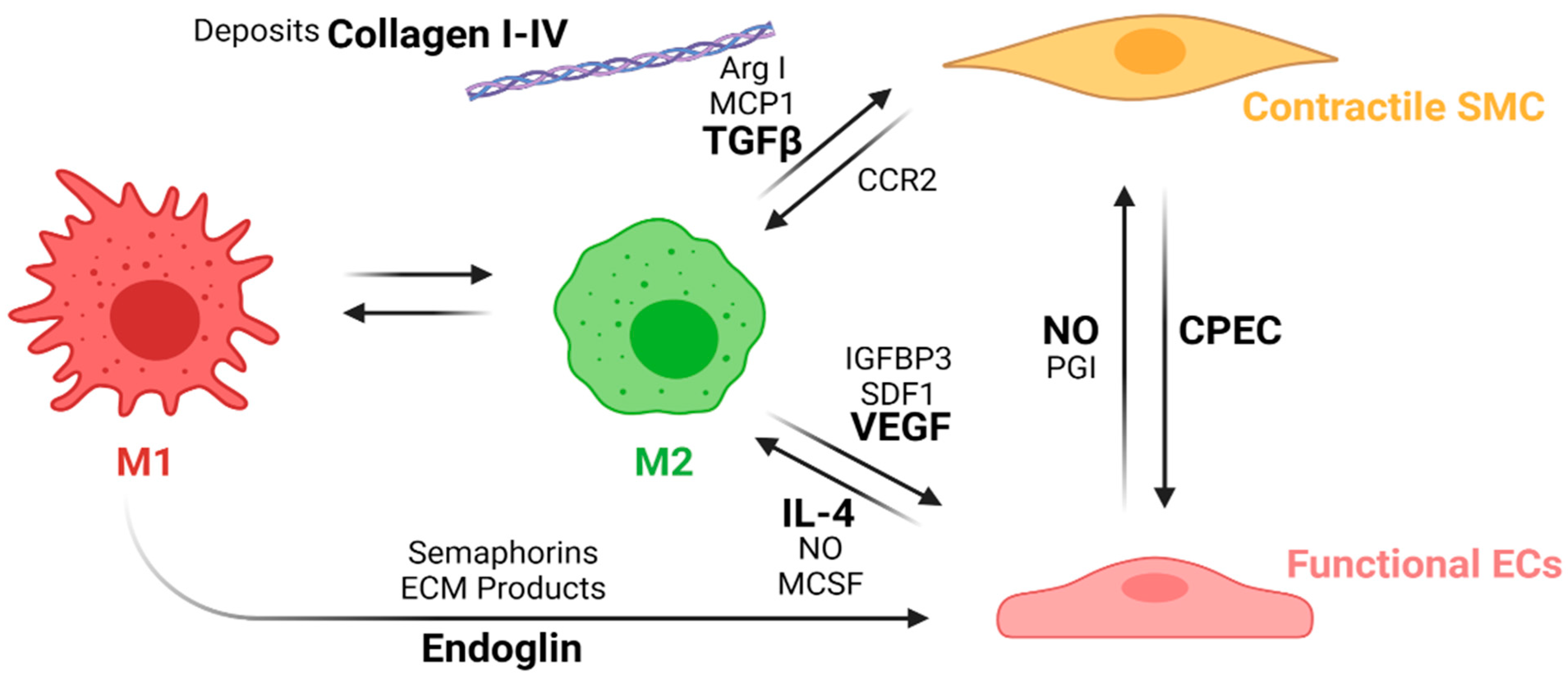
4.4.1. Macrophage’s Polarization
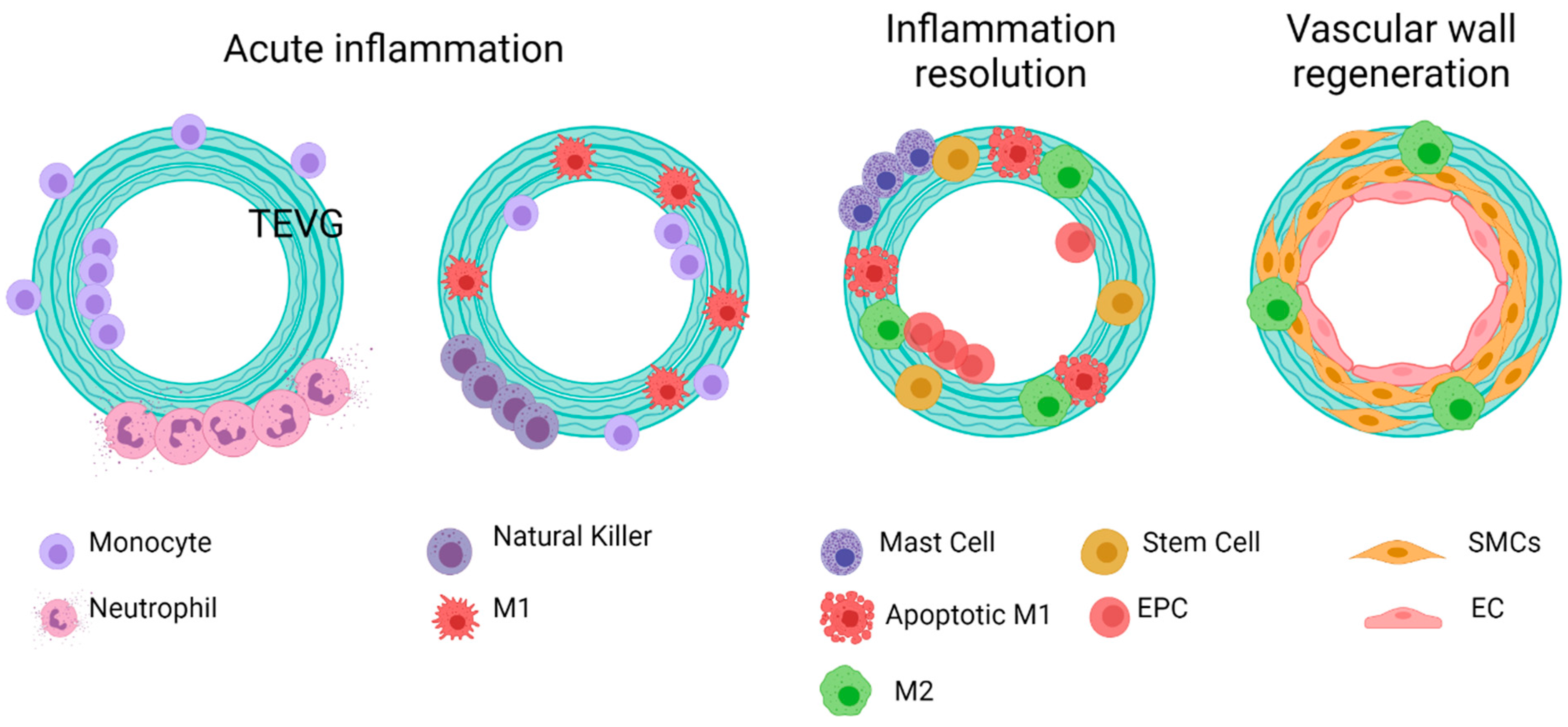
4.4.2. Inflammation Resolution
5. Conclusions
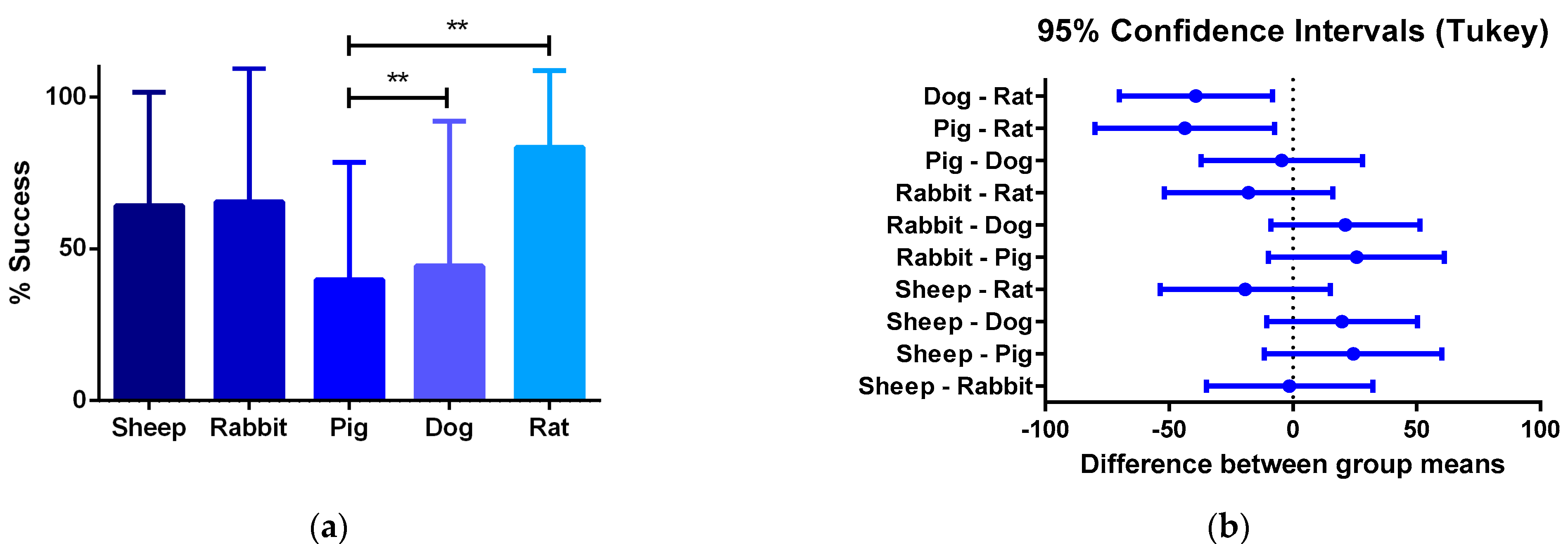
- Studies involving smaller animal models are characterized by shorter evaluation times, and therefore they arrive more quickly at later phases of integration. We found statistically significant differences in the percentages of success survival within smaller and larger animal models, as dog–rat and pig–rat. Vascular grafts that do not overcome the first phases of regeneration mainly fail due to thrombogenic events or bacterial infection in different ways. First, either the biomaterial is non-hemocompatible, the surface promotes the activation of the coagulation cascade and the platelet activation, or the released molecules from hemolyzed red blood cells activate the platelets. Second, altered hemodynamics will cause the von Willebrand factor (vWF) and vitronectin adhesion, causing platelet activation. Third, an exacerbated inflammatory response will generate platelet–leukocyte aggregates. Fourth, although rare, infections are also dependent on the biomaterial properties and might be life-threatening.
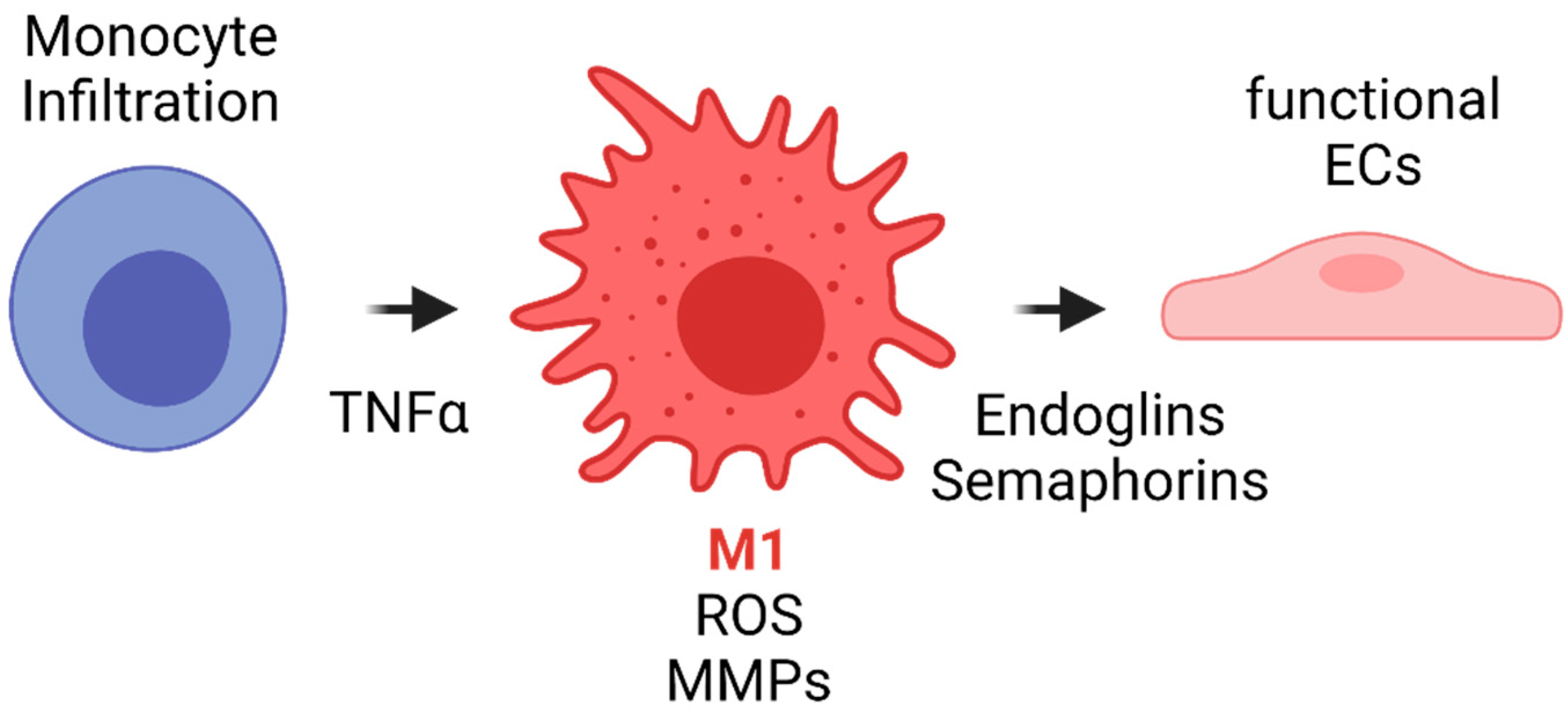
- Once the first phase of regeneration is crossed, there are two different events during the acute inflammatory response in TEVGs, considering that the cells’ sources for vascular wall regeneration can be the blood flow and the peripheral tissues. First, different kinds of cells, including monocytes and stem cells, infiltrate the vascular wall from the granulation tissue in the periphery, and regeneration will be allowed depending on their response and interaction,. Second, type-1 macrophages secrete integrins and semaphorins, which are chemoattractants for endothelial progenitors from the blood flow that will adhere to the lumen surface of the vascular graft and begin reendothelialization.
- If the regenerative pathway is activated, macrophage polarization occurs within the vascular wall, and type-2 macrophages release growth factors and cytokines that induce cell differentiation towards contractile smooth muscle cells and functional endothelial cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedin, U. Long-Term Results of PTFE Grafts. J. Vasc. Access 2015, 16, S87–S92. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft—Past, present, and future. Tissue Eng. Part. B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Zhang, K.; Chen, J.; Huang, N. Endothelialization of implanted cardiovascular biomaterial surfaces: The development from in vitro to in vivo. J. Biomed. Mater. Res. Part. A 2014, 102, 3754–3772. [Google Scholar] [CrossRef]
- Schneider, K.H.; Enayati, M.; Grasl, C.; Walter, I.; Budinsky, L.; Zebic, G.; Kaun, C.; Wagner, A.; Kratochwill, K.; Redl, H.; et al. Acellular vascular matrix grafts from human placenta chorion: Impact of ECM preservation on graft characteristics, protein composition and in vivo performance. Biomaterials 2018, 177, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Thamer, M.; Lee, T.C.; Wasse, H.; Glickman, M.H.; Qian, J.; Gottlieb, D.; Toner, S.; Pflederer, T.A. Medicare Costs Associated with Arteriovenous Fistulas Among US Hemodialysis Patients. Am. J. Kidney Dis. 2018, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ocak, G.; Rotmans, J.I.; Vossen, C.Y.; Rosendaal, F.R.; Krediet, R.T.; Boeschoten, E.W.; Dekker, F.W.; Verduijn, M. Type of arteriovenous vascular access and association with patency and mortality. BMC Nephrol. 2013, 14, 79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Handa, R.; Sharma, S. Vascular Graft Failure Of Leg Arterial Bypasses—A Review. J. Hypertens. Cardiol. 2014, 1, 17–21. [Google Scholar] [CrossRef]
- Gaudino, M.; Antoniades, C.; Benedetto, U.; Deb, S.; Di Franco, A.; Di Giammarco, G.; Fremes, S.; Glineur, D.; Grau, J.; He, G.-W.; et al. Mechanisms, Consequences, and Prevention of Coronary Graft Failure. Circulation 2017, 136, 1749–1764. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling During Platelet Adhesion and Activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.; Hong, J.; Drotz, K.; Risberg, B.; Sanchez, J.; Sellborn, A. An in vitro study of blood compatibility of vascular grafts made of bacterial cellulose in comparison with conventionally-used graft materials. J. Biomed. Mater. Res. Part. A 2011, 97A, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Vahidkhah, K.; Diamond, S.L.; Bagchi, P. Platelet Dynamics in Three-Dimensional Simulation of Whole Blood. Biophys. J. 2014, 106, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.-L.; Wautier, M.-P. Molecular basis of erythrocyte adhesion to endothelial cells in diseases. Clin. Hemorheol. Microcirc. 2013, 53, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, 1701461. [Google Scholar] [CrossRef]
- Saito, J.; Kaneko, M.; Ishikawa, Y.; Yokoyama, U. Challenges and Possibilities of Cell-Based Tissue-Engineered Vascular Grafts. Cyborg Bionic Syst. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Shoji, T.; Shinoka, T. Tissue engineered vascular grafts for pediatric cardiac surgery. Transl. Pediatr. 2018, 7, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Skovrind, I.; Harvald, E.B.; Juul Belling, H.; Jørgensen, C.D.; Lindholt, J.S.; Andersen, D.C. Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials. Stem Cells Transl. Med. 2019, 8, 671–680. [Google Scholar] [CrossRef]
- De Dios Ruiz-Rosado, J.; Lee, Y.; Mahler, N.; Yi, T.; Robledo-Avila, F.; Martinez-Saucedo, D.; Lee, A.Y.; Shoji, T.; Heuer, E.; Yates, A.R.; et al. Angiotensin II receptor I blockade prevents stenosis of tissue engineered vascular grafts. FASEB J. 2018, 32, 6822–6832. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Neff, L.P.; Corriere, M.A.; Christ, G.J.; Soker, S.; Atala, A.; Geary, R.L.; Yoo, J.J. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J. Vasc. Surg. 2012, 56, 783–793. [Google Scholar] [CrossRef]
- Kaushal, S.; Amiel, G.E.; Guleserian, K.J.; Shapira, O.M.; Perry, T.; Sutherland, F.W.; Rabkin, E.; Moran, A.M.; Schoen, F.J.; Atala, A.; et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 2001, 7, 1035–1040. [Google Scholar] [CrossRef]
- Cho, S.W.; Lim, S.H.; Kim, I.K.; Hong, Y.S.; Kim, S.S.; Yoo, K.J.; Park, H.Y.; Jang, Y.; Chang, B.C.; Choi, C.Y.; et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann. Surg. 2005, 241, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Koenneker, S.; Teebken, O.E.; Bonehie, M.; Pflaum, M.; Jockenhoevel, S.; Haverich, A.; Wilhelmi, M.H. A Biological Alternative to Alloplastic Grafts in Dialysis Therapy: Evaluation of an Autologised Bioartificial Haemodialysis Shunt Vessel in a Sheep Model. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 810–816. [Google Scholar] [CrossRef]
- Neff, L.P.; Tillman, B.W.; Yazdani, S.K.; Machingal, M.A.; Yoo, J.J.; Soker, S.; Bernish, B.W.; Geary, R.L.; Christ, G.J. Vascular smooth muscle enhances functionality of tissue-engineered blood vessels in vivo. J. Vasc. Surg. 2011, 53, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Flanagan, T.C.; Sachweh, J.S.; Tanios, F.; Schnoering, H.; Deichmann, T.; Ellä, V.; Kellomäki, M.; Gronloh, N.; Gries, T.; et al. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 2010, 31, 4731–4739. [Google Scholar] [CrossRef]
- Ju, Y.M.; Ahn, H.; Arenas-Herrera, J.; Kim, C.; Abolbashari, M.; Atala, A.; Yoo, J.J.; Lee, S.J. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater. 2017, 59, 58–67. [Google Scholar] [CrossRef]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Opfermann, J.; Ong, C.S.; Shinoka, T.; Breuer, C.K.; Krieger, A.; Johnson, J.; Hibino, N. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J. Thorac. Cardiovasc. Surg. 2017, 153, 924–932. [Google Scholar] [CrossRef]
- Row, S.; Peng, H.; Schlaich, E.M.; Koenigsknecht, C.; Andreadis, S.T.; Swartz, D.D. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: The role of cells in the vascular wall. Biomaterials 2015, 50, 115–126. [Google Scholar] [CrossRef]
- Aper, T.; Wilhelmi, M.; Gebhardt, C.; Hoeffler, K.; Benecke, N.; Hilfiker, A.; Haverich, A. Novel method for the generation of tissue-engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater. 2016, 29, 21–32. [Google Scholar] [CrossRef]
- Swartz, D.D.; Russell, J.A.; Andreadis, S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Circ. Physiol. 2005, 288, H1451–H1460. [Google Scholar] [CrossRef]
- Meier, L.A.; Syedain, Z.H.; Lahti, M.T.; Johnson, S.S.; Chen, M.H.; Hebbel, R.P.; Tranquillo, R.T. Blood Outgrowth Endothelial Cells Alter Remodeling of Completely Biological Engineered Grafts Implanted into the Sheep Femoral Artery. J. Cardiovasc. Transl. Res. 2014, 7, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Mathapati, S.; Galla, S.; Cherian, K.M.; Guhathakurta, S. Crosslinked acellular saphenous vein for small-diameter vascular graft. Asian Cardiovasc. Thorac. Ann. 2013, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Aussel, A.; Montembault, A.; Malaise, S.; Foulc, M.P.; Faure, W.; Cornet, S.; Aid, R.; Chaouat, M.; Delair, T.; Letourneur, D.; et al. In Vitro Mechanical Property Evaluation of Chitosan-Based Hydrogels Intended for Vascular Graft Development. J. Cardiovasc. Transl. Res. 2017, 10, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Reinhardt, S.; Eghbalzadeh, K.; Wacker, M.; Guschlbauer, M.; Maul, A.; Sterner-Kock, A.; Wahlers, T.; Wippermann, J.; Scherner, M. Patency and in vivo compatibility of bacterial nanocellulose grafts as small-diameter vascular substitute. J. Vasc. Surg. 2018, 68, 177S.e1–187S.e1. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Murphy, M.O.; Kielty, C.M.; Shuttleworth, C.A.; Black, R.A.; Humphries, M.J.; Walker, M.G.; Canfield, A.E. α 2 (VIII) Collagen Substrata Enhance Endothelial Cell Retention Under Acute Shear Stress Flow via an α 2 β 1 Integrin–Dependent Mechanism. Circulation 2006, 114, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Seo, J.W.; Rho, J.R.; Kim, W.G. Histopathologic Changes of Acellularized Xenogenic Carotid Vascular Grafts Implanted in a Pig-to-Goat Model. Int. J. Artif. Organs 2007, 30, 44–52. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Z.; Song, L.; Zhao, Q.; Zhang, J.; Li, D.; Wang, S.; Han, J.; Zheng, X.L.; Yang, Z.; et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012, 33, 2880–2891. [Google Scholar] [CrossRef]
- Cutiongco, M.F.A.; Kukumberg, M.; Peneyra, J.L.; Yeo, M.S.; Yao, J.Y.; Rufaihah, A.J.; Le Visage, C.; Ho, J.P.; Yim, E.K.F. Submillimeter diameter poly(vinyl alcohol) vascular graft patency in rabbit model. Front. Bioeng. Biotechnol. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Ju, R.; Chen, K.; Li, S.; Wang, W.; Yan, Y. In vivo biocompatibility and hemocompatibility of a polytetrafluoroethylene small diameter vascular graft modified with sulfonated silk fibroin. Am. J. Surg. 2017, 213, 87–93. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, W.; Pan, Y.; Ma, S.; Guan, Y.; Liu, R.; Zhu, M.; Zhou, X.; Zhang, J.; Zhao, Q.; et al. Three-Layered PCL Grafts Promoted Vascular Regeneration in a Rabbit Carotid Artery Model. Macromol. Biosci. 2016, 16, 608–918. [Google Scholar] [CrossRef]
- Wise, S.G.; Byrom, M.J.; Waterhouse, A.; Bannon, P.G.; Ng, M.K.C.; Weiss, A.S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011, 7, 295–303. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Hocking, K.M.; Osgood, M.J.; Voskresensky, I.; Dmowska, J.; Kilchrist, K.V.; Brophy, C.M.; Duvall, C.L. MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia. Sci. Transl. Med. 2015, 7, 291ra95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Bailey, S.R.; Elliott, J.; Li, X.; Escobar, G.P.; Rodriguez, E.M.; Agrawal, C.M. Development of a total atherosclerotic occlusion with cell-mediated calcium deposits in a rabbit femoral artery using tissue-engineering scaffolds. J. Tissue Eng. Regen. Med. 2012, 6, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Sakamoto, S.I.; Kronengold, R.T.; Virmani, R.; Rivera, E.A.; Goldman, S.M.; Prechtel, E.J.; Hill, J.G.; Damiano, R.J. A novel bioengineered small-caliber vascular graft incorporating heparin and sirolimus: Excellent 6-month patency. J. Thorac. Cardiovasc. Surg. 2008, 135, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A.M.; Khorramirouz, R.; Kameli, S.M.; Fendereski, K.; Daryabari, S.S.; Tavangar, S.M.; Azizi Garajegayeh, B. Three-year efficacy and patency follow-up of decellularized human internal mammary artery as a novel vascular graft in animal models. J. Thorac. Cardiovasc. Surg. 2019, 157, 1494–1502. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, L.; Ren, X.K.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Co-immobilization of ACH11 antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization. Acta Biomater. 2019, 97, 344–359. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, J.; Li, Q.; Guo, J.; Ren, X.; Xia, S.; Zhang, W.; Feng, Y. Biofunctionalized Electrospun PCL-PIBMD/SF Vascular Grafts with PEG and Cell-Adhesive Peptides for Endothelialization. Macromol. Biosci. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Koens, M.J.W.; Krasznai, A.G.; Hanssen, A.E.J.; Hendriks, T.; Praster, R.; Daamen, W.F.; van der Vliet, J.A.; van Kuppevelt, T.H. Vascular replacement using a layered elastin-collagen vascular graft in a porcine model: One week patency versus one month occlusion. Organogenesis 2015, 11, 105–121. [Google Scholar] [CrossRef]
- Rotmans, J.I.; Heyligers, J.M.M.; Verhagen, H.J.M.; Velema, E.; Nagtegaal, M.M.; de Kleijn, D.P.V.; de Groot, F.G.; Stroes, E.S.G.; Pasterkamp, G. In Vivo Cell Seeding With Anti-CD34 Antibodies Successfully Accelerates Endothelialization but Stimulates Intimal Hyperplasia in Porcine Arteriovenous Expanded Polytetrafluoroethylene Grafts. Circulation 2005, 112, 12–18. [Google Scholar] [CrossRef]
- Rothuizen, T.C.; Damanik, F.F.R.; Lavrijsen, T.; Visser, M.J.T.; Hamming, J.F.; Lalai, R.A.; Duijs, J.M.G.J.; van Zonneveld, A.J.; Hoefer, I.E.; van Blitterswijk, C.A.; et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials 2016, 75, 82–90. [Google Scholar] [CrossRef]
- Mahara, A.; Somekawa, S.; Kobayashi, N.; Hirano, Y.; Kimura, Y.; Fujisato, T.; Yamaoka, T. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials 2015, 58, 54–62. [Google Scholar] [CrossRef]
- Sánchez-Palencia, D.M.; Navarro, J.; Araque, J.C.; Umaña, J.B.; Guerrero, A.F.; Quijano, L.M.; López, R.D.P.; Sandoval, N.F.; Briceno, J.C. Effects of fabrication on early patency and regeneration of small intestinal submucosa vascular grafts. Asaio J. 2015, 61, 596–604. [Google Scholar] [CrossRef]
- Dahan, N.; Sarig, U.; Bronshtein, T.; Baruch, L.; Karram, T.; Hoffman, A.; Machluf, M. Dynamic Autologous Reendothelialization of Small-Caliber Arterial Extracellular Matrix: A Preclinical Large Animal Study. Tissue Eng. Part. A 2017, 23, 69–79. [Google Scholar] [CrossRef]
- Valencia, R.K.T.; Jaramillo, E.J.; Galvis, F.S.D.; Miranda, S.M.C.; del López, P.R.P.; Sandoval, R.N.F.; Briceño, T.J.C. New regenerative vascular grafts for hemodialysis access: Evaluation of a preclinical animal model. J. Investig. Surg. 2018, 31, 192–200. [Google Scholar] [CrossRef]
- Zavan, B.; Vindigni, V.; Lepidi, S.; Iacopetti, I.; Avruscio, G.; Abatangelo, G.; Cortivo, R. Neoarteries grown in vivo using a tissue-engineered hyaluronan-based scaffold. FASEB J. 2008, 22, 2853–2861. [Google Scholar] [CrossRef] [PubMed]
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary Results of Small Arterial Substitute Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Hinds, M.T.; Rowe, R.C.; Ren, Z.; Teach, J.; Wu, P.-C.; Kirkpatrick, S.J.; Breneman, K.D.; Gregory, K.W.; Courtman, D.W. Development of a reinforced porcine elastin composite vascular scaffold. J. Biomed. Mater. Res. Part. A 2006, 77A, 458–469. [Google Scholar] [CrossRef]
- Pellegata, A.F.; Dominioni, T.; Ballo, F.; Maestroni, S.; Asnaghi, M.A.; Zerbini, G.; Zonta, S.; Mantero, S. Arterial Decellularized Scaffolds Produced Using an Innovative Automatic System. Cells Tissues Organs 2015, 200, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Arts, C.H.P.; Hedeman Joosten, P.P.A.; Blankensteijn, J.D.; Staal, F.J.T.; Ng, P.Y.Y.; Heijnen-Snyder, G.J.; Sixma, J.J.; Verhagen, H.J.M.; De Groot, P.G.; Eikelboom, B.C. Contaminants from the transplant contribute to intimal hyperplasia associated with microvascular endothelial cell seeding. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 29–38. [Google Scholar] [CrossRef][Green Version]
- Xie, X.; Eberhart, A.; Guidoin, R.; Marois, Y.; Douville, Y.; Zhang, Z. Five types of polyurethane vascular grafts in dogs: The importance of structural design and material selection. J. Biomater. Sci. Polym. Ed. 2010, 21, 1239–1264. [Google Scholar] [CrossRef]
- Yokota, T.; Ichikawa, H.; Matsumiya, G.; Kuratani, T.; Sakaguchi, T.; Iwai, S.; Shirakawa, Y.; Torikai, K.; Saito, A.; Uchimura, E.; et al. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J. Thorac. Cardiovasc. Surg. 2008, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Wei, Z.; Liu, C.; Qiao, T.; Ran, F.; Bai, Y.; Jiang, X.; Ding, Y. Development and validation of small-diameter vascular tissue from a decellularized scaffold coated with heparin and vascular endothelial growth factor. Artif. Organs 2009, 33, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Liu, C.; Jiang, X.; Wei, Z.; Qiao, W.; Ran, F.; Wang, W.; Qiao, T.; Liu, C. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 111–120. [Google Scholar] [CrossRef]
- Zhou, M.; Qiao, W.; Liu, Z.; Shang, T.; Qiao, T.; Mao, C.; Liu, C. Development and in vivo evaluation of small-diameter vascular grafts engineered by outgrowth endothelial cells and electrospun chitosan/poly(ε- caprolactone) Nanofibrous Scaffolds. Tissue Eng. Part. A 2014, 20, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Soltani, M.; Maleki, M.A.; Kaboodrangi, A.H.; Mosadegh, B. Optimization of oxygen transport within a tissue engineered vascular graft model using embedded micro-channels inspired by vasa vasorum. Chem. Eng. Sci. 2018, 184, 1–13. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Nattich-Rak, M.; Dąbkowska, M.; Kujda-Kruk, M. Albumin adsorption at solid substrates: A quest for a unified approach. J. Colloid Interface Sci. 2018, 514, 769–790. [Google Scholar] [CrossRef]
- Buddhadasa, M.; Lerouge, S.; Girard-Lauriault, P.-L. Plasma polymer films to regulate fibrinogen adsorption: Effect of pressure and competition with human serum albumin. Plasma Process. Polym. 2018, 15, 1800040. [Google Scholar] [CrossRef]
- Thiruppathi, E.; Larson, M.K.; Mani, G. Surface Modification of CoCr Alloy Using Varying Concentrations of Phosphoric and Phosphonoacetic Acids: Albumin and Fibrinogen Adsorption, Platelet Adhesion, Activation, and Aggregation Studies. Langmuir 2015, 31, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Safiullin, R.; Christenson, W.; Owaynat, H.; Yermolenko, I.S.; Kadirov, M.K.; Ros, R.; Ugarova, T.P. Fibrinogen matrix deposited on the surface of biomaterials acts as a natural anti-adhesive coating. Biomaterials 2015, 67, 151–159. [Google Scholar] [CrossRef][Green Version]
- Manzi, B.M.; Werner, M.; Ivanova, E.P.; Crawford, R.J.; Baulin, V.A. Simulations of protein adsorption on nanostructured surfaces. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mott, E. Modelling the Adsorption of Fibrinogen and its Changes of Orientation Due to Surface Chemistry. 2016. Available online: https://vdocuments.net/modelling-the-adsorption-of-fibrinogen-and-its-changes-of-orientation-due-to.html (accessed on 10 January 2017).
- Fröhlich, S.M.; Eilenberg, M.; Svirkova, A.; Grasl, C.; Liska, R.; Bergmeister, H.; Marchetti-Deschmann, M. Mass spectrometric imaging of in vivo protein and lipid adsorption on biodegradable vascular replacement systems. Analyst 2015, 140, 6089–6099. [Google Scholar] [CrossRef] [PubMed]
- Lionello, A.; Josserand, J.; Jensen, H.; Girault, H.H. Dynamic protein adsorption in microchannels by “stop-flow” and continuous flow. Lab. Chip 2005, 5, 1096–1103. [Google Scholar] [CrossRef]
- Wentzel, J.J.; Chatzizisis, Y.S.; Gijsen, F.J.H.; Giannoglou, G.D.; Feldman, C.L.; Stone, P.H. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: Current understanding and remaining questions. Cardiovasc. Res. 2012, 96, 234–243. [Google Scholar] [CrossRef]
- Gogia, S.; Neelamegham, S. Role of fluid shear stress in regulating VWF structure, function and related blood disorders. Biorheology 2016, 52, 319–335. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Role of red blood cells in haemostasis and thrombosis. ISBT Sci. Ser. 2017, 12, 176–183. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The Clinical Sequelae of Intravascular Hemolysis and Extracellular Plasma Hemoglobin. JAMA 2005, 293, 1653. [Google Scholar] [CrossRef]
- Eriksson, O.; Mohlin, C.; Nilsson, B.; Ekdahl, K.N. The Human Platelet as an Innate Immune Cell: Interactions Between Activated Platelets and the Complement System. Front. Immunol. 2019, 10, 1590. [Google Scholar] [CrossRef]
- Patzelt, J.; Verschoor, A.; Langer, H.F. Platelets and the complement cascade in atherosclerosis. Front. Physiol. 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Conway, E.M. Platelets and Complement Cross-Talk in Early Atherogenesis. Front. Cardiovasc. Med. 2019, 6, 131. [Google Scholar] [CrossRef]
- Kim, H.-I.; Yu, J.E.; Lee, S.Y.; Sul, A.Y.; Jang, M.S.; Rashid, M.A.; Park, S.G.; Kim, S.J.; Park, C.-G.; Kim, J.H.; et al. The Effect of Composite Pig Islet-Human Endothelial Cell Grafts on the Instant Blood-Mediated Inflammatory Reaction. Cell Transplant. 2009, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Horbett, T.A. Fibrinogen adsorption to biomaterials. J. Biomed. Mater. Res. Part. A 2018, 106, 2777–2788. [Google Scholar] [CrossRef]
- Latour, R.A. Biomaterials: Protein-surface interactions. Encycl. Biomater. Biomed. Eng. 2005, 1, 270–284. [Google Scholar]
- Schepers, A.; de Vries, M.R.; van Leuven, C.J.; Grimbergen, J.M.; Holers, V.M.; Daha, M.R.; van Bockel, J.H.; Quax, P.H.A. Inhibition of Complement Component C3 Reduces Vein Graft Atherosclerosis in Apolipoprotein E3–Leiden Transgenic Mice. Circulation 2006, 114, 2831–2838. [Google Scholar] [CrossRef]
- de Vries, M.R.; Quax, P.H.A. Inflammation in Vein Graft Disease. Front. Cardiovasc. Med. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Kirkton, R.D.; Prichard, H.L.; Santiago-Maysonet, M.; Niklason, L.E.; Lawson, J.H.; Dahl, S.L.M. Susceptibility of ePTFE vascular grafts and bioengineered human acellular vessels to infection. J. Surg. Res. 2018, 221, 143–151. [Google Scholar] [CrossRef]
- Meyer, F.; Buerger, T.; Halloul, Z.; Lippert, H.; König, B.; Tautenhahn, J. Effects Of Gelatine-Coated Vascular Grafts On Human Neutrophils. Pol. J. Surg. 2015, 87, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Arun, M.Z.; Guzeloglu, M.; Onursal, C.; Gokce, G.; Korkmaz, C.G.; Reel, B. Low-dose doxycycline inhibits hydrogen peroxide-induced oxidative stress, MMP-2 up-regulation and contractile dysfunction in human saphenous vein grafts. Drug Des. Dev. Ther. 2019, 13, 1791–1801. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Abe, J.-I.; Pan, S.; Krovic, B.; Fujiwara, K. Shear stress-mediated signal transduction. In Hemodynamics and Mechanobiology of Endothelium; World Scientific: Singapore, 2010; pp. 39–68. [Google Scholar]
- Lambert, L.; Novakova, M.; Lukac, P.; Cechova, D.; Sukenikova, L.; Hrdy, J.; Mlcek, M.; Chlup, H.; Suchy, T.; Grus, T. Evaluation of the Immunogenicity of a Vascular Graft Covered with Collagen Derived from the European Carp (Cyprinus carpio) and Bovine Collagen. Biomed. Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Ramagiri-Vinod, N.; Tahir, H.; Narukonda, S.; Joshi, M. Prosthetic Arteriovenous Graft Contact Dermatitis Masquerading as an Arteriovenous Graft Infection in a Hemodialysis Patient. J. Investig. Med. High. Impact Case Rep. 2016, 4, 232470961665831. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Ryan, J.J.; Bowlin, G.L. Modulation of mast cell adhesion, proliferation, and cytokine secretion on electrospun bioresorbable vascular grafts. J. Biomed. Mater. Res. Part. A 2011, 97A, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.K.; Kwon, H.; Ko, G.-Y.; Kim, M.J.; Han, Y.; Chung, Y.S.; Park, H.; Kwon, T.-W.; Cho, Y.-P. Impact of graft composition on the systemic inflammatory response after an elective repair of an abdominal aortic aneurysm. Ann. Surg. Treat. Res. 2015, 88, 21. [Google Scholar] [CrossRef] [PubMed]
- Wübbeke, L.F.; Elshof, J.-W.; Conings, J.Z.M.; Scheltinga, M.R.; Daemen, J.-W.H.C.; Mees, B.M.E. A systematic review on the use of muscle flaps for deep groin infection following vascular surgery. J. Vasc. Surg. 2020, 71, 693–700.e1. [Google Scholar] [CrossRef] [PubMed]
- Arhuidese, I.J.; Beaulieu, R.J.; Aridi, H.D.; Locham, S.; Baldwin, E.K.; Malas, M.B. Age-related outcomes of arteriovenous grafts for hemodialysis access. J. Vasc. Surg. 2020, 72, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Milleret, V.; Hefti, T.; Hall, H.; Vogel, V.; Eberli, D. Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation. Acta Biomater. 2012, 8, 4349–4356. [Google Scholar] [CrossRef]
- Birinyi, L.K.; Douville, E.C.; Lewis, S.A.; Bjornson, H.S.; Kempczinski, R.F. Increased resistance to bacteremic graft infection after endothelial cell seeding. J. Vasc. Surg. 1987, 5, 193–197. [Google Scholar] [CrossRef]
- Hibino, N.; Mejias, D.; Pietris, N.; Dean, E.; Yi, T.; Best, C.; Shinoka, T.; Breuer, C. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB J. 2015, 29, 2431–2438. [Google Scholar] [CrossRef]
- Sánchez-Palencia, D.M.; González-Mancera, A.; Wagner, W.R.; Briceño, J.C.; Briceno, J. Effects of fabrication on the mechanics, microstructure and micromechanical environment of small intestinal submucosa scaffolds for vascular tissue engineering. J. Biomech. 2014, 47, 2766–2773. [Google Scholar] [CrossRef]
- Sánchez, P.F.; Brey, E.M.; Briceño, J.C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Beugels, J.; Molin, D.G.M.; Ophelders, D.R.M.G.; Rutten, T.; Kessels, L.; Kloosterboer, N.; de Grzymala, A.A.P.; Kramer, B.W.W.; van der Hulst, R.R.W.J.; Wolfs, T.G.A.M. Electrical stimulation promotes the angiogenic potential of adipose-derived stem cells. Sci. Rep. 2019, 9, 12076. [Google Scholar] [CrossRef] [PubMed]


| Caprine | Swine | Dog | Rabbit | Rat | |
|---|---|---|---|---|---|
| Av. Diameter (mm) | 5 | 4.3 | 4.4 | 2.8 | 1.7 |
| Av. Length (mm) | 47 | 50 | 45 | 21 | 8710 |
| Subjects | 2 to 20 | 3 to 10 | 1 to 20 | 3 to 18 | 4 to 24 |
| Following time | 1 to 9 months | 7 days to 5 months | 1 to 12 months | 1 month to 3 years | 1 to 12 months |
| Number of studies | 19 | 18 | 36 | 23 | 23 |
| Author, Year | Ref. | Cells Seeded | Luminal Cell Type | External Cell Type | Length (mm) | Diameter (mm) | Scaffold Type | Localization | |
|---|---|---|---|---|---|---|---|---|---|
| Sheep | Tillman, 2012 | [21] | Yes | PB-EC | None | 60 | 5.0 | Decellularized Artery | Carotid Artery AV Shunt |
| Kaushal, 2001 | [22] | Yes | PB-EC | None | 45 | 4.0 | Decellularized Artery | Carotid Artery | |
| Cho, 2005 | [23] | Yes | BMMNC-EC | BMNC-SMC | 40 | 3.0 | Decellularized Artery | Carotid Artery | |
| Koenneker, 2010 | [24] | Yes | PB-EC | None | 75 | 5.0 | Decellularized Artery | Carotid Artery AV Shunt | |
| Neff, 2011 | [25] | Yes | PB-EC | Vessel SMC | 60 | 5.0 | Decellularized Artery | Carotid Artery | |
| Koch, 2010 | [26] | Yes | None | Vessel SMC | 45 | 5.0 | Hybrid (Firbin-PLA) | Carotid Artery | |
| Ju, 2017 | [27] | Yes | PB-EC | Vessel SMC | 50 | 4.8 | Hybrid (Collagen-PCL) | Carotid Artery | |
| Fukunishi, 2017 | [28] | No | None | None | 15 | 12.0 | Synthetic (PGA-PCL) | Inferior vena cava | |
| Row, 2015 | [29] | Yes | EC | SMC | 45 | 5.0 | Natural (SIS- Fibrin) | Carotid Artery | |
| Aper, 2016 | [30] | Yes | PB-EC | PB-SMC | 90 | 5.6 | Natural (Fibrin + Coagulation Factor XIII) | Carotid Artery | |
| Swartz, 2005 | [31] | Yes | Vessel-EC | Vessel-SMC | 12 | 5.0 | Natural (Firin-Thrombin) | Yugular Vein | |
| Meier, 2014 | [32] | Yes | PB-EC | None | 25 | 4.0 | Natural (Fibrin gel) | Femoral Artery | |
| Ramesh, 2013 | [33] | No | None | None | 130 | 4.0 | Decellularized Vein | Carotid Artery | |
| Aussel, 2017 | [34] | No | None | None | 35 | 6.0 | Natural (Chitosan) | Carotid Artery | |
| Weber, 2018 | [35] | No | None | None | 10 | 4.5 | Natural (Nanocellulose) | Carotid Artery | |
| Goat | Turner, 2006 | [36] | Yes | Vessel-EC | None | 45 | 4.5 | Hybrid (PU + cells) | Carotid Artery |
| Kim, 2007 | [37] | No | None | None | 40 | 4.5 | Decellularized Artery | Carotid Artery | |
| Rabbit | Zheng, 2012 * | [38] | No | None | None | 15 | 2.2 | Synthetic (PCL) | Carotid Artery |
| Cutiongco, 2016 * | [39] | No | None | None | Not reported | 0.9–1 | Synthetic (PVA) | Femoral Artery | |
| Zhang, 2017 * | [40] | No | None | None | 30 | 4.0 | Synthetic (ePTFE) | Abdominal Aorta | |
| Wang, 2016 * | [41] | No | None | None | 10 | 2.2 | Synthetic (PCL) | Carotid Artery | |
| Wise, 2011 * | [42] | No | None | None | 20 | 2.8 | Hybrid (PCL—Elastin) | Carotid Artery | |
| Tillman, 2009 * | [43] | No | None | None | 40 | 5.0 | Hybrid (PCL—collagen) | Aortoiliac Bypass | |
| Evans, 2015 * | [44] | No | None | None | 30–40 | 2.0 | Autologous vein | Yugular Vein | |
| Zhu, 2012 * | [45] | Yes | HOB | None | 5 | 3 | Synthetic (PCL) | Femoral Artery | |
| Ishii, 2008 * | [46] | No | None | None | 24 | 3.6 | Hybrid (PU—collagen—Hyaluronic acid) | Abdominal Aorta | |
| Kajbafzadeh, 2019 * | [47] | No | None | None | Not reported | Not reported | Decellularized Artery | Femoral Arteries | |
| Zhao, 2019 * | [48] | No | None | None | 20 | 2 | Synthetic (PLCL) | Carotid Artery | |
| Bai, 2019 * | [49] | No | None | None | 20 | 2 | Synthetic (PS) | Carotid Artery | |
| Bai, 2018 * | [49] | No | None | None | 20 | 2 | Synthetic (PS) | Carotid Artery | |
| Pig | Koens, 2015 | [50] | No | None | None | 35 | 4 | Natural (elastin-collagen) | Iliac Artery |
| Rotmans, 2005 | [51] | No | None | None | 70 | 5 | Synthetic (PTFE+ CD34 coating) | Carotid Artery AV Shunt | |
| Rothuizen, 2016 | [52] | No | None | None | 40 | 4.2 | Fibrotic capsule tube + PCL | Carotid Artery | |
| Mahara, 2015 | [53] | No | None | None | 250 | 3 | Decellularized Artery | Femoral Artery | |
| Sánchez-Palencia, 2018 * | [54] | No | None | None | 17 | 4.5 | Decellularized SIS | Carotid Artery | |
| Dahan, 2017 | [55] | No | None | None | 45 | 4.0 | Decellularized Artery | Carotid Artery | |
| Valencia Rivero, 2017 | [56] | No | None | None | 150 | 4.0 | Decellularized SIS | Carotid Artery AV Shunt | |
| Zavan, 2008 | [57] | No | None | None | 50 | 4.0 | Natural (Hyaluronan) | Carotid Artery | |
| Wippermann, 2009 | [58] | No | None | None | 10 | 3.4 | Natural (Cellulose) | Carotid Artery | |
| Hinds, 2006 | [59] | No | None | None | 40 | 4.3 | Natural (Ellastin) | Carotid Artery | |
| Pellegata, 2015 | [60] | Yes | hIAECS | None | 50 | 6.0 | Decellularized Artery | Iliac Artery | |
| Dog | Arts, 2002 | [61] | Yes | MVEC | None | 50 | 4.0 | Synthetic (ePTFE) | Carotid Artery |
| Xie, 2010 | [62] | No | None | None | 50 | 6.0 | Poly (carbonate urethane) (PCU) filaments | Infra-renal aorta | |
| Yokota, 2008 | [63] | No | None | None | 30 | 4.0 | Collagen microsponge with a biodegradable woven polyglycolic acid (core) and poly-L-lactic acid (sheath) fibers. | Carotid Artery | |
| Zhou, 2009 * | [64] | No | None | None | 45 | 3.0 | Decellularized/hybrid | Carotid Artery | |
| Zhou, 2012 | [65] | Yes | PBMC-EC | None | 45 | 3.0 | Decellularized Artery | Carotid Artery | |
| Zhou, 2014 * | [66] | No | None | None | 45 | 3.0 | Hybrid chitosan/poly(e-caprolactone) (CS/PCL) nanofibers | Carotid Artery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Soto, M.A.; Suarez Vargas, N.; Riveros, A.; Camargo, C.M.; Cruz, J.C.; Sandoval, N.; Briceño, J.C. Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response. Cells 2021, 10, 3140. https://doi.org/10.3390/cells10113140
Rodriguez-Soto MA, Suarez Vargas N, Riveros A, Camargo CM, Cruz JC, Sandoval N, Briceño JC. Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response. Cells. 2021; 10(11):3140. https://doi.org/10.3390/cells10113140
Chicago/Turabian StyleRodriguez-Soto, Maria A., Natalia Suarez Vargas, Alejandra Riveros, Carolina Muñoz Camargo, Juan C. Cruz, Nestor Sandoval, and Juan C. Briceño. 2021. "Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response" Cells 10, no. 11: 3140. https://doi.org/10.3390/cells10113140
APA StyleRodriguez-Soto, M. A., Suarez Vargas, N., Riveros, A., Camargo, C. M., Cruz, J. C., Sandoval, N., & Briceño, J. C. (2021). Failure Analysis of TEVG’s I: Overcoming the Initial Stages of Blood Material Interaction and Stabilization of the Immune Response. Cells, 10(11), 3140. https://doi.org/10.3390/cells10113140









