Cortactin Modulates Lung Endothelial Apoptosis Induced by Cigarette Smoke
Abstract
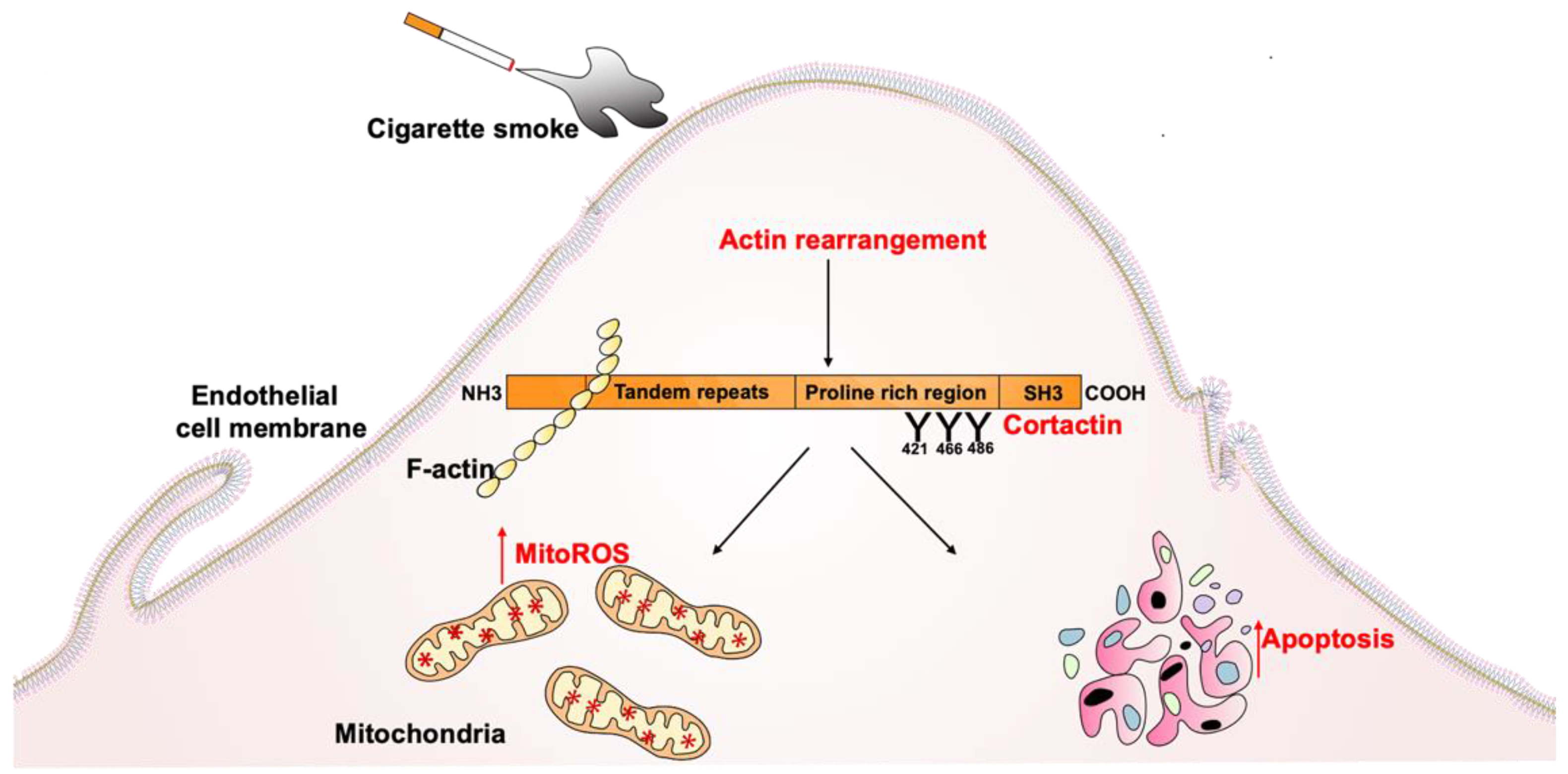
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Human Lung Tissue Specimens
2.3. Cell Culture
2.4. Cigarette Smoke Condensate (CSC)
2.5. E-Cigarette
2.6. siRNA Transfection
2.7. Blocking Peptide Experiments
2.8. Determination of Apoptosis by Flow Cytometry
2.9. RNA Isolation and Quantitative Real-Time PCR Analysis (qPCR)
2.10. Western Blotting
2.11. Immunofluorescence Microscopy
2.12. Mitochondrial ROS Generation
2.13. Statistical Analysis
3. Results
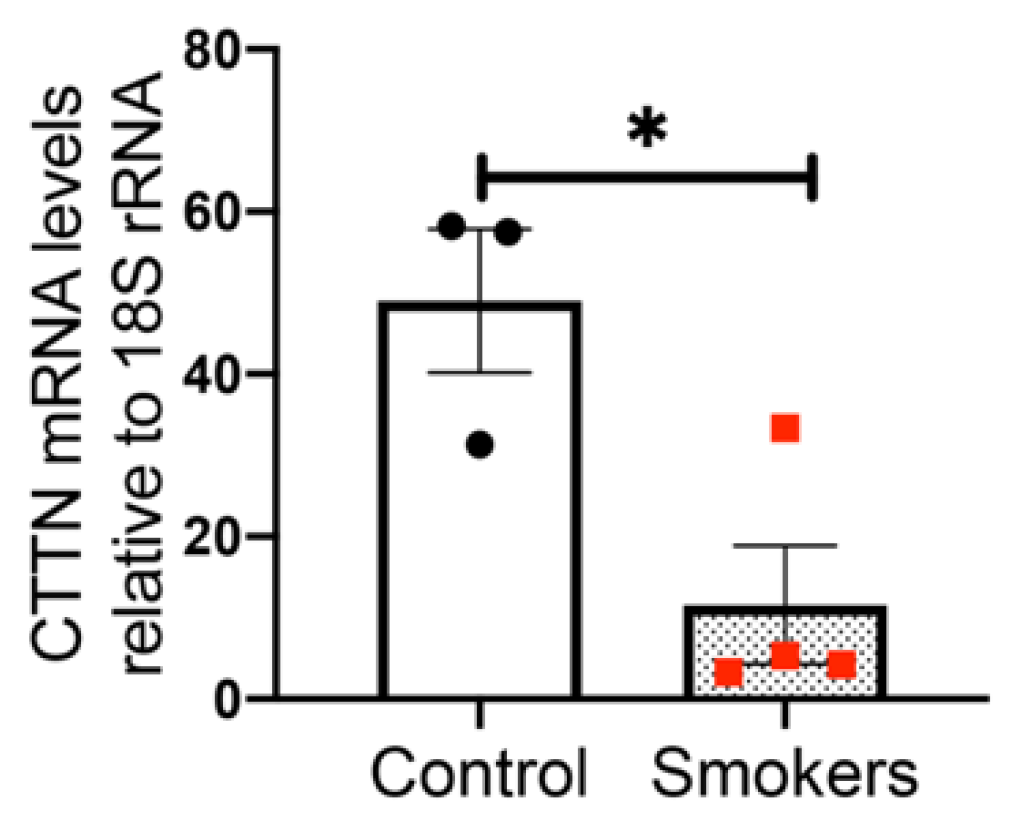
3.1. Cortactin mRNA Levels Are Decreased in Human Lung Tissues from Smokers Compared to Non-Smokers
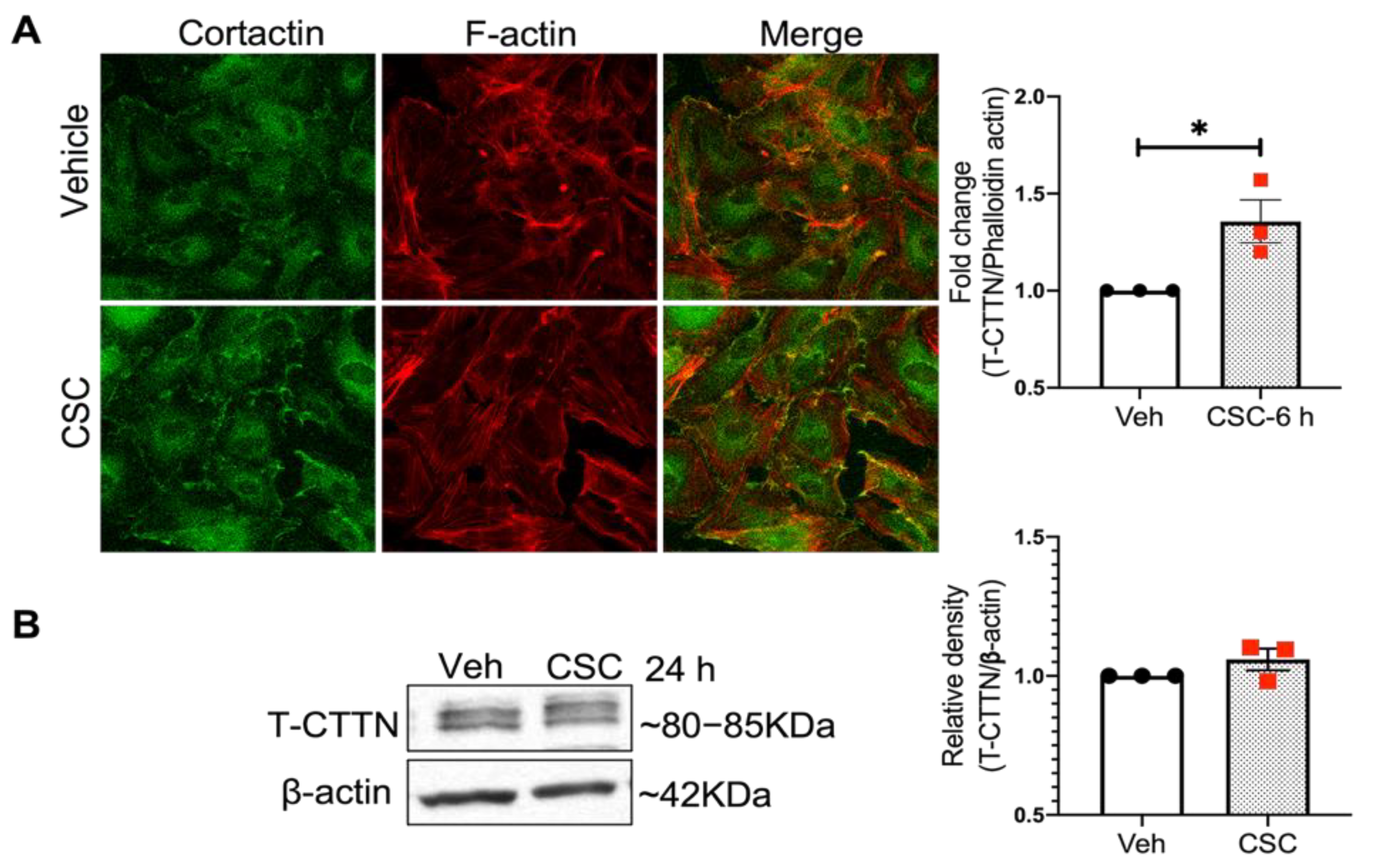
3.2. Cigarette Smoke Condensate Induces Cytoskeletal Rearrangement in Human Lung Endothelial Cells
3.3. CSC Induces CTTN Tyrosine Phosphorylation in Human Lung EC
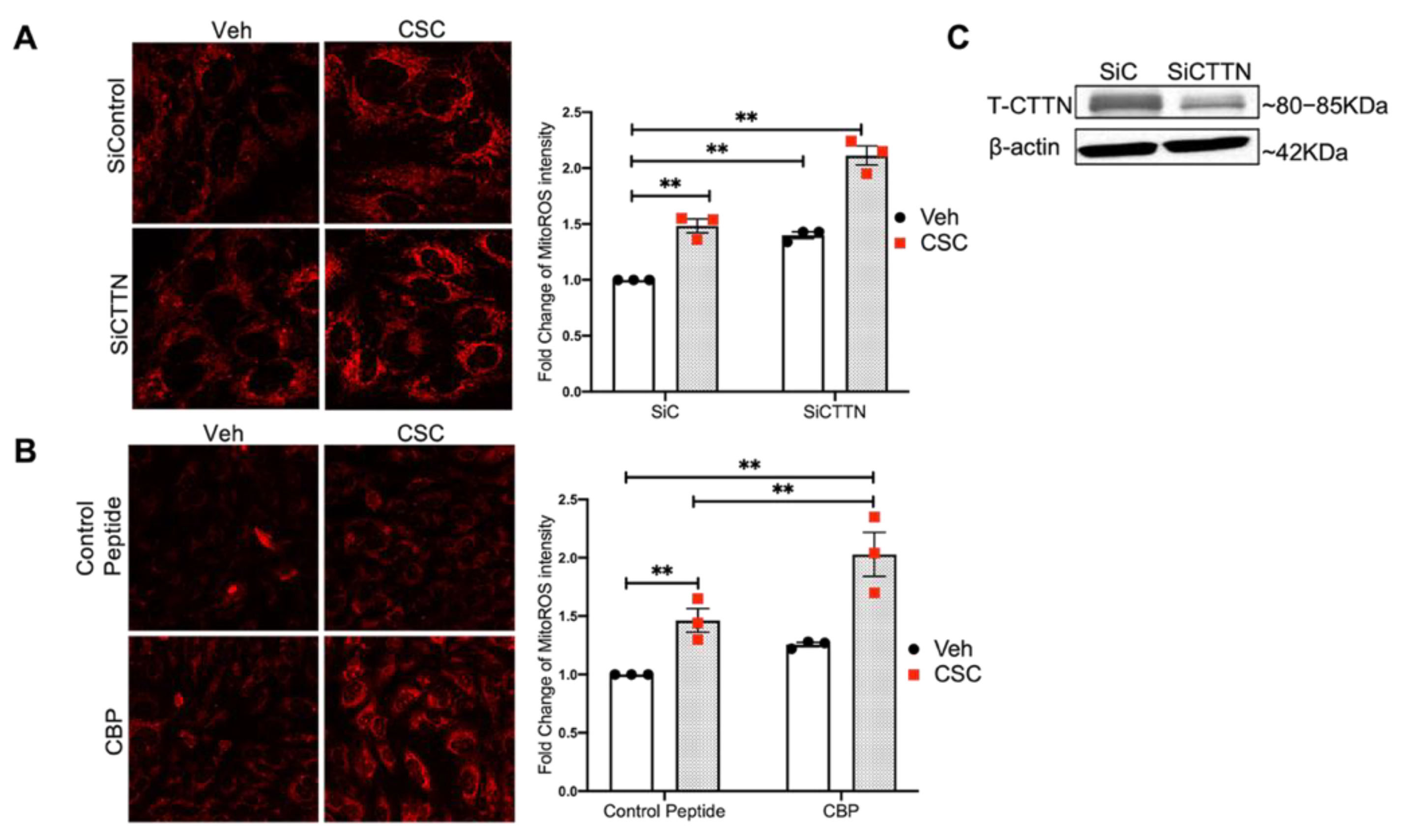
3.4. Inhibition of CTTN Expression or Its SH3 Domain Augments CSC-Induced MitoROS in Lung EC
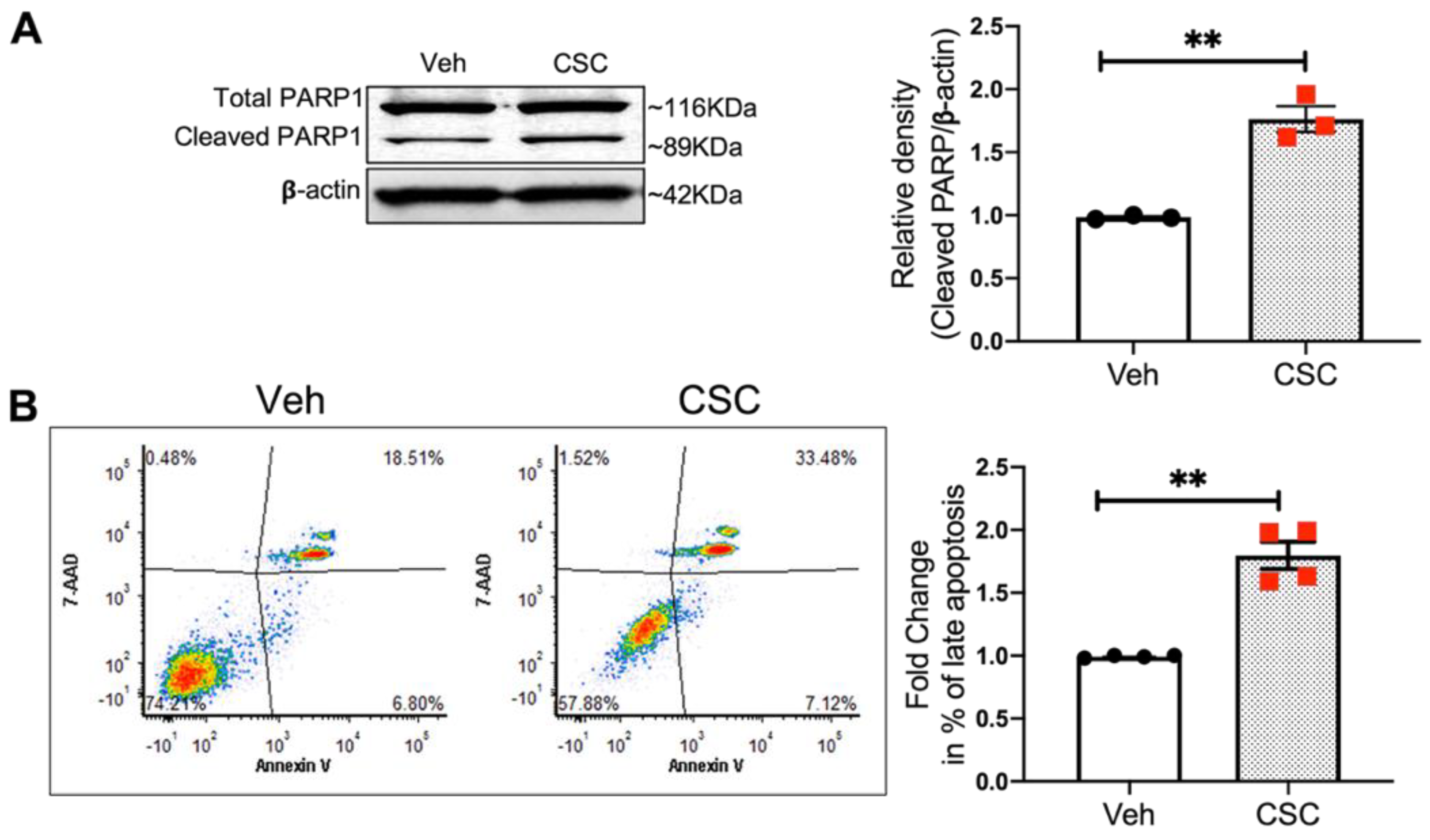
3.5. CSC Induces Apoptosis in Lung EC
3.6. CTTN Expression Regulates CSC-Induced Apoptosis in Lung EC
3.7. CTTN SH3 Domain Interactions Regulate CSC-Induced Apoptosis in Lung EC
3.8. CTTN Expression Regulates E-Cigarette-Induced Apoptosis in Lung EC
3.9. CTTN SH3 Domain Interactions Regulate E-Cigarette-Induced Apoptosis in Lung EC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.; Wrobel, J. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 871–888. [Google Scholar] [CrossRef]
- Barnes, P.J. Chronic obstructive pulmonary disease: A growing but neglected global epidemic. PLoS Med. 2007, 4, e112. [Google Scholar] [CrossRef]
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140. [Google Scholar] [CrossRef]
- Lu, Q.; Gottlieb, E.; Rounds, S. Effects of cigarette smoke on pulmonary endothelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L743–L756. [Google Scholar] [CrossRef]
- Chambers, E.; Rounds, S.; Lu, Q. Pulmonary Endothelial Cell Apoptosis in Emphysema and Acute Lung Injury. Adv. Anat. Embryol. Cell Biol. 2018, 228, 63–86. [Google Scholar]
- Messner, B.; Frotschnig, S.; Steinacher-Nigisch, A.; Winter, B.; Eichmair, E.; Gebetsberger, J. Apoptosis and necrosis: Two different outcomes of cigarette smoke condensate-induced endothelial cell death. Cell Death Dis. 2012, 3, e424. [Google Scholar] [CrossRef]
- Jacobson, J.R.; Dudek, S.M.; Singleton, P.A.; Kolosova, I.A.; Verin, A.D.; Garcia, J.G. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am. J. Physiol. Cell. Mol. Physiol. 2006, 291, L289–L295. [Google Scholar] [CrossRef][Green Version]
- Tomar, A.; Lawson, C.; Ghassemian, M.; Schlaepfer, D.D. Cortactin as a target for FAK in the regulation of focal adhesion dynamics. PLoS ONE 2012, 7, e44041. [Google Scholar] [CrossRef]
- Delaguillaumie, A.; Lagaudriere-Gesbert, C.; Popoff, M.R.; Conjeaud, H. Rho GTPases link cytoskeletal rearrangements and activation processes induced via the tetraspanin CD82 in T lymphocytes. J. Cell Sci. 2002, 115 Pt 2, 433–443. [Google Scholar] [CrossRef]
- Ammer, A.G.; Weed, S.A. Cortactin branches out: Roles in regulating protrusive actin dynamics. Cell Motil. Cytoskelet. 2008, 65, 687–707. [Google Scholar] [CrossRef]
- Schnoor, M.; Stradal, T.E.; Rottner, K. Cortactin: Cell Functions of A Multifaceted Actin-Binding Protein. Trends Cell Biol. 2018, 28, 79–98. [Google Scholar] [CrossRef]
- Dudek, S.M.; Garcia, J.G.N. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 2001, 91, 1487–1500. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Liao, K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011, 12, 49. [Google Scholar] [CrossRef]
- Tehrani, S.; Tomasevic, N.; Weed, S.; Sakowicz, R.; Cooper, J.A. Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 11933–11938. [Google Scholar] [CrossRef] [PubMed]
- Joehanes, R.; Just, A.C.; Marioni, R.E.; Pilling, L.C.; Reynolds, L.M.; Mandaviya, P.R. Epigenetic Signatures of Cigarette Smoking. Circ. Cardiovasc. Genet. 2016, 9, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; Navarette, K.A. E-cigarette, or vaping, product use associated lung injury (EVALI): Case series and diagnostic approach. Lancet Respir. Med. 2019, 7, 1017–1026. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, W.; Moshensky, A.; Shakir, Z.; Khan, R.; Crotty Alexander, L.E. Cigarette Smoke and Nicotine-Containing Electronic-Cigarette Vapor Downregulate Lung WWOX Expression, Which Is Associated with Increased Severity of Murine Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 2021, 64, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.W. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Final Report. N. Engl. J. Med. 2020, 382, 903–916. [Google Scholar] [CrossRef]
- Morjaria, J.; Mondati, E.; Polosa, R. E-cigarettes in patients with COPD: Current perspectives. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3203–3210. [Google Scholar] [CrossRef][Green Version]
- Khalil, C.; Chahine, J.B.; Haykal, T.; Al Hageh, C.; Rizk, S.; Khnayzer, R.S. E-cigarette aerosol induced cytotoxicity, DNA damages and late apoptosis in dynamically exposed A549 cells. Chemosphere 2020, 263, 127874. [Google Scholar] [CrossRef]
- Anderson, C.; Majeste, A.; Hanus, J.; Wang, S. E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicol. Sci. 2016, 154, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Landeck, M.; Koyama, T.; Zhao, Z.; Singer, J.; Kern, R. A randomized trial of the effects of nebulized albuterol on pulmonary edema in brain-dead organ donors. Arab. Archaeol. Epigr. 2014, 14, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Dudek, S.M.; Jacobson, J.R.; Chiang, E.T.; Birukov, K.G.; Wang, P.; Zhan, X. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: Roles for cortactin and myosin light chain kinase. J. Biol. Chem. 2004, 279, 24692–24700. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Meyer, N. Annexin V/7-AAD staining in keratinocytes. Methods Mol. Biol. 2011, 740, 57–63. [Google Scholar] [PubMed]
- Bikkavilli, R.K.; Zerayesus, S.A.; Van Scoyk, M.; Wilson, L.; Wu, P.Y.; Baskaran, A. K-homology splicing regulatory protein (KSRP) promotes post-transcriptional destabilization of Spry4 transcripts in non-small cell lung cancer. J. Biol. Chem. 2017, 292, 7423–7434. [Google Scholar] [CrossRef]
- Ryter, S.W.; Rosas, I.O.; Owen, C.A.; Martinez, F.J.; Choi, M.E.; Lee, C.G. Mitochondrial Dysfunction as a Pathogenic Mediator of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S4), S266–S272. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.K.; Wu, P.P.; Wang, Y.; Ren, L.Y.; Xu, A.H. Necroptosis Mediates Cigarette Smoke-Induced Inflammatory Responses in Macrophages. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Aravamudan, B.; Kiel, A.; Freeman, M.; Delmotte, P.; Thompson, M.; Vassallo, R. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L840–L854. [Google Scholar] [CrossRef]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef]
- Rahman, I.; Adcock, I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006, 28, 219–242. [Google Scholar] [CrossRef]
- Suryadevara, V.; Huang, L.; Kim, S.J.; Cheresh, P.; Shaaya, M.; Bandela, M. Role of phospholipase D in bleomycin-induced mitochondrial reactive oxygen species generation, mitochondrial DNA damage, and pulmonary fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L175–L187. [Google Scholar] [CrossRef]
- Huang, L.S.; Kotha, S.R.; Avasarala, S.; VanScoyk, M.; Winn, R.A.; Pennathur, A. Lysocardiolipin acyltransferase regulates NSCLC cell proliferation and migration by modulating mitochondrial dynamics. J. Biol. Chem. 2020, 295, 13393–13406. [Google Scholar] [CrossRef]
- Weaver, A.M.; Heuser, J.E.; Karginov, A.V.; Lee, W.L.; Parsons, J.T.; Cooper, J.A. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr. Biol. 2002, 12, 1270–1278. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, K.; Hao, J.J.; Liu, J.; Smith, N.; Zhan, X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J. Cell Sci. 2005, 118 Pt 4, 807–817. [Google Scholar] [CrossRef]
- Dudek, S.M.; Birukov, K.G.; Zhan, X.; Garcia, J.G. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 2002, 298, 511–519. [Google Scholar] [CrossRef]
- Belvitch, P.; Htwe, Y.M.; Brown, M.E.; Dudek, S. Cortical Actin Dynamics in Endothelial Permeability. Curr. Top. Membr. 2018, 82, 141–195. [Google Scholar]
- Chen, Y.R.; Kori, R.; John, B.; Tan, T.H. Caspase-mediated cleavage of actin-binding and SH3-domain-containing proteins cortactin, HS1, and HIP-55 during apoptosis. Biochem. Biophys. Res. Commun. 2001, 288, 981–989. [Google Scholar] [CrossRef]
- Hiemstra, P.; Bals, R. Basic science of electronic cigarettes: Assessment in cell culture and in vivo models. Respir. Res. 2016, 17, 127. [Google Scholar] [CrossRef]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Nyunoya, T.; Mebratu, Y.; Contreras, A.; Delgado, M.; Chand, H.S.; Tesfaigzi, Y. Molecular processes that drive cigarette smoke-induced epithelial cell fate of the lung. Am. J. Respir. Cell Mol. Biol. 2014, 50, 471–482. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Gao, H.; Zeng, Y. DNA Methylation in Pulmonary Fibrosis. Adv. Exp. Med. Biol. 2020, 1255, 51–62. [Google Scholar]
- Gao, X.; Zhang, Y.; Breitling, L.P.; Brenner, H. Tobacco smoking and methylation of genes related to lung cancer development. Oncotarget 2016, 7, 59017–59028. [Google Scholar] [CrossRef]
- Lin, B.H.; Tsai, M.H.; Lii, C.K.; Wang, T.S. IP3 and calcium signaling involved in the reorganization of the actin cytoskeleton and cell rounding induced by cigarette smoke extract in human endothelial cells. Environ. Toxicol. 2016, 31, 1293–1306. [Google Scholar] [CrossRef]
- Sakhatskyy, P.; Gabino Miranda, G.A.; Newton, J.; Lee, C.G.; Choudhary, G.; Vang, A. Cigarette smoke-induced lung endothelial apoptosis and emphysema are associated with impairment of FAK and eIF2alpha. Microvasc. Res. 2014, 94, 80–89. [Google Scholar] [CrossRef]
- Carnevali, S.; Petruzzelli, S.; Longoni, B.; Vanacore, R.; Barale, R.; Cipollini, M. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L955–L963. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, C.W.; Hwang, K.A.; Choi, D.W.; Choi, K.C. Three components of cigarette smoke altered the growth and apoptosis of metastatic colon cancer cells via inducing the synthesis of reactive oxygen species and endoplasmic reticulum stress. Environ. Toxicol. Pharmacol. 2016, 45, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Munakata, S.; Ishimori, K.; Kitamura, N.; Ishikawa, S.; Takanami, Y.; Ito, S. Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco- and nicotine-containing products. Regul. Toxicol. Pharmacol. 2018, 99, 122–128. [Google Scholar] [CrossRef]
- Ito, S.; Araya, J.; Kurita, Y.; Kobayashi, K.; Takasaka, N.; Yoshida, M. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 2015, 11, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, S.; Roelofs, B.A.; Boyman, L.; Lederer, W.J.; Sesaki, H. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 2015, 208, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Uruno, T.; Haudenschild, C.; Dudek, S.M.; Garcia, J.G.; Zhan, X. Interaction of cortactin and Arp2/3 complex is required for sphingosine-1-phosphate-induced endothelial cell remodeling. Exp. Cell Res. 2004, 298, 107–121. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Ye, J.; Li, X.; Zhang, D.; Liang, D. Aberrant dynamin 2-dependent Na(+)/H(+) exchanger-1 trafficking contributes to cardiomyocyte apoptosis. J. Cell Mol. Med. 2013, 17, 1119–1127. [Google Scholar] [CrossRef]
- Unsicker, C.; Cristian, F.B.; von Hahn, M.; Eckstein, V.; Rappold, G.A.; Berkel, S. SHANK2 mutations impair apoptosis, proliferation and neurite outgrowth during early neuronal differentiation in SH-SY5Y cells. Sci. Rep. 2021, 11, 2128. [Google Scholar] [CrossRef]
- Man, W.; Gu, J.; Wang, B.; Zhang, M.; Hu, J.; Lin, J. SHANK3 Co-ordinately Regulates Autophagy and Apoptosis in Myocardial Infarction. Front. Physiol. 2020, 11, 1082. [Google Scholar] [CrossRef]
- Petrache, I.; Birukov, K.; Zaiman, A.L.; Crow, M.T.; Deng, H.; Wadgaonkar, R. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J. 2003, 17, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Husain, M. Caspase-mediated degradation of host cortactin that promotes influenza A virus infection in epithelial cells. Virology 2016, 497, 146–156. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Husain, M. Caspase-Mediated Cleavage of Human Cortactin during Influenza A Virus Infection Occurs in Its Actin-Binding Domains and Is Associated with Released Virus Titres. Viruses 2020, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Husain, M. Cortactin Mediates Apoptosis of Gastric Epithelial Cells Induced by VacA Protein of Helicobacter pylori. Dig. Dis. Sci. 2015, 61, 80–90. [Google Scholar]
- Wu, X.; Zhang, H.; Qi, W.; Zhang, Y.; Li, J.; Li, Z. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018, 9, 171. [Google Scholar] [CrossRef]
- Zeidler, R.; Albermann, K.; Lang, S. Nicotine and apoptosis. Apoptosis 2007, 12, 1927–1943. [Google Scholar] [CrossRef]
- Garcia-Arcos, I.; Geraghty, P.; Baumlin, N.; Campos, M.; Dabo, A.J.; Jundi, B. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 2016, 71, 1119–1129. [Google Scholar] [CrossRef]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef]
- Weaver, C.V.; Liu, S.-P. Differentially expressed pro- and anti-apoptogenic genes in response to benzene exposure: Immunohistochemical localization of p53, Bag, Bad, Bax, Bcl-2, and Bcl-w in lung epithelia. Exp. Toxicol. Pathol. 2008, 59, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.P.; Hamon, R.; Macowan, M.G.; Thredgold, L.; Reynolds, P.N.; Hodge, S.J. Effects of E-cigarette E-liquid components on bronchial epithelial cells: Demonstration of dysfunctional efferocytosis. Respirology 2019, 25, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Jordt, S.E.; McConnell, R.; Tarran, R. What are the respiratory effects of e-cigarettes? BMJ 2019, 366, l5275. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Polosa, R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Saf. 2014, 5, 67–86. [Google Scholar] [CrossRef]
- Glantz, S.A.; Bareham, D.W. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annu. Rev. Public Health 2018, 39, 215–235. [Google Scholar] [CrossRef]
- Cooper, M.; Harrell, M.B.; Perry, C.L. Comparing young adults to older adults in e-cigarette perceptions and motivations for use: Implications for health communication. Health Educ. Res. 2016, 31, 429–438. [Google Scholar] [CrossRef]
- Glynos, C.; Bibli, S.I.; Katsaounou, P.; Pavlidou, A.; Magkou, C.; Karavana, V. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L662–L672. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.S.; Chen, S.X.; Law, S.; Van Demark, M.; Poirier, C.; Justice, M.J. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L175–L187. [Google Scholar] [CrossRef]
- Lee, W.H.; Ong, S.G.; Zhou, Y.; Tian, L.; Bae, H.R.; Baker, N. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J. Am. Coll. Cardiol. 2019, 73, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.J.; Semlali, A.; Zakrzewski, A.; Chmielewski, W.; Chakir, J. E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway. J. Cell. Physiol. 2016, 232, 1539–1547. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandela, M.; Letsiou, E.; Natarajan, V.; Ware, L.B.; Garcia, J.G.N.; Singla, S.; Dudek, S.M. Cortactin Modulates Lung Endothelial Apoptosis Induced by Cigarette Smoke. Cells 2021, 10, 2869. https://doi.org/10.3390/cells10112869
Bandela M, Letsiou E, Natarajan V, Ware LB, Garcia JGN, Singla S, Dudek SM. Cortactin Modulates Lung Endothelial Apoptosis Induced by Cigarette Smoke. Cells. 2021; 10(11):2869. https://doi.org/10.3390/cells10112869
Chicago/Turabian StyleBandela, Mounica, Eleftheria Letsiou, Viswanathan Natarajan, Lorraine B. Ware, Joe G. N. Garcia, Sunit Singla, and Steven M. Dudek. 2021. "Cortactin Modulates Lung Endothelial Apoptosis Induced by Cigarette Smoke" Cells 10, no. 11: 2869. https://doi.org/10.3390/cells10112869
APA StyleBandela, M., Letsiou, E., Natarajan, V., Ware, L. B., Garcia, J. G. N., Singla, S., & Dudek, S. M. (2021). Cortactin Modulates Lung Endothelial Apoptosis Induced by Cigarette Smoke. Cells, 10(11), 2869. https://doi.org/10.3390/cells10112869





